Abstract
Plant growth-promoting rhizobacteria (PGPR) are increasingly accepted and important in agricultural production worldwide. There is rising demand for food produced without synthetic chemical products, for sustainable agricultural methods, and for a holistic vision of development associated with environmental protection. In this study, we found that the effects of PGPR inoculation and volatile organic compound (VOC) emission on various aromatic plant species (Origanum majorana [sweet marjoram], Origanum x majoricum [Italian oregano], Ocimum basilicum [sweet basil], Tagetes minuta [wild marigold], Mentha x piperita [peppermint]) varied depending on the inoculated strain (Pseudomonas fluorescens WCS417r, Azospirillum brasilense Sp7, Bacillus subtilis GB03, or their combination). PGPR-inoculated plant species display host response specificity. The responses we observed were diverse. In most cases, the growth parameters evaluated (leaf number, shoot fresh weight, root dry weight) were significantly increased by P. fluorescens inoculation. Essential oil (EO) yield was increased to varying degrees in O. majorana, O. x majoricum, and T. minuta inoculated with P. fluorescens. Monoterpene accumulation was increased ~2-fold in most cases and 24-fold in O. majorana.
The effects of VOCs emitted by P. fluorescens evaluated in M. piperita showed the same tendency that of direct inoculation. In contrast, inoculation with B. subtilis showed different effects upon the affected plant: O. majorana and O. majoricum did not show any change in EO yield in contrast with O. basilicum. The same result was observed with the exposition to B. subtilis VOCs in O. basilicum but in M. piperita did not affect the total monoterpene accumulation. The total EO yield was increased by direct root inoculation with A. brasilense in O. x majoricum but not in T. minuta. Our findings indicate that inoculation with certain PGPR causes systemic induction of monoterpene pathways in various aromatic plants species, suggesting that PGPR inoculation can significantly increase productivity and reduce the amount of fertilizer required for economically viable aromatic crop production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aromatic plants
- Mentha x piperita
- Tagetes minuta
- Origanum majorana
- Origanum x majoricum
- Ocimum basilicum
- Rhizobacteria
- Volatile organic compounds
- Essential oils
- Pulegone
- Menthone
1 Introduction
Bacteria are by far the most abundant organisms in soil, where they play essential roles in nutrient cycling and soil fertility. Root-colonizing bacteria are commonly referred to as “rhizobacteria.” Many rhizobacterial strains, collectively termed “plant growth-promoting rhizobacteria” (PGPR), enhance plant growth when inoculated on seeds. PGPR species and strains in the genera Acetobacter, Acinetobacter, Alcaligenes, Arthrobacter, Azoarcus, Azospirillum, Azotobacter, Bacillus, Beijerinckia, Burkholderia, Derxia, Enterobacter, Gluconacetobacter, Herbaspirillum, Klebsiella, Ochrobactrum, Pantoea, Pseudomonas, Rhodococcus, Serratia, Stenotrophomonas, and Zoogloea have been the subjects of extensive research for many decades (Babalola 2010). PGPR promote plant growth by both direct and indirect mechanisms (Kloepper 1993; Niranjan et al. 2006; Van Loon 2007). Direct mechanisms include production of stimulatory bacterial volatile organic compounds (VOCs) and phytohormones, reduction of ethylene level in plants, improvement of plant nutrient status (release of phosphates and micronutrients from insoluble sources; nonsymbiotic nitrogen fixation), and enhancement of disease-resistance mechanisms (induced systemic resistance). Indirect effects of PGPR include functioning as biocontrol agents to reduce diseases, promotion of other beneficial symbioses, and protection of plants by degrading xenobiotics in contaminated soils (Figueiredo et al. 2010). Studies during the past 5 years have shown that some PGPR are capable of releasing functional VOCs that trigger growth promotion and induced resistance (Ryu et al. 2004). Depending on the PGPR species, two or more of the above growth-promoting mechanisms may be present (Vessey 2003).
During the past three decades, medicinal and aromatic plants have undergone a transition from unknown or minor agricultural plantings to major crops that farmers may consider as alternatives to traditional food or feed crops. The steadily increasing agricultural role is driven by consumer interest in these plants for culinary, medicinal, and other anthropogenic applications.
Aromatic plant species are a highly diverse group whose common characteristic is the production of essential oils (EOs) (Guenther 1948). EOs are active compounds that can modify behavioral or physiological responses in other organisms (Langenheim 1994). The major EOs in Lamiaceae, a large plant family that includes many aromatic and medicinal species, are terpenes, particularly monoterpenes (C10 members of the terpenoid class).
Terpenes are responsible for the characteristic fragrances of aromatic plants (Chen et al. 2011) and are typically emitted when plant structures are damaged (Wittstock and Gershenzon 2002). Lamiaceae accumulate EOs in specific structures, glandular trichomes (also termed secretory or peltate trichomes), which are lipophilic glands consisting of secretory cells and a cuticle-enclosed cavity that becomes filled with the secreted compound (Werker 2000). The plastids in glandular trichomes have less-defined membrane structures in comparison with chloroplasts and may be associated with synthesis and/or secretion of secondary metabolites such as terpenoids (Werker 2000).
Monoterpenes are among the best studied plant secondary metabolites with defensive functions. These colorless, lipophilic, volatile substances are the major constituents of plant EOs and display defensive effects (toxic, repellent, anti-feeding, anti-ovipositing) against a variety of harmful insects and pathogens (Harrewijn et al. 2001; Chen et al. 2011). Some monoterpenes are involved in plant intraspecific communication (Wittstock and Gershenzon 2002).
Inducible chemical changes are of particular interest in medicinal and aromatic plants, not only in relation to defensive mechanisms as above but also because the altered compounds may have aromatic or therapeutic properties that enhance the economic value of the plant (Banchio et al. 2005). Increased knowledge of factors that affect EO quantity and quality in aromatic plants will be useful for improving production of these natural products and in pest management strategies (Kogan and Fischer 1991).
Chemical fertilizers and pesticides have been used increasingly in recent decades to maximize agricultural production. However, they are responsible for a variety of ecologically and agriculturally deleterious effects, e.g., depletion of nonrenewable energy resources, pollution of watersheds, elimination of beneficial microorganisms and insects, increasing the susceptibility of the crop to disease, and reducing soil fertility (Babalola 2010).
Interest in environmentally safe, sustainable, and organic agricultural practices that reduce negative environmental effects associated with food and feed production is steadily increasing (Lind et al. 2004). “Organic agriculture” is a production system that avoids or minimizes the use of synthetic fertilizers, pesticides, and growth regulators, relying instead on biofertilization, crop rotation, crop residues, mechanical cultivation, and biological pest control to maintain soil productivity. Reduced yield is a major problem and concern in organic production systems. For many medicinal and aromatic plants that are consumed without further processing following harvest, it is important that synthetic compounds not be present.
