Abstract
Clinical studies have suggested that use of bicarbonate-containing substitution and dialysis fluids during continuous kidney replacement therapy may result in excessive increases in the carbon dioxide concentration of blood; however, the technical parameters governing such changes are unclear. The current work used a mathematical model of acid–base chemistry of blood to predict its composition within and exiting the extracorporeal circuit during continuous veno-venous hemofiltration (CVVH) and continuous veno-venous hemodiafiltration (CVVHDF). Model predictions showed that a total substitution fluid infusion rate of 2 L/h (33% predilution) with a bicarbonate concentration of 32 mEq/L during CVVH at a blood flow rate of 200 mL/min resulted in only modest increases in plasma bicarbonate concentration by 2.0 mEq/L and partial pressure of dissolved carbon dioxide by 4.4 mmHg in blood exiting the extracorporeal circuit. The relative increase in bicarbonate concentration (9.7%) was similar to that in partial pressure of dissolved carbon dioxide (8.2%), resulting in no significant change in plasma pH in the blood exiting the CVVH circuit. The changes in plasma acid–base levels were larger with a higher infusion rate of substitution fluid but smaller with a higher blood flow rate or use of substitution fluid with a lower bicarbonate concentration (22 mEq/L). Under comparable flow conditions and substitution fluid composition, model predicted changes in acid–base levels during CVVHDF were similar, but smaller, than those during CVVH. The described mathematical model can predict the effect of operating conditions on acid–base balance within and exiting the extracorporeal circuit during continuous kidney replacement therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Patients with acute kidney injury (AKI) treated by continuous kidney replacement therapy (CKRT) require the use of fluids or solutions containing bicarbonate or other buffer bases to correct metabolic acidosis, and a large majority of these critically-ill patients also receive mechanical ventilatory support [1, 2]. The effect of the administration of large amounts of bicarbonate, either by using substitution (also called replacement) or dialysis fluids, on acid–base balance and overall clinical outcomes in these patients is however unclear. For instance, Kashani et al. [3] used propensity score matching in a retrospective analysis of AKI patients treated by continuous veno-venous hemofiltration (CVVH) using regional citrate anticoagulation, in which over 80% also required mechanical ventilatory support, to show that patients treated with a substitution fluid containing a high concentration of bicarbonate (32 mEq/L versus 22 mEq/L) was associated with significantly higher in-hospital and 90-day mortality. These investigators hypothesized that administration of large amounts of bicarbonate resulted in increased production of dissolved carbon dioxide potentially leading to cerebral acidosis or intracranial hypertension. A subsequent retrospective analysis by this same group [4] showed that use of substitution fluid containing a high concentration of bicarbonate was also associated with hypotension during the first hour of CKRT initiation. In contrast, Allegretti et al. [5] studied 19 mechanically-ventilated patients who were subsequently treated by CVVH with substitution fluids containing 32 mEq/L of bicarbonate. These investigators showed that arterial partial pressure of carbon dioxide (pCO2) remained stable during the initial 96 h after initiation of CVVH. These two reports were limited to critically-ill AKI patients without significant hypercarbia.
The effect of bicarbonate-containing substitution fluids during CKRT on acid–base parameters in critically-ill patients with impaired respiratory function is also incompletely understood, although it is known that both mechanical ventilatory support and acute respiratory distress syndrome are associated with subsequent AKI [6, 7]. Such patients are also administered large amounts of bicarbonate independent of whether the patients are treated by hemodialysis or CKRT, and the clinical consequences of such bicarbonate administration is unclear. For example, Nentwich et al. [8] recently reported that mechanically-ventilated patients with hypercapnic acidosis requiring kidney replacement therapy only achieved modest reductions in the arterial pCO2 (even after applying extracorporeal carbon dioxide removal), presumably because of the loading of blood with CO2 via use of bicarbonate-rich substitution fluids during CVVH. Better understanding of acid–base chemistry of blood during the administration of bicarbonate-containing substitution fluids in extracorporeal circuits will likely assist in developing new approaches to improve the outcome of AKI patients.
