Abstract
Water stress is the most important environmental factor that limits plant growth and yield. However, plant growth-promoting rhizobacteria (PGPR) can stimulate resistance of the plant host in unsuitable environmental conditions such as water stress. In order to evaluate whether PGPR improve morphological, physiological, and phytochemical traits of the savory plant Satureja hortensis L., the effects of two PGPR strains of Pseudomonas fluorescens Migula (PF-135 and PF-108) under two water conditions (well-watered and 50 % field capacity) were studied by performing a factorial experiment based on randomized complete block design with three replications under commercial greenhouse. The highest values of root and shoot dry matter, root length, plant height, leaf number, and branch number were observed in PF-135-inoculated plants under well-watered conditions, whereas the above-mentioned parameters were found to be the lowest in non-inoculated plants under water stress condition. Chlorophyll a, b, total chlorophyll, and carotenoid contents significantly changed under water stress conditions. The H2O2 and MDA contents of root and shoot significantly decreased in plants inoculated with PF-135, whereas their contents increased in non-inoculated plants under water stress condition. The highest shoot oil yield was observed in plants inoculated with PF-135 under water stress condition, while the lowest shoot oil yield was observed in plants inoculated with PF-108 under well-watered condition. Twenty-eight components were found in the essential oils of S. hortensis. Carvacrol (56.81–78.15 %), γ-terpinene (9.08–22.87 %), and p-cymene (5.78–14.28 %) were identified as the major components in all treatments. Plants under water stress conditions showed the highest yield of these components when inoculated with bacteria. Thus, we could suggest that the promising strains of P. fluorescens are able to minimize the deleterious effects of water stress on plant growth and improve the morphological and physiological traits of plants as well as increase the essential oil yield and quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microorganisms can affect (positive or negative) plant growth and induce specific secondary metabolite pathways in plants (Sánchez-Blanco et al. 2004). Among the various bacteria in the rhizosphere, plant growth-promoting rhizobacteria (PGPR) have positive influences on plant growth and yield via direct and indirect mechanisms (Glick 1995). The improved growth and productivity of plants take place through the root system architecture modulation, and the shoot growth is promoted by the production of phytohormones such as auxins and cytokinins. PGPR can indirectly stimulate plant growth by producing some metabolites, such as antibiotics and hydrogen cyanide, which decrease the inhibitory effects of various pathogens and deleterious microorganisms in the rhizosphere. In addition, PGPR may activate plant defense response by inducing a systemic resistance in the plant against both root and foliar pathogens as well as induce systemic tolerance to abiotic stress (Ortíz-Castro et al. 2009). PGPR comprise a diverse group of rhizosphere-colonizing bacteria which can stimulate plant resistance to unsuitable environmental conditions (Glick 1995; Vessey 2003). PGPR are able to induce the synthesis of osmoprotectants such as proline and glycine betaine and help in maintaining cell membrane integrity, all of which contribute to improve stress tolerance in plants (Glick et al. 2007).
Plants produce and release a broad range of both volatile and non-volatile substances, such as sugars, amino acids, organic acids, and vitamins, which can be used by microorganisms as nutrient sources or environmental signals. Microorganisms also release both volatile and non-volatile compounds, such as phytohormones and small molecules that can directly or indirectly activate plant immunity defense pathways, and promote plant growth and morphogenesis (Ortíz-Castro et al. 2009). Also, higher plants are able to synthesize wide types of compounds entitled secondary metabolites. Secondary metabolites are not essential for plant growth, but they play an important role in defense responses of plants to adapt to their environment (Wink 1988).
Yield and chemical compositions of medicinal plants are influenced by biotic and abiotic factors (Heywood 2002). Some environmental factors (e.g., drought stress) severely reduce the yield of plants and increase reactive oxygen species (ROS) in plants (Neill et al. 2002; Chaves et al. 2003; Apel and Hirt 2004). ROS such as superoxide radical, hydrogen peroxide, and hydroxyl radical have a role in lipid peroxidation (Imlay 2003), membrane damage, and consequently cell death (Jones 2000; Vranová et al. 2002).