Unconventional techniques such as inoculation with PGPR must be considered and investigated in the search for new strategies of plant production with high yield but without undesirable compounds or effects. The effects of PGPR inoculation in medicinal and aromatic plants have received very little research attention to date. New, less aggressive biotechnological methods involving the application of beneficial microorganisms as biofertilizers are a viable alternative to the use of chemical fertilizers. There are economic, environmental, and health-related justifications for research on PGPR strains as inoculants for cultivation of medicinal and aromatic plants. Application of these techniques may contribute to environmental conservation, increased crop productivity, and sustainable agricultural practices.
We present here an integrated summary of our experimental findings on induced responses to PGPR in various aromatic plant species of the families Lamiaceae and Asteraceae. Our focus is on the changes in plant EO/VOC composition (particularly of monoterpenes, the major EOs) induced by inoculation with various PGPR species.
2 Materials and Methods
2.1 Bacterial Strains, Culture Conditions, Media, and Treatments
Three bacterial strains well known as PGPR were used. Pseudomonas fluorescens WCS417r and Azospirillum brasilense Sp7 (Van Loon 2007) were grown on LB medium. Bacillus subtilis was grown on TSA for routine use and maintained in nutrient broth with 15 % glycerol at −80 °C for long-term storage.
Bacterial cultures were grown overnight at 30 °C with rotation (120 rpm) until reaching exponential phase. Each culture was then washed twice in 0.9 % NaCl by centrifugation (4,300×g, 10 min, 4 °C) in an Eppendorf centrifuge, resuspended in sterile water, and adjusted to a final concentration of ~109 CFU/ml for use as inoculum. Plants were grown in plastic pots (diameter 12 cm, depth 22 cm) containing 250 g sterilized vermiculite. Seeds were surface sterilized in 70 % ethanol for 5 min, rinsed 5× with sterile water, dipped in 1 % NaCl for 1 min, rinsed 5× with sterile water, planted in vermiculite (one seed per pot), and inoculated with 1 ml bacterial suspension.
2.2 Greenhouse Experiments
Plants were grown in a growth chamber under controlled conditions of light (16/8 h light/dark cycle), temperature (22 ± 2 °C), and relative humidity (~70 %). Bacterial suspensions as described above were applied to experimental seedlings, and sterile water was applied to control seedlings. All plants were watered with Hoagland’s nutrient medium (20 ml/pot) once per week (Banchio et al. 2008). All experiments were performed under non-sterile conditions.
Each experiment was replicated (ten pots per treatment; one plant per pot). Pots were arranged randomly in the growth chamber. Ninety days after inoculation, plants were removed from pots, roots were washed to remove vermiculite, and standard growth parameters (leaf number, shoot fresh weight, root dry weight) were measured.
2.3 Micropropagation of Plants
Young shoots from Mentha x piperita plants grown in Traslasierra Valley (Córdoba province, Argentina) were surface disinfected by soaking for 1 min in 17 % sodium hypochlorite solution and rinsed 3× in sterile distilled water. Disinfected shoots were cultured in 100 ml MS culture medium containing 0.7 % (w/v) agar and 1.5 % (w/v) sucrose (Murashige and Skoog 1962). All culture media contained 30 g/L sucrose and 7.5 g/L agar.
Stage I Initial shoot-tip culture: After 30 days, apical meristems with foliar primordia and no sign of contamination were removed aseptically from terminal buds of shoots obtained as above. Explants were cultured in test tubes containing 40 ml MS medium with 0.66 mg/L indolebutyric acid.
Stage II Growth and in vitro multiplication: Plantlets obtained from shoot tips as above were multiplied by single-node culture, and MS medium was adjusted to pH 5.6–5.8 prior to autoclaving (20 min, 121 °C). Explants were placed in a growth chamber under controlled conditions as in Sect. 13.2.2.
2.4 Exposure to VOCs
One node from an aseptically cultured plantlet or one sterilized O. basilicum seed was placed on one side of a specialized plastic Petri dish (90 × 15 mm) containing a center partition (I-plate; Fisher Scientific). Both sides of the dish contained 50 % strength MS solid medium. 20 μL suspension cultures of various PGPR strains in sterile distilled water were applied one drop at a time to the side of the dish opposite the plant node. By this method, plants were exposed to bacterial VOCs without physical contact. Dishes were sealed with Parafilm, arranged in a completely randomized design, and placed in a growth chamber under controlled conditions as in Sect. 13.2.2. Plants were harvested after 30 days. Ten plants were used for each treatment, and experiments were replicated 4× (Santoro et al. 2011).
2.5 Extraction of EOs
The shoot samples were individually weighed and subjected to hydrodistillation in a Clevenger-like apparatus for 40 min, and the volatile fraction was collected in dichloromethane. Delta-dodecalactone (0.1 μL in 50 μL ethanol) was added as an internal standard.
The major EOs (accounting for ~75 % of the total EO volume) were identified and quantified relative to the delta-dodecalactone standard. Flame ionization detector (FID) response factors for each compound generated equivalent areas with negligible differences (<5 %).
Chemical analyses were performed using a PerkinElmer Clarus 600 gas chromatograph (GC) equipped with a CBP-1 capillary column (30 m × 0.25 mm, film thickness 0.25 μm) and mass-selective detector. Analytical conditions: injector/detector temperatures 250/270 °C; oven temperature programmed from 60 °C (3 min) to 240 °C at 4 °C/min; carrier gas = helium at constant 0.9 ml/min flow; source 70 eV. EO components were identified based on mass spectra and retention times, in comparison with standards (Banchio et al. 2005). GC analysis was performed using a PerkinElmer Clarus 500 GC fitted with a 30 m × 0.25 mm fused silica capillary column coated with Supelcowax 10 (film thickness 0.25 μm). GC operating conditions: oven temperature programmed from 60 °C (3 min) to 240 °C at 4 °C/min; injector/detector temperature 250 °C; detector FID; carrier gas = nitrogen at 0.9 ml/min constant flow.
2.6 Determination of Total Phenols
Total phenols were determined as described by Singleton and Rossi (1965). Plant extracts (each 0.5 ml) or gallic acid (standard phenolic reference compound) were mixed with Folin-Ciocalteu reagent (0.5 ml, diluted with 8 ml distilled water) and aqueous Na2CO3 (1 ml, 1 M). After 1 h, the level of total phenols was determined by colorimetry at wavelength 760 nm and expressed in terms of mg gallic acid equivalent per g plant dry weight (Lan et al. 2007).