The current work describes a mathematical model of acid–base balance during CVVH and continuous veno-venous hemodiafiltration (CVVHDF) based on a model of acid–base chemistry of blood by Rees and Andreassen [9, 10]. The main objective of this work is to predict changes in the acid–base composition of blood within a CKRT circuit as a result of using substitution and dialysis fluids containing high bicarbonate concentrations; as a first step, this work is limited to predicting changes in blood chemistry within the extracorporeal circuit only.
2 Methods
2.1 Total carbon dioxide mass balance
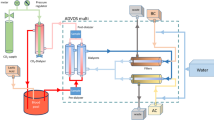
CVVH and CVVHDF extracorporeal circuits are shown in Fig. 1 where, for simplicity, it has been assumed that there is negligible net fluid removal from the patient, and only steady state conditions were considered. During CVVH, the total substitution fluid flow rate (Qs) is thus equal to the ultrafiltration rate (Qf), and the fraction of substitution fluid that is infused prefilter in predilution mode is denoted by the letter f. During CVVHDF, the sum of the predilution substitution fluid flow rate and dialysis fluid flow rate is equal to the total substitution fluid flow rate; as with CVVH, the fraction of the total substitution fluid infused prefilter is denoted by the letter f. In both cases, the inlet and outlet blood flow rates to the extracorporeal circuit indicated by Qbi are equal. Consideration here is restricted to conditions where the composition of all substitution and dialysis fluids during a given treatment are assumed to be the same.
Schematics of the flow of blood, substitution fluid and dialysis fluid in CVVH and CVVHDF circuits. The blood flow rate from the patient is denoted by Qbi; it contains a total carbon dioxide concentration of Cbi. The total substitution/dialysate fluid flow rate is denoted by Qs; it contains a total carbon dioxide concentration of Cs. The symbol f denotes the fraction of substitution fluid that is infused before the filter (predilution). The total carbon dioxide concentration entering the filter is denoted by Ci (see Eq. 1). The total carbon dioxide concentration in the outflow dialysis fluid during CVVHDF is denoted by Cse; in general, that concentration is not used in any model calculation
Mass balance during both CVVH and CVVHDF requires that total carbon dioxide concentration in the blood inlet of the hemofilter (Ci) is modified from that in the patient’s blood by the following relationship (see Appendix)
where Cbi and Cs denote total carbon dioxide concentrations in patient blood entering the extracorporeal CKRT circuit and in substitution fluid, respectively. During CVVH the relationship between the acid–base composition of blood entering and exiting the hemofilter is determined by the ultrafiltration rate and the overall sieving coefficient. Here, it was assumed that the overall sieving coefficient for total carbon dioxide is equal to unity; stated in other words, it is assumed that the concentration of total carbon dioxide in the ultrafiltrate is equal to that in plasma. Under such conditions, the total carbon dioxide concentration in blood exiting the hemofilter (Co) can be readily calculated since the acid–base composition of plasma and red blood cells does not change within the hemofilter; there is only a change in the hematocrit (see Supplementary Materials). The term overall sieving coefficient used here is unconventional but represents the combined effects of the true sieving coefficients for dissolved carbon dioxide and bicarbonate, the volume of protein occupied by plasma proteins, and electrostatic forces from the Donnan effect. An improved description of the overall sieving coefficient requires one to identify the true sieving coefficients for a specific hemofilter. Likewise, the total carbon dioxide concentration in blood exiting the extracorporeal circuit (Cbo) after infusion of postdilution substitution fluid during CVVH can be expressed by the following (see Appendix)
Equations (1) and (2) were used to determine the total carbon dioxide composition of blood at different positions within the extracorporeal CVVH circuit (for example, Ci, Co and Cbo) using the mathematical model of acid–base chemistry of blood described below.