Summer savory (Satureja hortensis L.), a herbaceous plant belonging to the family Lamiaceae, is used as a spice and traditional herb in Iran (Zargari 1999). The antispasmodic, antidiarrheal, antioxidant, sedative, and antimicrobial properties of summer savory oils and extracts resulted in the extensive application of them in the food and pharmaceutical industries (Svoboda and Greenaway 2003; Gursoy et al. 2009). Essential oil (EO) of summer savory has a high percentage of Carvacrol, which is mainly responsible for biological activities, including antimicrobial, antioxidant, antidiabetic, antihyperlipidemic, antispasmodic, antinociceptive, antiinflammatory, antiproliferative, sedative, and reproduction stimulatory (Martini et al. 1996; Adorjan and Buchbauer 2010).
The concentrations and compositions of secondary metabolites in savory oils (e.g., y-terpinene, p-cymene, Carvacrol methyl ether, caryophyllene) play important ecological roles as they possess insecticidal, antifungal, and antibacterial properties (Sangwan et al. 2001; del Rosario Cappellari et al. 2013; Farzaneh et al. 2015; Farzaneh and Carvalho 2015). In other studies, terpene emissions and subsequent attracting mechanisms have been shown to play an indirect role in plant defense mechanisms (Zwenger 2008). Moreover, some volatile compounds may act as airborne signals that can directly or indirectly induce systemic resistance in the plant and also induce defense responses in neighboring plants (Heil and Bueno 2007; Ton et al. 2007).
By considering the positive effects of PGPR on the plants, it could be suggested that the use of PGPR in agriculture systems can noticeably improve plant health and productivity especially under biotic and abiotic stresses conditions. In addition, PGPR may alleviate water stress in medicinal plants, thus increasing the plant yield and EOs quality. The main goals of this study were to (1) evaluate the influences of two PGPR strains on some morphological, physiological, and phytochemical characteristics as well as the EO composition of summer savory plant under well-watered and water stress conditions, and (2) show that the use of PGPR improves the yield and quality of savory EO under water stress conditions.
Materials and methods
Plant materials
Satureja hortensis seeds were prepared in the Agricultural and Natural Resources Center in Esfahan, Iran. Seeds were surface-sterilized by shaking them for 5 min in 1 % sodium hypochlorite (NaOCl) solution and then washed with distilled water for 10 min. Subsequently, the seeds were placed between two layers of the filter paper (Whatman no. 1) in petri dishes moistened with 5 mL of distilled water. After 3 days, the germinated seeds (with 1–2 mm radical length) appeared.
Bacterial strains and screening
Twenty PGPR strains (belonging to the fluorescent pseudomonads group) were obtained from the Department of Plant Protection, Azarbaijan Shahid Madani University, Tabriz, Iran. The PGPR strains were screened for their ability to enhance the summer savory seedling growth under in vitro and sand culture assays. Briefly, the promising PGPR isolates were grown in 100-mL Erlenmeyer flasks containing 25 mL of tryptone soybean broth (TSB) for 72 h at 28 °C on a rotating shaker incubator (120 rpm). The bacterial suspension was centrifuged (6000 rpm for 15 min) and then washed and diluted in sterile 0.85 % saline solution (NaCl) to achieve a final concentration of 109 colony-forming units (CFU) per mL. The prepared suspensions were inoculated into savory radicals and culture media under aseptic conditions.
Greenhouse PGPR activity assay
Five germinated seeds (with 1–2 mm radical length) of summer savory were placed in the soil (a mixture of clay, sand, and silt in the ratios of 3.5, 75.5, and 21 %, respectively) surface in polyethylene pots. Then, 100 µL of bacterial inocula (109 CFU mL−1) of two promising strains, P. fluorescens PF-135 and P. fluorescens PF-108, were poured over each seed. First, watering was done by spraying water on the soil surface. After seedling emergence, pots were watered by adjusting the water level on the underneath tray at a 4-day interval. An extra light was provided in the greenhouse for 16 h per day with a temperature of 28/18 °C (day/night). At the 3–4 leaf stage, plants were thinned to four per pot.