2.7 Statistical Analyses
Data were pooled and subjected to analysis of variance (ANOVA) followed by comparison of multiple treatment levels with controls using Fisher’s post hoc LSD (least significant difference) test. Differences between means were considered to be significant for p < 0.05. The Infostat software program, version 2008 (Group Infostat, Universidad Nacional de Córdoba, Argentina), was used for all statistical analyses.
3 Results
3.1 Sweet Marjoram (Origanum majorana)
Sweet marjoram is an herb native to Asia Minor (Turkey) and now abundant throughout the Mediterranean region and southern Europe. It is a small woody-stemmed shrub that grows best in well-drained alkaline soil. It reaches a height of ~75 cm and has a hairy stem, soft oval-shaped dark-green leaves, and tiny pinkish-white flowers. The leaves are typically harvested just after flower bud formation but before flowering. For blanching, harvested stems are hung in a dark, dry room ~7–10 days, and leaves are stripped from the stems and stored in an airtight container. O. majorana is an economically important species (Werker et al. 1993). Its EOs are used as flavoring in foods and beverages, as fragrances, and as fungicides or insecticides in pharmaceutical and industrial products (Deans and Svoboda 1990). O. majorana has strong antioxidant activity, primarily because of its high content of phenolic acids and flavonoids; this activity makes it useful in health supplements and food preservation (Vági et al. 2005). O. majorana contains up to 3 % volatile oils, comprising more than 40 distinct compounds. The major EOs, accounting for ~85 % of the total oil volume, are terpinen-4-ol, cis-sabinene hydrate, α-terpineol, and trans-sabinene hydrate (Banchio et al. 2008).
The effects of inoculation on plant development differed between P. fluorescens and B. subtilis (Table 13.1, Fig. 13.1). Some differences among treatments were observed even after 90 days of growth. Leaf number was 80 % higher in plants inoculated directly with P. fluorescens than in controls (p < 0.05) (Table 13.1). Shoot fresh weight and root dry weight were, respectively, 3.2-fold and 6-fold higher in P. fluorescens-inoculated plants than in controls (p < 0.05) (Table 13.1).
In terms of EO composition, PGPR inoculation caused increased production of certain terpenes (Fig. 13.2). The total EO yield in P. fluorescens-treated plants was ~24-fold higher than in controls (p = 0.001) (Fig. 13.1).
The EO components that were affected most notably by P. fluorescens inoculation (Fig. 13.2) were terpinen-4-ol, cis-sabinene hydrate, trans-sabinene hydrate, and α-terpineol. PGPR inoculation caused increases of not only EO synthesis but also relative percentages (R%) of the EO components. Terpinen-4-ol showed an increase of 66.65 % in P. fluorescens-treated plants as compared with 53.9 % in controls. Percent increases for trans-sabinene hydrate (17.33 %, 15.50 %) showed a similar trend.
3.2 Italian Oregano (Origanum x majoricum)
Oregano, a member of the family Lamiaceae, is used extensively in the food industry because of its aromatic and antioxidant properties (Petersen and Simmonds 2003). One economically important species is Origanum x majoricum Cambess. (Italian oregano), a hybrid of O. majorana L. x O. vulgare L. ssp. virens Ietswaart (Werker et al. 1993). O. x majoricum is a bushy, semiwoody subshrub with upright or spreading stems and branches. It grows in mats and spreads by rhizomes. The aromatic leaves are oval-shaped, ~3.8 cm long, and usually pubescent. The plant bears tiny purple tube-shaped flowers ~0.3 cm long throughout the summer. The flowers peek out from whorls of purplish-green leafy 2.5 cm long bracts that resemble tiny pinecones. The abundant EOs located in the leaf trichomes are lipophilic VOCs (mostly monoterpenes, sesquiterpenes, and phenylpropanoid metabolites) that are widely used as flavoring in foods and beverages, as fragrances, and as fungicides or insecticides in pharmaceutical and industrial products (Harrewijn et al. 2001). O. x majoricum contains up to 3 % volatile oils, comprising more than 35 different compounds (Tabanca et al. 2004). The major EOs, accounting for ~55 % of the total oil volume, are cis- and trans-sabinene hydrate, terpinene, carvacrol, and thymol (Banchio et al. 2010).
The effects of direct PGPR inoculation on O. x majoricum development differed for the three PGPR species examined (B. subtilis, P. fluorescens, A. brasilense) (Table 13.2, Fig. 13.3). Leaf numbers did not differ significantly (p > 0.05), but certain differences among the treatments were evident even after 90 days’ growth. Shoot fresh weight in all inoculated plants was ~50 % higher than in controls (Table 13.2). This increase was due to a combination of increased leaf size and internode elongation. Root dry weight was promoted by all three treatments and was ~2-fold higher (p < 0.05) in P. fluorescens-treated and A. brasilense-treated plants than in controls (Table 13.2).
The total EO yield for P. fluorescens- and A. brasilense-treated plants was 3.57 and 3.41 μg/mg fresh weight, respectively, ∼2.5-fold higher than for controls (p = 0.001) (Fig. 13.3). PGPR inoculation caused increased production of certain terpenes. No change of monoterpene production was observed in B. subtilis-treated plants.
Concentrations of γ-terpinene, trans-sabinene hydrate, cis-sabinene hydrate, and thymol were higher in PGPR-inoculated plants than in controls in most cases (Fig. 13.4). Concentrations of trans- and cis-sabinene hydrate, the major EO components, were ~3-fold and 2-fold higher, respectively, in P. fluorescens- and A. brasilense-treated plants than in controls. The thymol content was increased by all treatments. γ-terpinene showed a significant increase only in P. fluorescens-treated plants. Carvacrol showed a significant increase (~9-fold; p < 0.05) only in A. brasilense-treated plants (Banchio et al. 2010).
3.3 Sweet Basil (Ocimum basilicum)
Ocimum basilicum is an aromatic, annual herb, generally 0.3–0.5 m tall (as high as 1 m tall for certain cultivars). The leaves of some cultivars have leaves and stems with a deep purple color. The leaves are ovate, often puckered, the flowers are white or pink, and the fruits have four small nutlets that become mucilaginous when wet. O. basilicum is used in perfumery, soapmaking, and flavoring liqueurs. The seeds are edible and become mucilaginous when soaked in water. The leaves are used to make an insecticide that protects stored crops from beetle damage. O. basilicum is rich in stored EOs and is commonly utilized in the spice industry (Werker et al. 1993). The abundant EOs located in leaf trichomes are lipophilic VOCs that consist mostly of monoterpenes, sesquiterpenes, and phenylpropanoid metabolites. O. basilicum EOs contain ~40 different metabolites. Two components, R-terpineol and eugenol, account for almost 60 % of the total VOC content (Simon et al. 1990; Zheljazkov et al. 2008).