Equations describing mass balance of total carbon dioxide concentration during CVVHDF differ because there is both diffusive and convective transport of solutes with this modality. We assumed the equation proposed by Sargent and Gotch [11] describes diffusive and convective transport of total carbon dioxide within the filter (J), defined as positive in the dialysate-to-blood direction, as follows (see Appendix).
where D denotes the dialysance, Qf denotes the ultrafiltration rate, defined as positive in the blood-to-dialysate direction, α denotes the Donnan factor (1.05 for a monovalent anion) [12], Cs denotes the total carbon dioxide concentration in the dialysis fluid, Qi denotes the blood flow rate into the hemofilter (Qbi + fQs), and Ci denotes total carbon dioxide concentration in the blood entering the hemofilter as described in Eq. (1). As the dialysate flow rate is relatively small compared with both the blood flow rate into the hemofilter and the dialysance, it will be assumed that the dialysance is equal to the inlet dialysate flow rate or (1-f)Qs; under these assumptions therefore, Eq. (3) becomes
This equation represents an upper bound to the actual transport rate; the latter will be less than that predicted by Eq. (4).
Mass balance across the CVVHDF filter then allows the calculation of the total carbon dioxide concentration from the outlet of the extracorporeal circuit (Cbo) as (see Appendix)
where Ci is described in Eq. (1).
2.2 Non-bicarbonate buffer and hematocrit mass balance
Albumin is the major non-bicarbonate buffer in plasma [9, 13]. As albumin is not present in substitution fluids used during CKRT, it can be assumed that the concentration of non-bicarbonate buffer in substitution fluid is zero. Thus, in general, Eq. (1) can be used to calculate the dilution of total non-bicarbonate buffer concentration in plasma entering the hemofilter (using the plasma, not the blood, flow rate) and the total non-bicarbonate buffer concentration exiting the extracorporeal circuit can be assumed to be equal to that entering the extracorporeal circuit as net fluid removal is zero and the hemofilter sieving coefficient of albumin is assumed equal to zero. A similar argument applies to changes in hematocrit; these changes in total non-bicarbonate buffer concentration and hematocrit assume that bicarbonate buffer tonicity is identical to that in plasma such that the substitution fluid does not enter red blood cells.
The total non-bicarbonate buffer concentration in plasma exiting the hemofilter was determined by assuming a sieving coefficient is equal to zero and realizing that ultrafiltration only removes plasma water. The hematocrit exiting the hemofilter was calculated by a cell balance analysis as described in Supplementary Materials. Specific equations used for calculating the total non-bicarbonate buffer concentration in plasma and hematocrit within and exiting the extracorporeal circuit are specified in Supplementary Materials.
2.3 Buffer base mass balance and base excess
The model of acid–base chemistry of blood described below requires that base excess be determined at each position within the extracorporeal circuit. The concept of base excess is only germane to blood and other body fluids; however, the buffer base (concentration) of any fluid is equal to the strong ion difference [14], and base excess is a parameter that can be derived from the buffer base of a solution [9]. Substitution fluids typically contain only bicarbonate, dissolved carbon dioxide and various electrolytes; thus, the buffer base and strong ion difference of such solutions can be assumed to be equal to their bicarbonate concentration. Because mass balance considerations of buffer base are comparable to those for total carbon dioxide, we can use Eqs. (1, 2, 3, 4 and 5) to determine buffer base at positions within a CKRT extracorporeal circuit by using buffer base concentrations instead of total carbon dioxide concentrations. The determination of buffer base exiting the hemofilter is more complex since the sieving coefficient of the two main buffer base components, bicarbonate and albumin (or non-bicarbonate buffer), differ. The equations used to calculated the buffer base concentration of blood exiting the hemofilter are specifically described in Supplementary Methods.
We then used the definition of base excess as the calculated buffer base of blood under the conditions within the extracorporeal circuit minus the buffer base of the same blood sample (i.e., the same hematocrit and total non-bicarbonate concentration in plasma) under normal conditions defined as full oxygenation, a pCO2 of 40 mmHg and a pH equal to 7.4 [9]. The latter can be also be calculated using the model of acid–base chemistry of blood under different applied conditions as described in Supplementary Materials.