A factorial experiment based on randomized complete block design was carried out with three replications. The experiment involved the following treatments (N = 3): (1) non-inoculated plants; (2) plants inoculated with Pseudomonas fluorescens strains (PF-135); and (2) plants inoculated with Pseudomonas fluorescens strains (PF-108). Treatments were subjected to two levels of irrigation: (i) well-watered condition, irrigated every 2 days to achieve field capacity (FC), and (ii) water stress condition, with 50 % of FC. Plants were exposed to water stress during initial flowering (80 days after planting for 6 weeks) with daily weighting pots.
Plant morphological and physiological parameters
After the experiment period, plant height, the number of leaves and branches, and shoot and root dry weight were characterized. Subsequently, plants were harvested individually at the flowering stage and were immediately frozen in liquid nitrogen for 2 min, and then stored at −70 °C for the physiological measurements such as chlorophyll a, b, total chlorophyll and carotenoids, malondialdehyde (MDA), H2O2, and proline contents.
Leaf relative water content determination
Relative water content (RWC) from fresh leaves was determined according to the method of Barrs and Weatherley (1962):
where FW, DW, and TW mean fresh weight, dry weight, and turgor weight, respectively.
Plastid pigment measurements
Chlorophyll (Chl) and carotenoids were extracted from 0.1 g of the youngest fully expanded fresh leaves which were grounded in 0.5 mL of acetone (80 % v/v). The absorption was recorded at 645 nm (Chl a), 663 nm (Chl b), and 470 nm (carotenoids) in a spectrophotometer (PG Instrument LTD T80+UV/VIS). Photosynthetic pigment contents were calculated using the following equations as described by Lichtenthaler and Wellburn (1983):
Determination of H2O2 content
Hydrogen peroxide (H2O2) content in the leaves of the savory plant was determined according to the method of Velikova et al. (2000). Briefly, fresh tissues of leaves (0.1 g) were homogenized in an ice bath with 5 mL of TCA (0.1 % w/v). The homogenate was centrifuged at 12,000×g for 15 min. Then 0.5 mL of supernatant was supplemented with 0.5 mL of 10 mm potassium phosphate buffer (pH 7.0) and 1 mL of 1 m KI. Finally, the absorbance of the supernatant was recorded at 390 nm in a spectrophotometer (PG Instrument LTD T80+ UV/VIS). The content of H2O2 was estimated by comparison with a standard calibration curve which was previously made by various H2O2 concentrations.
Determination of the MDA content
The level of malondialdehyde (MDA) content (as an end product of lipid peroxidation) was assessed according to the method of Heath and Packer (1968). Briefly, 0.1 g of fresh tissues of leaves were homogenized in 5 mL of 0.1 % (w/v) TCA solution and centrifuged at 12,000×g for 15 min at 25 °C. Then, 2 mL of supernatant was added to 2 mL of 0.6 % (w/v) TBA. The mixture was incubated at 95 °C for 30 min, cooled on ice, and the samples were then centrifuged at 4000×g for 20 min. The absorbance of the supernatant was recorded at 532 nm. The MDA content was calculated based on its extinction coefficient of 155 mM−1 cm−1.
Determination of proline content
The proline contents in leaf tissue were measured according to the method of Bates et al. (1973). The homogenized fresh leaf material (0.1 g) in 3 % aqueous sulfosalicylic acid (10 mL) was centrifuged at 10,000 rpm. Then, 2 mL of supernatant fluid was mixed in the glass test tube containing 2 mL of acid ninhydrin and 2 mL of glacial acetic acid and incubated in water bath for 1 h at 100 °C. The proline content of mixture was extracted with 4 mL toluene, cooled to room temperature, and estimated based on the color change at 520 nm in a spectrophotometer (PG Instrument LTD T80+ UV/VIS). Appropriate proline standards were included in the calculation of proline in the samples.