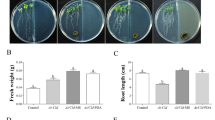
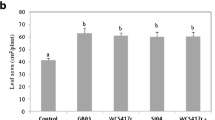
O. basilicum was exposed to direct root inoculation with B. subtilis GB03 culture medium and to VOCs emitted by GB03 (Banchio et al. 2009). To investigate whether GB03 VOCs affected O. basilicum growth, the plants and bacteria were grown on the same dish with physical separation such that VOCs but not solutes from the bacteria could reach the plant. Leaf number was increased by both root inoculation and VOC exposure in comparison with controls (p < 0.05) (Table 13.3). Leaf area was increased 2-fold in plants exposed to GB03 VOCs. Fresh shoot weight was increased 3-fold and 2-fold by root inoculation and VOC exposure, respectively (p < 0.05). Root dry weight was increased only in root-inoculated plants (Table 13.3).
EO production was increased by both GB03 medium root inoculation and exposure to GB03 VOCs (Fig. 13.5). The total EO yield measured on a fresh weight basis was 2-fold less for root inoculation than for VOC exposure.
Increases in the major EO components were observed for both experimental treatments. Terpineol yield was increased ~2-fold for both treatments. Eugenol yield was increased ~8-fold for root inoculation and ~6-fold for VOC exposure (Fig. 13.6).
3.4 Wild Marigold (Tagetes minuta)
Wild marigold (Tagetes minuta) is an important member of the Asteraceae family. It has tiny involucres, toxic flowers, and a unique odor. T. minuta is native to the temperate grasslands and mountain regions of southern South America but is now distributed worldwide; it is a “weed” with the ability to grow in environments ranging from extreme temperate to tropical (Singh and Singh 2003). T. minuta is an annual plant, 50–150 cm high, with a glabrous, erect, branched stem and opposite branches. The leaves are opposite and pinnately parted; the upper leaves are alternate. The leaves have a length of 4–8 cm, width of 3–4.5 cm, and margins that are acute and serrate. There are corymbiform dense inflorescences at the ends of branches. The phyllaries form a cylindrical tube that is naked at the base. There are three florets that are ligulate, dark brown, or lemon colored. Tubular florets are orange. The achene is dark brown and covered with appressed hairs. In tropical regions, T. minuta is grown for EO production (Shahzadi et al. 2010). The EO, known as “Tagetes oil” to retailers and end users, is a commercially valuable product (Singh and Singh 2003) used primarily in the preparation of high-grade perfumes (Kaul et al. 2000). Because of the high demand for Tagetes oil, there has been increasing cultivation of T. minuta for commercial production (Ghera and Leon 1999).
The effects of PGPR inoculation on T. minuta growth and development varied depending on the inoculated strain (P. fluorescens WCS417r, A. brasilense, or their combination) (Table 13.4, Fig. 13.7). Most of the growth parameters evaluated were significantly (p < 0.05) increased by each of the three treatments (Table 13.4).
Leaf number, shoot fresh weight, and root dry weight were all increased significantly by A. brasilense treatment (Table 13.4); root dry weight was 80 % higher than in controls. The increase of root weight was due primarily to an increased number of lateral roots (data not shown). Shoot fresh weight was increased significantly (~50 %) by single inoculation of P. fluorescens or co-inoculation of P. fluorescens and A. brasilense. Leaf number showed a similar trend (Table 13.4). Leaf number was 33 % higher in P. fluorescens-inoculated and co-inoculated plants than in controls, as reflected by the increased shoot fresh weight. Root dry weight in these treated plants was significantly (~35 %) increased, partly because of an increase in root length.
The total phenol content was 2-fold higher (p < 0.005) in single-inoculated or co-inoculated plants than in controls (Table 13.4). The total EO yield was 50 % higher (p = 0.02) in P. fluorescens single-inoculated or co-inoculated plants than in controls (Fig. 13.7). Single inoculation with A. brasilense did not significantly affect the total monoterpene content.
Levels of the major EO components analyzed, i.e., (Z)-(E)-tagetone, (Z)-(E)-ocimenone, (Z)-β-ocimene, and limonene (which together accounted for ~60 % of the total EO content), were usually different in inoculated plants than in controls (Fig. 13.8). (E)-ocimenone was by far the predominant component (accounting for ~50 % of the total EO content) and was increased affected by each of the experimental treatments. A. brasilense single inoculation increased the levels of (E)-ocimenone and (E)-tagetone by 71 and 66 %, respectively (p < 0.005) (Fig. 13.8). P. fluorescens single inoculation caused increases of each of the EO components except (Z)-β-ocimene. The effects of co-inoculation were similar to those of P. fluorescens single inoculation.
Single inoculation with A. brasilense or (to a greater degree) P. fluorescens affected plant growth and development. Co-inoculation caused greater increases in plant growth/development parameters and secondary metabolites, indicating a synergistic effect of the two PGPR. The population size of P. fluorescens increased from 105 CFU/ml at day 0 to 108 CFU/ml at day 7 and remained roughly constant thereafter (p > 0.05 for comparison between days 7 and 14). The population size of A. brasilense increased from 105 to 106 CFU/ml during the same period (p < 0.05 for comparison between days 7 and 14). Copresence of the two strains was observed throughout the co-inoculation experiments. P. fluorescens showed the same behavior in co-inoculation as in single inoculation (108 CFU/ml; p > 0.05). In contrast, A. brasilense in co-inoculation increased its population during days 0–7 and maintained its population thereafter (106 CFU/ml) (Cappellari et al. 2013).
3.5 Peppermint (Mentha x piperita)
The genus Mentha, which includes >25 species, is responsible for ~2,000 t of EO production worldwide, making it the second most important genus (after Citrus) in this regard (Mucciarelli et al. 2003). Peppermint, a naturally occurring hybrid of water mint (Mentha aquatica) and spearmint (Mentha spicata), was first cultivated in England in the late seventeenth century. It is an herbaceous rhizomatous perennial plant 30–90 cm tall, with smooth stems that are square in cross section. The rhizomes are wide-spreading, fleshy, and bare fibrous roots. The leaves are 4–9 cm long and 1.5–4 cm wide, dark green with reddish veins, with an acute apex and coarsely toothed margins. The leaves and stems are usually slightly fuzzy. The flowers are purple, 6–8 mm long, with a four-lobed corolla ~5 mm in diameter; they are produced in whorls around the stem, forming thick, blunt spikes. Flowering is from middle to late summer. M. x piperita is a fast-growing plant and spreads very quickly. Plants growing in vitro contain 3 % volatile oils, consisting of >50 different compounds. The EOs, which account for 60 % of the total oil volume, are (+) pulegone, (−) menthone, (−) menthol, and (+) menthofuran (Santoro et al. 2011).