2.4 Assumed clinical conditions
The purpose of this work is to use a mathematic model to predict acid–base balance within and exiting the extracorporeal circuit during CKRT for an assumed acid–base composition of blood entering the extracorporeal circuit. Current CKRT devices typically access venous, not arterial, blood; thus, venous blood composition will be selected to be representative. Blood flow rates during CKRT are typically 150–200 mL/min but under some conditions can be up to 400 mL/min. The volume of substitution fluid used is typically based on patient body weight but is generally about 2 L/h, although it may be increased to increase uremic toxin removal. Substitution fluid composition can vary significantly to control blood electrolyte composition (for example, serum potassium or phosphorus); however, many of the elements of blood do not influence acid–base balance directly and will be neglected further here. The main components of substitution fluid considered will be the bicarbonate concentration of either 32 or 22 mEq/L; these concentrations of bicarbonate were chosen since they have been evaluated in previous clinical studies [3, 4]. As described above, these substitution fluids have a strong ion difference and buffer base of 32 or 22 mEq/L, respectively. These solutions also contain dissolved carbon dioxide at low but somewhat variable concentrations such that the pH of commercial solutions vary from 7.0 to 8.5. It will be assumed that the dissolved carbon dioxide concentration in these solutions is such that the pH is 7.4; based on that assumption and the Henderson-Hasselbalch equation [9], the substitution fluids with bicarbonate concentrations of 32 and 22 mEq/L will be assumed to have pCO2 of 52.2 and 35.9 mmHg, respectively. The total carbon dioxide concentration in the substitution and dialysis fluids is then the sum of the bicarbonate and dissolved carbon dioxide concentrations.
2.5 Calculation of blood acid–base composition
We used the model described by Rees and Andreassen [9, 10] to determine the acid–base chemistry of blood within the extracorporeal circuits of CVVH and CVVHDF. Once the concentrations of total carbon dioxide and base excess in whole blood and that of the total non-bicarbonate buffer in plasma are derived as above, the model can calculate the complete acid–base composition in plasma and red blood cells; these calculations are not detailed here but have been completely described elsewhere [9, 10]. In Supplementary Materials, we describe the equations used to determine the acid–base composition of blood based on this approach. Briefly, this model calculates the total carbon dioxide concentration in blood as the volume-weighted sum of that in plasma and red blood cells. The total carbon dioxide concentration in plasma is the sum of the concentrations of dissolved carbon dioxide and bicarbonate, and the total carbon dioxide concentration in red blood cells is the sum of the concentrations of dissolved carbon dioxide and bicarbonate within red blood cells plus that of carbamino hemoglobin (hemoglobin with bound carbon dioxide moieties). The total concentration of oxygen in blood is similarly quantified; it includes concentrations of dissolved oxygen and oxygenated hemoglobin, the latter within red blood cells only. The concentration of total non-bicarbonate buffer in plasma is the sum of both the protonated and non-protonated forms of the buffer (or buffers) that are in equilibrium; the fraction of each are dependent on plasma pH. The whole blood base excess is the weighted sum of that in plasma and red blood cells as defined elsewhere [9]. All chemical reaction equilibrium constants and other parameters were assumed as previously described [9] and are listed in Supplementary Materials. Throughout this work, the fraction of red blood cells (hematocrit) of whole blood was assumed to be 0.3, and the oxygen saturation of venous blood was assumed to be 0.7. The oxygen content of blood was assumed constant throughout the extracorporeal circuit.
Using the above equations, the acid–base composition of blood at any position in the extracorporeal circuit can be determined by simultaneously solving 19 equations according to the model of Rees and Andreassen [9, 10]. We performed these calculations using Matlab (Version R2018a, MathWorks, Natick, MA, USA).