Essential oils analyses
The aerial parts of each plant had their oil yields (w/w) and components extracted by hydro-distillation in a Clevenger type apparatus for 3–4 h, dried over anhydrous sodium sulfate, and were kept in dark sealed vials at 4 °C until analysis (Farzaneh et al. 2015). Gas chromatography equipped with flame ionization detector (GC-FID) analyses of the essential oils were carried out using a Thermoquest-Finnigan instrument. The analyses were performed on DB-5 fused silica capillary column (30 m × 0.25 mm i.d., film thickness 0.25 µm). The carrier gas used was nitrogen at a constant flow rate of 1.1 mL min−1. The oven temperature program was set at 60–250 °C at the rate of 4 °C min−1 and held isothermally for 10 min in the final. The injector and detector (FID) temperature were set at 250 and 280 °C, respectively. The split ratio was set at 1:50. Gas chromatography–mass spectrometry (GC–MS) analyses were performed on Thermoquest-Finnigan Trace GC–MS instrument equipped with the same gas chromatography condition as mentioned for GC. The carrier gas used was helium at the flow rate of 1.1 mL min−1 with the split ratio at 1:50. The transfer line and ion source temperature were set at 200 and 250 °C, respectively. The ionization voltage was set at 70 eV.
The components of essential oils were identified by comparing their mass spectra and retention indices (RI) with those in the published data and with authentic compounds (Adams 2007). Retention indices of the essential oils’ components were calculated by homologous n-alkanes (C6–C24) under the same conditions.
Statistical analysis
The data were analyzed using SAS statistical software. All response variables were analyzed using a General Linear Model (GLM) with Tukey HSD post hoc test for pairwise comparisons.
Results and discussion
Our results showed that the water stress reduced the plant height, the number of subsidiary branches per plant, root length, leaf number, and root and shoot dry weight (Table 1, Table S1). However, water stress improved malondialdehyde (MDA), H2O2, and proline contents on shoots and roots, and also improved total chlorophyll, chlorophyll a, b, and carotenoid contents (Table 1, Table S1). On the other hand, the maximum amount of these traits was observed in well-watered plants (Table 1).
Savory plants inoculated with PF-135 increased the plant height, the number of subsidiary branches per plant, root length, leaf number, relative water content, and proline contents on roots and shoots under water stress (Table 1). On the other hand, inoculation reduced the root and shoot MDA and H2O2 contents, while it did not affect root dry weight, and chlorophyll and carotenoid contents (Table 1). The plant growth-promoting rhizobacteria (PGPR) stimulated the root growth by improving the nutrient status or by influencing other beneficial symbiotic relationships (Vessey 2003). H2O2 and malondialdehyde (MDA) contents decreased in plants under well-watered and water stress conditions when inoculated with bacteria (Table 1). These results may be due to a considerable increment in the antioxidant enzyme expression that reduced the malondialdehyde levels and electrolyte leakage (Lu et al. 2015). In the plants’ cells, some compounds such as lipid soluble antioxidants (α-tocopherol and carotenoids), water-soluble reductants (glutathione and ascorbate), and antioxidant enzymes could protect the plants from the harmful effects of reactive oxygen species (ROS) (Desikan et al. 2004). In addition, accumulation of some osmolytes (e.g., for example proline) in plant cells could scavenge free radicals and protect enzymes (Krishnan et al. 2008). As a consequence, inoculation with bacteria seems to ameliorate cell membrane damage from oxidative stress caused by water stress conditions.
Water stress reduced root dry weight in general, even in inoculated treatments, but the effect was more prominent in plants inoculated with PF-108 (Table 1), which may be due to an overall decrease in newly synthesized cell wall polysaccharides such as pectins, hemicelluloses, and cellulose (Piro et al. 2003). Similarly, Liu et al. (2011) found that drought reduced both shoot and root dry weight in Asian red sage, but the effect was more severe on shoots. In our study, bacterial inoculation did not affect root dry weight under well-watered conditions, whereas at water stress condition, significant difference in root dry weight was observed due to bacterial strain inoculation.