To investigate the effect of VOCs from three PGPR on M. x piperita growth, plants and bacteria were grown in I-plates. The effect of VOC emission on plant development varied depending on the PGPR species (Table 13.5, Fig. 13.9). Clear differences among the treatments were detectable after 30 days’ growth.
Exposure to B. subtilis VOCs caused a 2-fold increase (p < 0.05) in shoot fresh weight, and similar effects were observed for P. fluorescens treatment (Table 13.5). Root dry weight in B. subtilis-treated plants was 3.5-fold higher than in controls and significantly (p < 0.05) higher than in plants exposed to VOCs of P. fluorescens or A. brasilense. The increased shoot fresh weight of B. subtilis-treated plants was due to a 2-fold increase in leaf area in combination with internode elongation (data not shown). Leaf number was not changed significantly by any of the treatments (Table 13.5).
EO yields for P. fluorescens- and A. brasilense-treated plants were, respectively, 4.46 and 3.22 mg/mg fresh weight, ~2-fold higher than for controls (Fig. 13.9). Yields of the major EOs (+) pulegone, (−) menthone, (−) menthol, and (+) menthofuran were generally higher in treated plants than in controls (Fig. 13.10). Pulegone concentration was significantly increased (3.14-fold; p < 0.05) only by P. fluorescens treatment. Menthone was increased 15.4- and 13.5-fold (p < 0.05) in P. fluorescens- and A. brasilense-treated plants, respectively. Menthofuran was increased significantly in P. fluorescens-treated plants. The only decreases in EO yield (~5-fold) were observed for menthol and menthofuran in A. brasilense-treated plants. Exposure to PGPR VOCs led to changes in relative percentage (R%), as well as yield, of EOs. R% for pulegone, the major EO component, increased to 59.9 % in P. fluorescens-treated plants, compared with 45.3 % in controls. R% for menthone increased in all cases. R% for menthol was lower in P. fluorescens- and A. brasilense-treated plants (6.1 %; 5.9 %) than in controls (9.6 %) but was higher in B. subtilis-treated plants (11.3 %). The only EO that showed a significant R% decrease in A. brasilense-treated plants was menthofuran.
4 Discussions
Enhanced growth and development following inoculation with PGPR has been reported for a number of plant species (Vessey 2003; Gray and Smith 2005; Van Loon 2007). The possible causes vary depending on the species and may include both direct and indirect mechanisms (Glick 1995; Gupta et al. 2002). Some examples of these mechanisms, which may be active simultaneously or sequentially at different stages of plant growth, are (1) increased mineral nutrient solubilization and nitrogen fixation, which make nutrients available for the plant; (2) suppression of soilborne pathogens (through production of hydrogen cyanide, siderophores, antibiotics, and/or competition for nutrients); (3) enhancement of plant tolerance to stress factors such as drought, salinity, and metal toxicity; and (4) production of phytohormones such as indole-3-acetic acid (IAA) (Gupta et al. 2002). Some PGPR have the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase, which hydrolyzes ACC, the immediate precursor of ethylene in plants. By lowering ethylene concentration (and thereby the inhibitory effect of ethylene) in seedlings, these PGPR increase seedling root length (Glick 1995).
The effects of PGPR inoculation or VOC emission on the plant species (O. majorana, O. x majoricum, O. basilicum, T. minuta, M. x piperita) evaluated in this study varied depending on the inoculated strain (P. fluorescens WCS417r, A. brasilense Sp7, Bacillus subtilis GB03, or their combination). Previous studies have demonstrated host response specificity in plant species treated with PGPR (O’Neal et al. 2002) and diverse responses to PGPR inoculation.
In our study, the growth parameters evaluated were significantly modified in most cases by P. fluorescens single inoculation and by P. fluorescens/A. brasilense co-inoculation. A. brasilense single inoculation promoted all growth parameters in O. x majoricum, but only enhanced root dry weight in T. minuta.
B. subtilis inoculation caused significant increases in shoot fresh weight and root dry weight in O. x majoricum and O. basilicum, but had no significant effect on O. majorana.
Exposure of O. basilicum and M. x piperita to B. subtilis VOCs caused increases in shoot fresh weight whereas exposure of M. x piperita to A. brasilense VOCs had no such effect.
All plants in the study received Hoagland’s nutrient solution and were grown on a sterilized, inert substrate in which nitrogen and other nutrients were available. The growth stimulatory effects observed were therefore not due to solubilization of phosphates, oxidation of sulfates, increased nitrate availability, extracellular production of antibiotics, or induction of plant systemic resistance (Kloepper 1993). Rather, the enhanced growth of the plant species observed following PGPR inoculation was presumably due to increased production of growth hormones and/or VOCs emitted by the PGPR.
Consistent with our findings, fluorescent pseudomonads were reported to promote overall growth of various crop species (Vikram 2007). P. fluorescens enhanced plant growth through production of growth-promoting substances such as IAA and cytokinins (Vikram 2007; De Salamone et al. 2001). The role of auxins and cytokinins in enhancing plant cell division and root development is well documented (Arshad and Frankenberger 1993). IAA is involved in root initiation, cell division, and cell enlargement (Gray and Smith 2005) and increases root surface area and consequent access to soil nutrients. Cytokinins promote cell division, cell enlargement, and tissue expansion in certain plant parts (Gray and Smith 2005). A. brasilense, in addition to its nitrogen-fixing ability, secretes phytohormones such as auxins, cytokinins, and gibberellins. Auxins are quantitatively the most abundant phytohormones secreted by Azospirillum. Auxin production, rather than nitrogen fixation, is considered to be the major factor responsible for stimulation of rooting and enhancement of plant growth (Bloemberg and Lugtenberg 2001).
We found that the effects of PGPR inoculation and VOCs on the formation of plant secondary compounds are species specific. The total phenol content in T. minuta was increased by single inoculation or co-inoculation with P. fluorescens and A. brasilense. Phenolic compounds are a major class of plant secondary metabolites and one of the most common and widespread groups of plant components in general. They are essential for plant growth and reproduction. Some phenolic compounds are produced constitutively; others are induced as a plant defensive response. In contrast to basic metabolism, which refers to the anabolic and catabolic processes required for cell maintenance and proliferation, secondary metabolism refers to compounds present in specialized cells that are not directly essential for basic photosynthetic or respiratory metabolism, but are considered to be necessary for plant survival in the external physical environment (Lattanzio et al. 2006). There has been recent interest in phenolic acids because of their potential protective role, via ingestion of fruits and vegetables, against oxidative damage diseases (coronary heart disease, stroke, cancers). Recent studies have clearly demonstrated the important antioxidant activities of phenolic compounds and the advantages of their use as natural antioxidants in processed foods (Lattanzio et al. 2006). Phenolic compounds also act as defensive compounds (against herbivores, microbes, viruses, or competing plants) and as signaling compounds (to attract pollinating or seed-dispersing animals) and protect the plant from ultraviolet (Kutchan 2001).