3 Results
The acid–base composition of venous blood entering the extracorporeal circuit assumed a total carbon dioxide concentration of 19 mmol/L and a base excess of −8 mmol/L, comparable to that which occurs for a patient with metabolic acidosis with normocarbia in previously reported clinical studies [3, 5]; the complete acid–base composition is detailed in Supplementary Materials. Figure 2 shows the effect of blood flow rate and substitution fluid flow rate during CVVH on the calculated values of plasma bicarbonate concentration and pCO2 in blood entering the extracorporeal circuit, after infusion of predilution substitution fluid (i.e., entering the hemofilter), and at the outflow of the extracorporeal circuit. Here, results are shown for a substitution fluid with a bicarbonate concentration of 32 mEq/L, 33% infused in predilution mode; the venous blood composition was assumed the same in each. Plasma bicarbonate concentration and pCO2 were only modestly higher after infusion of substitution fluid, both in blood entering the hemofilter and that exiting the extracorporeal circuit. These trends were accentuated when using higher rates of substitution fluid but lessened when the blood flow rate was higher. Additional model predictions regarding the effect of substitution flow rate on total carbon dioxide concentration in blood, base excess in blood, total non-bicarbonate buffer concentration in plasma and hematocrit at several positions within the extracorporeal circuit under these same conditions are listed in Table 1. As expected, higher substitution fluid flow rates resulted in higher total carbon dioxide concentrations in blood and more positive base excess values in blood exiting the extracorporeal circuit.
Plasma concentration of bicarbonate ([bicarbonate]) and partial pressure of carbon dioxide (pCO2) in venous blood entering the CVVH circuit, after infusion of substitution fluid in predilution mode and in the outflow from CVVH circuit. Blood flow rate and substitution fluid flow rates were 200 mL/min and 2 L/h for black bars, 200 mL/min and 3 L/h for white bars and 400 mL/min and 2 L/h for gray bars. Results are shown in solid bars for [bicarbonate] and in dotted bars for pCO2. The total carbon dioxide concentration and base excess in blood entering the CVVH circuit were assumed as 19 and −8 mmol/L, respectively
Figure 3 shows plasma bicarbonate concentration and pH under the same conditions as in Fig. 2. Although plasma bicarbonate concentration increases after both predilution and postdilution infusion of substitution fluid, plasma pH is not substantially altered. The lack of a change in plasma pH resulted from approximately proportional increases in plasma bicarbonate concentration and pCO2. At a blood flow rate of 200 mL/min and a substitution flow rate of 2 L/hr, for example, the relative increase at the exit of the CVVH circuit in plasma bicarbonate concentration was 9.7% and the relative increase in pCO2 was 8.2%, resulting in an unaltered plasma pH in blood exiting the CVVH circuit.
Plasma concentration of bicarbonate ([bicarbonate]) and pH in blood entering the CVVH circuit, after infusion of substitution fluid in predilution mode and in the outflow from CVVH circuit. The fraction of substitution fluid infused predilution was 0.33. Blood flow rate and substitution fluid flow rates were 200 mL/min and 2 L/h for black bars, 200 mL/min and 3 L/h for white bars and 400 mL/min and 2 L/h for gray bars. Results are shown in solid bars for [bicarbonate] and in dotted bars for pH. The total carbon dioxide concentration and base excess in blood entering the CVVH circuit were assumed as 19 and − 8 mmol/L, respectively
Figure 4 compares the effect of the bicarbonate concentration in substitution fluid on plasma bicarbonate concentration and pCO2 within the extracorporeal CVVH circuit. As expected, using a substitution fluid with a lower bicarbonate concentration of 22 mEq/L blunted increases in both plasma bicarbonate concentration and pCO2 within and exiting the CVVH circuit. Indeed, there was virtually no increase in pCO2 within and exiting the CVVH circuit with the lower bicarbonate concentration in the substitution fluid. Figure 5 compares the effect of CVVHDF instead of CVVH on plasma bicarbonate concentration and pCO2 with a substitution/dialysis fluid containing a bicarbonate concentration of 32 mEq/L, 33% infused into the blood in the predilution mode. Use of CVVHDF instead of CVVH using the same substitution/dialysis fluid composition had a minor effect on plasma bicarbonate concentration and pCO2 within and exiting the extracorporeal circuit. Smaller increases in plasma bicarbonate concentration during CVVHDF resulted from the lower rates of bicarbonate transfer to blood due to the Donnan effect, reducing the transfer rate of bicarbonate anions into blood. Figure 5 also shows the effect of using 100%, instead of only a fraction, of the substitution fluid in the predilution mode. Infusion of all substitution fluid in the predilution mode resulted in similar, but slightly lower, plasma bicarbonate concentration and pCO2 exiting the extracorporeal circuit.