Relative water content, which is a good indicator of plant water status and even a relevant screening tool of drought tolerance (Teulat et al. 2003), has been reduced significantly in water-stressed plants in this study (Table 1). However, inoculation with PF-135 caused an increase in RWC in both well-watered and water stress conditions (Table 1), possibly due to the accumulation of compatible solutes such as proline. In fact, root and shoot proline contents also increased after inoculation with PF-135 and PF-108. The results showed that inoculation with bacteria increased the proline contents under water stress conditions (Table 1). The accumulation of nitrogenous compounds such as amino acids (e.g., proline), amides, proteins, quaternary ammonium compounds, and polyamines in response to abiotic stresses has frequently been reported (Amudha and Balasubramani 2011). Also, proline has been proposed to act as a compatible osmolyte and ROS scavenger (Maggio et al. 2002). However, this increment in proline content could prevent chlorophyll and carotenoids degradation presumably because proline molecules are accumulated in the cytosol and, regard to osmotic adjustment, is the net increase in intercellular solutes in response to water stress, which allows turgor maintenance at lower water potential (Chaves et al. 2003). Under drought condition, the plant cell lost intracellular water as a result of which cellular dehydration occurred. In order to prevent degradation, plant cells may accumulate many organic compounds such as proline, quaternary and other amines, a variety of sugars (mainly fructose and sucrose), and sugar alcohols (Valliyodan and Nguyen 2006). These osmoprotectants may be accumulated to high levels without disturbing the intracellular biochemistry (Ford 1984), and therefore, water loss would be prevented and osmotic adjustment would be facilitated (Delauney and Verma 1993).
Photosynthetic pigments (chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids) significantly increased with water stress (Table 1), but inoculation did not affect any pigment content. This could be related to chloroplast development, as it has been reported by other authors (Chang et al. 1997). In addition, non-inoculated and inoculated plants showed similarities in their photosynthetic pigments (Table 1).
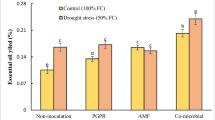
Essential oil yields varied in our study from 0.78 to 1.32 % (Table 1). Plants inoculated with PF-135 increased their percentage of essential oils under well-watered and water stress conditions, while plants inoculated with PF-108 showed a reduction in their essential oil yields under water stress conditions (Table 1). Other studies have reported that the oil contents of different Iranian accessions of S. hortensis are between 0.5 and 2.9 % (Sefidkon et al. 2006; Hadian et al. 2010), and can accumulate under severe water stress at the flowering stage (Baher et al. 2002). However, when the plants are exposed to drought stress, they could probably accumulate a higher concentration of secondary metabolites which are the backbone of their essential oils (Selmar and Kleinwächter 2013). Medicinal plants inoculated with rhizobacteria such as P. fluorescens or Bradyrhizobium sp. increase their essential oils in response to biotic and abiotic stress as a result of terpene biosynthesis (Banchio et al. 2008).