EO yield was increased to varying degrees by P. fluorescens inoculation in O. majorana, O. x majoricum, and T. minuta. Monoterpene production was increased 2-fold in some plants and 24-fold in O. majorana. VOCs emitted by P. fluorescens had the same effect on M. x piperita as direct root inoculation, whereas the effects of B. subtilis VOCs vs. inoculation were different. O. majorana and O. x majoricum did not show changes in EO yield, whereas EO yield in O. basilicum was increased 2-fold. Similar results were observed for VOC exposure in O. basilicum. VOC exposure did not affect the total monoterpene accumulation in M. x piperita. An increase in the total EO yield by root inoculation with A. brasilense was observed in O. x majoricum, but not in T. minuta.
The enhanced EO accumulation response was not due to increased biomass. It may have resulted from increased terpene biosynthesis, although we did not measure this process. In addition to increased EO synthesis, relative percentages (R%) of EO components were changed significantly by inoculation in several cases.
Our findings indicate that effects of PGPR VOCs on plants are species specific; i.e., VOCs from a particular bacterial strain do not cause the same effects, or to the same degree, in all plant species. A particular plant–bacteria combination has its own characteristic responses. Possible explanations for this phenomenon are as follows: (1) different plants respond to different component(s) of VOC mixtures; (2) reactive sites are different; (3) plants differ in their ability to metabolize VOCs.
The concentration and composition of oils in plants serve important ecological roles. Increased EO synthesis provides a defensive response to colonization by microorganisms; several EOs have antimicrobial properties (Sangwan et al. 2001). Analogously, monoterpene synthesis is induced by herbivore feeding in Minthostachys mollis (Banchio et al. 2005) and other plant species, apparently to protect damaged leaves from further attack (Harrewijn et al. 2001).
There have been few attempts to elucidate the relative quantitative and qualitative contributions of rhizobacteria to formation of plant secondary compounds. Induction of secondary metabolite responses has been reported in other beneficial microbe–plant interactions involving arbuscular mycorrhizal (AM) fungi. Gupta et al. (2002) inoculated the AM fungus Glomus fasciculatum in cultivars of wild mint (Mentha arvensis) and observed increased plant height, shoot growth, and oil content. Khaosaad et al. (2006) observed changes of EO concentration (but not composition) following mycorrhizal inoculation of Origanum sp. Copetta et al. (2006) reported increases of glandular hair abundance and EO yield in inoculated O. basilicum. The increased EO yield was associated with a larger number of peltate glandular trichomes, the primary site of EO synthesis. Belowground AM fungi cause changes in leaf isoprenoid content that favor EO production, particularly under drought stress condition or following jasmonic acid (JA) application (Asensio et al. 2012). AM fungi increase plant growth and EO production because mycorrhization allows the root system to exploit a greater volume of soil by (1) extending the root zone, (2) reaching smaller soil pores not accessible by root hairs, and (3) acquiring organic phosphates through production of extracellular acid phosphatases (Bouwmeester et al. 2007).
Terpene compounds help the plant’s photosynthetic apparatus recover from brief episodes of high temperature. Isoprene may physically stabilize thylakoid membranes at high temperature or quench reactive oxygen species (e.g., ozone) that cause membrane damage (Pichersky and Gershenzon 2002). Enhanced biosynthesis of secondary metabolites can be triggered by certain stress factors (Ramomoorthy et al. 2001). Nonpathogenic rhizobacteria have been shown to stimulate secondary metabolism in plants through a mechanism termed ISR (induced systemic resistance) (van Oosten et al. 2008; Pozo et al. 2008; Pieterse et al. 2009; Pineda et al. 2012). The occurrence of ISR has been demonstrated in various plants inoculated with various species of rhizobacteria (Pineda et al. 2013). ISR may be local or systemic (when it is expressed at sites not directly exposed to the inducing agent). The inducing agent may be a chemical activator or an extract of cells of living organisms or microorganisms. ISR has been described as “activation of the host plant’s physical or chemical defenses by an inducing agent” (Kloepper 1993). Interestingly, PGPR simultaneously induce an ISR response and promote plant growth (Kloepper et al. 2004; Yi et al. 2013).
Both direct and indirect defenses are under the control of a complex network of signal transduction pathways that are regulated by various phytohormones, of which JA is a central regulator (Snoeren et al. 2009; Kusnierczyk et al. 2011). JA exerts its protective effects by regulating a wide range of defense-related processes, including the synthesis of toxic secondary metabolites (Pauwels et al. 2009). JA also triggers the biosynthesis of mono- and sesquiterpenes (Arimura et al. 2000) that are presumed to act as master switches for plant responses stimulated by root-colonizing bacteria, leading to activation of distinct sets of defense genes responsible for terpenoid formation (Pineda et al. 2012).
Some of the roles of EO components are relatively straightforward; e.g., they play numerous generalized protective roles (antioxidant, free radical scavenging, UV light absorbing, antiproliferative, etc.) and defend the plant against microorganisms (bacteria, fungi, viruses). EO components also help modulate interplant relationships, acting as allelopathic defenders of the plant’s growing space against competing plants. More complex roles include defining or modifying the plant’s relationship with herbivores (Tahara 2007; Wink 2000). The primary role of EO components is often viewed as feeding deterrence; to this end, many phytochemicals are bitter and/or toxic to potential herbivores. The toxic effects often extend to direct interactions with the herbivore’s central and/or peripheral nervous systems (Rattan 2010). Secondary metabolites often act as agonists or antagonists of neurotransmitter systems (Wink 2000; Rattan 2010) or form structural analogs of endogenous hormones (Miller and Heyland 2010).
Biosynthesis of terpenoids depends on primary metabolism (e.g., photosynthesis) and oxidative pathways for carbon and energy supply (Singh et al. 1990). Giri et al. (2003) found that net photosynthesis of PGPR host plants increases as a result of improved nutritional status. Factors that increase dry matter production may influence the interrelationship between primary and secondary metabolism, leading to increased biosynthesis of secondary products (Shukla et al. 1992). Increased plant biomass may result in greater availability of substrate for monoterpene biosynthesis (Harrewijn et al. 2001). The increased concentration of monoterpenes in inoculated plants may be caused by growth-promoting substances produced by the inoculated microorganism that affect plant metabolic processes. Because the plants in the present study were grown in enriched medium containing nitrogen and other nutrients, bacterial metabolites are the most likely growth-promoting substance.
Knowledge of the adaptive mechanisms of plants is of interest from an ecophysiological point of view. These mechanisms also provide an important (probably crucial) starting point for improvement of plant production, including optimization of secondary metabolite production. The use of fungal and bacterial inoculants is an efficient biotechnological alternative for stimulating secondary metabolism in plants. Studies of such inoculants will also clarify certain adaptive processes that are poorly understood at present.
5 Conclusions
The present findings show that inoculation of certain PGPR causes systemic induction of monoterpene pathways in various aromatic plants species, suggesting that PGPR inoculation can significantly increase productivity and reduce the amount of fertilizer required for economically viable aromatic crop production. The markets for medicinal plants, aromatic plants, and organic foods are steadily expanding (Adam 2005; Hartman Group 2006). As consumers become more concerned and knowledgeable about their own health and wellness, there is increasing demand for quality plant material, produced by sustainable methods and uncontaminated by synthetic pesticides or genetically modified organisms (Craker 2007).
Abbreviations
- EOs:
-
Essential oils
- PGPR:
-
Plant growth-promoting rhizobacteria
- VOCs:
-
Volatile organic compounds
References
Adam KI (2005) Herb production in organic systems. A publication of ATTRA – national sustainable agricultural information service. www.attra.ncat.org
Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory induced volatiles elicit defense genes in lima bean leaves. Nature 406:512–515
Arshad M, Frankenberger WP Jr (1993) Microbial production of plant growth regulators. In: Metting FB (ed) Soil microbial ecology – application in agricultural and environmental management. Dekker, New York, pp 307–347
Asensio D, Curiel J, Mattana S, Ribas A, Llusià J, Peñuelas J (2012) Litter VOCs induce changes in soil microbial biomass C and N and largely increase soil CO2 efflux. Plant Soil 360:163–174
Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett 32:1559–1570
Banchio E, Zygadlo J, Valladares G (2005) Quantitative variations in the essential oil of Minthostachys mollis (Kunth.) Griseb. in response to insects with different feeding habits. J Agric Food Chem 53:6903–6906
Banchio E, Bogino P, Zygadlo J, Giordano W (2008) Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem Syst Ecol 36:766–771
Banchio E, Xie X, Zhang H, Paré PW (2009) Soil bacteria elevate essential oil accumulation and emissions in sweet basil. J Agric Food Chem 57:653–657
Banchio E, Bogino P, Santoro M, Torres L, Zygadlo J, Giordano W (2010) Systemic induction of monoterpene biosynthesis in Origanum majoricum by soil bacteria. J Agric Food Chem 58:650–665
Bloemberg GV, Lugtenberg BJJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Bouwmeester HJ, Roux C, Lopez-Raez JA, Becard G (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12:224–230
Cappellari L, Santoro MV, Nievas F, Giordano W, Banchio E (2013) Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl Soil Ecol 70:16–22
Chen F, Tholl D, Bohlmann J, Pichersky E (2011) The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J 66:212–229
Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 16:485–494
Craker LE (2007) Medicinal and aromatic plants—future opportunities. In: Janick J, Whipkey A (eds) New crops and new uses. ASHS Press, Alexandria, VA, pp 248–257
De Salamone IEG, Hynes RK, Nelson LM (2001) Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can J Microbiol 47:404–411
Deans SG, Svoboda KP (1990) The antimicrobial properties of Marjoram (Origanum majorana L.) volatile oil. Flav Fragrance J 5:187–190
Figueiredo MVB, Seldin L, de Araujo FF, Mariano RLR (2010) Plant growth promoting rhizobacteria: fundamentals and applications. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. doi:10.1007/978-3-642-13612-2_2
Ghera CM, Leon RJC (1999) Successional changes in the agroecosystems of the rolling pampas. In: Walker LR (ed) Ecosystems of disturbed ground. Amsterdam, Elsevier, pp 487–502
Giri B, Kapoor R, Mukerji KG (2003) Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass and mineral nutrition of Acacia auriculiformis. Biol Fertil Soils 38:170–175
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 4:109–117
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signalling processes. Soil Biol Biochem 37:395–412
Guenther E (1948) The essential oil, vol 1. D van Nostrand Co., London, pp 1–342
Gupta ML, Prasad A, Ram M, Kumar S (2002) Effect of the vesicular-arbuscular mycorrhizal (VAM) fungus Glomus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Bioresour Technol 81:77–79
Harrewijn P, Van Oosten AM, Piron PGM (2001) Natural terpenoids as messengers: a multidisciplinary study of their production, biological functions, and practical applications. Springer, Dordrecht, p 440
Hartman Group Inc (2006) Trends to watch in 2007. www.harman-group.com.products/HB/2006_12_13.html
Kaul VK, Singh B, Singh V (2000) Damask rose and marigold: prospective industrial crops. J Med Aromat Plant Sci 22:313–318
Khaosaad T, Vierheilig H, Nell M, Zitterl-Eglseer K, Novak J (2006) Arbuscular mycorrhiza alters the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 16:443–446
Kloepper JW (1993) Plant growth promoting rhizobacteria as biological control agents. In: Metting FB (ed) Soil microbial ecology – applications in agricultural and environmental management. Dekker, New York, pp 255–274
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus species. Phytopathology 94:1259–1266
Kogan M, Fischer DC (1991) Inducible defenses in soybean against herbivorous insects. In: Douglas WT, Raupp MJ (eds) Phytochemical induction by herbivores. Wiley, New York, pp 347–378
Kusnierczyk A, Tran DHT, Winge P, Jorstad TS, Reese JC, Troczynska J, Bones AM (2011) Testing the importance of jasmonate signalling in induction of plant defences upon cabbage aphid Brevicoryne brassicae attack. BMC Genomics 12:423
Kutchan TM (2001) Ecological arsenal and developmental dispatcher: the paradigm of secondary metabolism. Plant Physiol 125:58–60
Lan S, Jun-Jie Y, Denys C, Kequan Z, Moore J, Liangli Y (2007) Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem 100:990–997
Langenheim JH (1994) Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol 20:1223–1280
Lattanzio V, Lattanzio VMT, Cardinal A (2006) Role of phenolics in the resistance mechanism of plant against fungal pathogens and insects. In: Imperato F (ed) Phytochemistry: advances in research. Research Signpost, Trivandrum, pp 23–67