Twenty-eight compounds were identified representing around 98.72–99.68 % of essential oils (Table 2). The major components of essential oils were Carvacrol (56.81–78.15 %), γ-terpinene (9.08–22.87 %), and p-cymene (5.78–14.28 %). Hadian et al. (2010) have reported that chemical constituents of essential oils of different Iranian accessions of S. hortensis consisting of Carvacrol ranged between 42.0 and 83.3 %. In our study, plants inoculated with the strain PF-135 accumulated a great quantity of Carvacrol under well-watered and water stress conditions, while plants inoculated with the strain PF-108 showed high concentrations of Carvacrol under well-watered conditions. In addition, other treatments could also increase (11.3–19.3 %) Carvacrol content. On the other hand, γ-terpinene content decreased to 48–60 % in all treatments. The strain PF-108 under well-watered condition and the strain PF-135 under well-watered and water stress conditions resulted in a considerable reduction (48–60 %) in γ-terpinene content. The results on p-cymene content showed that the effect of water stress is stronger than that of bacterial inoculation, as water stress caused 51–57 % of reduction in p-cymene content. Furthermore, water stress and bacterial inoculation could considerably influence the biosynthesis and accumulation of other volatile compounds in the plant. Water stress resulted in the increase of 1,8-cineol (2.14-fold), cis-sabinene hydrate (3.75-fold), and trans-sabinene hydrate contents (6.44-fold), remarkably. Bacterial inoculation only increased the contents of 1,8-cineol (0.714- to 0.857-fold), cis-sabinene hydrate (0.875- to 1.87-fold), and trans-sabinene hydrate (1.33- to 3.77-fold) under well-watered conditions. In addition, plants inoculated with PF-135 and PF-108 showed a reduction in their contents of α-pinene, β-myrcene, and thymol under well-watered and water stress conditions. Interestingly, the variations in thymoquinone contents depended on the bacterial strain. The strain PF-135 decreased the thymoquinone content, while PF-108 increased it under well-watered conditions. It was similarly demonstrated that in the presence of mycorrhiza or rhizobacteria, the production of certain terpenes in the plant would also change (Gupta et al. 2002; Banchio et al. 2008). It was also reported that the inoculation of sweet marjoram plants with rhizobacteria increased the concentrations of some compounds in EO such as terpinene-4-ol, cis-sabinene hydrate, trans-sabinene hydrate, and α-terpineolin (Banchio et al. 2008). Essential oil biosynthesis is relevant to the production of certain terpenes that can be considered as a defensive response to biotic and abiotic stresses (Sangwan et al. 2001; Banchio et al. 2008; Selmar and Kleinwächter 2013).
Our results suggest that inoculation of S. hortensis with certain P. fluorescens strains can significantly increase the plant biomass and some essential oil yields under water stress condition. However, the inoculation with P. fluorescens (PF-135) not only improves the activity of antioxidant enzymes but also induces the synthesis of proline which is one of the osmolytes responsible for maintaining cell turgor under water stress condition. In addition, the promising strains of P. fluorescens improved essential oil yield and Carvacrol content under water stress and well-watered condition. Finally, P. fluorescens (PF-135) strain seems to minimize the deleterious effects of water stress on plant growth parameters, improving essential oil yield and quality in low input system. Unfortunately, few studies have attempted to elucidate the relative quantitative and qualitative contributions of rhizobacteria to the formation of secondary compounds in aromatic plants. Therefore, future studies under greenhouse and field conditions will be required to find the potential of both promising strains as PGPR agents for commercial production of aromatic plants in low input system.
References
Adams R (2007) Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured, Carol Stream
Adorjan B, Buchbauer G (2010) Biological properties of essential oils: an updated review. Flavour Fragr J 25:407–426
Amudha J, Balasubramani G (2011) Recent molecular advances to combat abiotic stress tolerance in crop plants. Biotechnol Mol Biol Rev 6:31–58
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann Rev Plant Biol 55:373–399
Baher ZF, Mirza M, Ghorbanli M, Bagher Rezaii M (2002) The influence of water stress on plant height, herbal and essential oil yield and composition in Satureja hortensis L. Flavour Frag J 17:275–277
Banchio E, Bogino PC, Zygadlo J, Giordano W (2008) Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem Syst Ecol 36:766–771
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Chang CC, Locy RD, Smeda R, Sahi SV, Singh NK (1997) Photoautotrophic tobacco cells adapted to grow at high salinity. Plant Cell Rep 16:495–502
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