Lind K, Lafer G, Schloffer K, Innerhoffer G, Meister H (2004) Organic fruit growing. CABI Publishing, Wallingford
Miller AE, Heyland A (2010) Endocrine interactions between plants and animals: implications of exogenous hormone sources for the evolution of hormone signaling. Gen Comp Endocrinol 166:455–461
Mucciarelli M, Scannerini S, Bertea C, Maffei M (2003) In vitro and in vivo peppermint (Mentha piperita) growth promotion by nonmycorrhizal fungal colonization. New Phytol 158:579–591
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assay with tobacco tissue culture. Physiol Plant 15:473–497
Niranjan RS, Shetty HS, Reddy MS (2006) Plant growth promoting rhizobacteria: potential green alternative for plant productivity. In: Siddiqui ZA (ed) PGPR: biocontrol and biofertilization. Springer, The Netherlands, pp 197–216
O’Neal ME, Landis DA, Isaacs R (2002) An inexpensive, accurate method for measuring leaf area and defoliation through digital image analysis. J Econ Entomol 95:1190–1194
Pauwels L, Inze D, Goossens A (2009) Jasmonate-inducible gene: what does it mean? Trends Plant Sci 114:87–91
Petersen M, Simmonds MSJ (2003) Rosmarinic acid. Phytochemistry 62:121–125
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Pieterse CMJ, Leon-Reyes A, Ent S, Wees SCM (2009) Networking by small-molecules hormones in plant immunity. Nat Chem Biol 5:308–316
Pineda A, Zheng SJ, van Loon JJ, Dicke M (2012) Rhizobacteria modify plant-aphid interactions: a case of induced systemic susceptibility. Plant Biol 14:83–90
Pineda A, Soler R, Weldegergis BT, Shimwela MM, van Loon JJ, Dicke M (2013) Non-pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via jasmonic acid signalling. Plant Cell Environ 36:393–404
Pozo MJ, Ent S, Loon LC, Pieterse CMJ (2008) Transcription factor MYC2 is involved in priming for enhanced defense during rhizobacteria-induced systemic resistance in Arabidopsis thaliana. New Phytol 180:511–523
Ramomoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R (2001) Induction of systemic resistance by plant growth promoting rhizobacteria in crop plant against pest and diseases. Crop Prot 20:1–11
Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot 29:913–920
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Sangwan NS, Farooqi AHA, Shabih F, Sangwan RS (2001) Regulation of essential oil production in plants. Plant Growth Regul 34:3–21
Santoro MV, Zygadlo J, Giordano W, Banchio E (2011) Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita). Plant Physiol Biochem 49:1177–1182
Shahzadi I, Hassan A, Khan UW, Shah MM (2010) Evaluating biological activities of the seed extracts from Tagetes minuta L. found in Northern Pakistan. J Med Plant Res 4:2108–2112
Shukla A, Farooqi AH, Shukla YN, Sharma S (1992) Effect of triacontanol and chlormequat on growth, plant hormones and artemisinin yield in Artemisia annua L. Plant Growth Regul 11:165–171
Simon JE, Quinn J, Murray RG (1990) Basil: a source of essential oils. In: Janick J, Simon JE (eds) Advances in new crops. Timber, Portland, pp 484–489
Singh V, Singh B (2003) Domestication of wild marigolds as a potential economic crop in Western Himalaya and North Indian Plains. Econ Bot 57:535–544
Singh N, Luthra R, Sangwan RS (1990) Oxidative pathways of essential oil biosynthesis in the developing Cymbopogon flexuosus leaf. Plant Physiol Biochem 28:703–710
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Snoeren TAL, Van Poecke RMP, Dicke M (2009) Multidisciplinary approach to unravelling the relative contribution of different oxylipins in indirect defense of Arabidopsis thaliana. J Chem Ecol 35:1021–1031
Tabanca N, Ozek T, Baser KHC, Tumen G (2004) Comparison of the essential oils of Origanum majorana L. and Origanum majoricum Cambess. J Essent Oil Res 16:248–252
Tahara S (2007) A journey of twenty-five years through the ecological biochemistry of flavonoids. Biosci Biotechnol Biochem 71:1387–1404
Vági E, Rapavi E, Hadolin M, Vasarhelyine Peredi K, Balazs A, Blazovics A, Simandi B (2005) Phenolic, triterpenoid antioxidants from Origanum majorana L. herb and extracts obtained with different solvents. J Agric Food Chem 12:17–21
Van Loon LC (2007) Plant response to plant growth-promoting rhizobacteria. Eur J Plant Pathol 119:243–254
Van Oosten VR, Bodenhausen N, Reymond P, Pelt JA, Loon LC, Dicke M, Pieterse CMJ (2008) Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis. Mol Plant Microbe Interact 21:919–930
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vikram A (2007) Efficacy of phosphate solubilizing bacteria isolated from vertisols on growth and yield parameters of sorghum. Res J Microbiol 2:550–559
Werker E (2000) Trichome diversity and development. Adv Bot Res 31:1–35
Werker E, Putievsky E, Ravid U, Dudai N, Katzir I (1993) Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae). Ann Bot 71:43–50
Wink M (2000) Interference of alkaloids with neuroreceptors and ion channels. Stud Nat Prod Chem 21:3–122
Wittstock U, Gershenzon J (2002) Constitutive plant toxins and their role in plant defense. Curr Opin Plant Biol 5:300–307
Yi H-S, Yang JW, Ryu CM (2013) ISR meets SAR outside: additive action of the endophyte Bacillus pumilus INR7 and the chemical inducer, benzothiadiazole, on induced resistance against bacterial spot in field-grown pepper. Front Plant Sci 4:122
Zheljazkov VD, Cantrell CL, Tekwani B, Khan S (2008) Content, composition, and bioactivity of the essential oil of three basil genotypes as a function of harvesting. J Agric Food Chem 56:380–385
Acknowledgments
This study was supported by a grant from Consejo Nacional de Investigaciones Científicas y Tecnológicas. MS and LC have fellowships from Consejo Nacional de Investigaciones Científicas y Técnicas of the República Argentina (CONICET). EB and WG are Career Member of CONICET. The authors are grateful to Dr. S. Anderson for English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Santoro, M.V., Cappellari, L., Giordano, W., Banchio, E. (2015). Systemic Induction of Secondary Metabolite Biosynthesis in Medicinal Aromatic Plants Mediated by Rhizobacteria. In: Egamberdieva, D., Shrivastava, S., Varma, A. (eds) Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants. Soil Biology, vol 42. Springer, Cham. https://doi.org/10.1007/978-3-319-13401-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-13401-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13400-0
Online ISBN: 978-3-319-13401-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)