del Rosario Cappellari L, Santoro MV, Nievas F, Giordano W, Banchio E (2013) Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl Soil Ecol 70:16–22
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55:205–212
Farzaneh V, Carvalho IS (2015) A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind Crop Prod 65:247–258
Farzaneh M, Kiani H, Sharifi R, Reisi M, Hadian J (2015) Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol Technol 109:145–151
Ford CW (1984) Accumulation of low molecular weight solutes in water-stressed tropical legumes. Phytochemistry 23:1007–1015
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41:109–117
Glick WH, Miller CC, Cardinal LB (2007) Making a life in the field of organization science. J Organ Behav 28:817–835
Gupta M, Prasad A, Ram M, Kumar S (2002) Effect of the vesicular–arbuscular mycorrhizal (VAM) fungus Glomus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Bioresour Technol 81:77–79
Gursoy UK, Gursoy M, Gursoy OV, Cakmakci L, Könönen E, Uitto VJ (2009) Anti-biofilm properties of Satureja hortensis L. essential oil against periodontal pathogens. Anaerobe 15:164–167
Hadian J, Ebrahimi SN, Salehi P (2010) Variability of morphological and phytochemical characteristics among Satureja hortensis L. accessions of Iran. Ind Crop Prod 32:62–69
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Heil M, Bueno JCS (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci 104:5467–5472
Heywood VH (2002) The conservation of genetic and chemical diversity in medicinal and aromatic plants. In: Şener B (ed) Biodiversity: biomolecular aspects of biodiversity and innovative utilization. Springer Science + Business Media, New York, pp 13–22
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
Jones A (2000) Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trend Plant Sci 5:225–230
Krishnan N, Dickman MB, Becker DF (2008) Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic Biol Med 44:671–681
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Liu H, Wang X, Wang D, Zou Z, Liang Z (2011) Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind Crop Prod 33:4–88
Lu YY, Deng XP, Kwak SS (2015) Over expression of CuZn superoxide dismutase (CuZn SOD) and ascorbate peroxidase (APX) in transgenic sweet potato enhances tolerance and recovery from drought stress. Afr J Biotechnol 9:8378–8391
Maggio A, Miyazaki S, Veronese P, Fujita T, Ibeas JI, Damsz B, Narasimhan ML, Hasegawa PM, Joly RJ, Bressan RA (2002) Does proline accumulation play an active role in stress-induced growth reduction? Plant J 31:699–712
Martini H, Weidenbörner M, Adams S, Kunz B (1996) Eugenol and carvacrol: the main fungicidal compounds in clove and savory. Ital J Food Sci 8:63–67
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4(8):701–712
Piro G, Leucci MR, Waldron K, Dalessandro G (2003) Exposure to water stress causes changes in the biosynthesis of cell wall polysaccharides in roots of wheat cultivars varying in drought tolerance. Plant Sci 165:559–569
Sánchez-Blanco MJ, Ferrández T, Morales MA, Morte A, Alarcón JJ (2004) Variations in water status, gas exchange, and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. J Plant Physiol 161:675–682
Sangwan N, Farooqi A, Shabih F, Sangwan R (2001) Regulation of essential oil production in plants. Plant Growth Regul 34:3–21
Sefidkon F, Abbasi K, Khaniki GB (2006) Influence of drying and extraction methods on yield and chemical composition of the essential oil of Satureja hortensis. Food Chem 99:19–23
Selmar D, Kleinwächter M (2013) Stress enhances the synthesis of secondary plant products: the impact of the stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol 54:817–826
Svoboda K, Greenaway R (2003) Investigation of volatile oil glands of Satureja hortensis L. (summer savory) and phytochemical comparison of different varieties. Int J Aromather 13:196–202
Teulat B, Zoumarou-Wallis N, Rotter B, Salem MB, Bahri H, This D (2003) QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor Appl Genet 108:181–188
Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch Mani B, Turlings TC (2007) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49:16–26
Valliyodan B, Nguyen HT (2006) Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr Opin Plant Biol 9:189–195
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vranová E, Inzé D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Wink M (1988) Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor Appl Genet 75:225–233
Zargari A (1999) Medicinal plants, V.3, 6th edn. Tehran University, Iran
Zwenger S (2008) Plant terpenoids: applications and future potentials. Biotechnol Mol Biol Rev 3:1–7
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, H., Dashi, R., Farzaneh, M. et al. Effects of beneficial root pseudomonas on morphological, physiological, and phytochemical characteristics of Satureja hortensis (Lamiaceae) under water stress. Braz. J. Bot 40, 41–48 (2017). https://doi.org/10.1007/s40415-016-0319-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0319-2