Plasma concentration of bicarbonate ([bicarbonate]) and partial pressure of carbon dioxide (pCO2) in venous blood entering the CVVH circuit, after infusion of substitution fluid in predilution mode and in the outflow from CVVH circuit. Blood flow rate and substitution fluid flow rates were 200 mL/min and 2 L/h with a bicarbonate concentration of 32 mEq/L (black bars) and 22 mEq/L (white bars). Results are shown in solid bars for [bicarbonate] and in dotted bars for pCO2. The total carbon dioxide concentration and base excess in blood entering the CVVH circuit were assumed as 19 and −8 mmol/L, respectively
Plasma concentration of bicarbonate ([bicarbonate]) and partial pressure of carbon dioxide (pCO2) in venous blood entering the extracorporeal circuit, after infusion of substitution fluid in predilution mode and in the outflow from extracorporeal circuit. Blood flow rate and substitution fluid flow rates were 200 mL/min and 2 L/h during CVVH for black bars and during CVVHDF for white bars; the fraction of substitution fluid in the predilution mode was 0.33 for both. Blood flow rate and substitution fluid flow rates were 200 mL/min and 2 L/h during CVVH with all substitution fluid infused in the predilution mode for gray bars. Results are shown in solid bars for [bicarbonate] and in dotted bars for pCO2. The total carbon dioxide concentration and base excess in blood entering the CVVH circuit were assumed as 19 and −8 mmol/L, respectively
The above results assumed only a single acid–base composition of venous blood entering the extracorporeal circuit; however, this may differ depending on the patient’s underlying medical condition and may vary over days of treatment for a given patient. Figure 6 shows the effect of blood and substitution fluid flow rates during CVVH when venous blood contained a high pCO2, representing a patient with potential lung dysfunction. This example assumed a total carbon dioxide concentration of 25 mmol/L and a base excess of -2 mmol/L in venous blood; the complete acid–base composition is detailed in Supplementary Materials. The model predictions in Fig. 6 are qualitatively similar to those shown in Fig. 2. Similar calculated results were predicted when the assumed acid–base composition of blood during CVVHDF was as described in Fig. 6 when compared to those in Fig. 5 (results not shown).
Plasma concentration of bicarbonate ([bicarbonate]) and partial pressure of carbon dioxide (pCO2) in blood entering the CVVH circuit, after infusion of substitution fluid in predilution mode and in the outflow from CVVH circuit. Blood flow rate and substitution fluid flow rates were 200 mL/min and 2 L/h for black bars, 200 mL/min and 3 L/h for white bars and 400 mL/min and 2 L/h for gray bars. Results are shown in solid bars for [bicarbonate] and in dotted bars for pCO2. These predictions are comparable to those in Fig. 2 but for a different composition of venous blood entering the CVVH circuit. The total carbon dioxide concentration and base excess in blood entering the CVVH circuit were assumed as 25 and −2 mmol/L, respectively
4 Discussion
Several clinical studies have evaluated acid–base balance during CKRT but have focused primarily on comparing the advantages and disadvantages of bicarbonate versus lactate as the substitution fluid buffer base [15,16,17,18,19,20,21]. Virtually all such studies have been concerned with overall effects on whole body acid–base balance with little effort to understand the direct effects of buffer base administration on acid–base chemistry in the blood of the extracorporeal circuit per se. A recent study by Jonckheer et al. [22] reported blood acid–base and oxygen chemistry of blood at various positions within a CVVH extracorporeal circuit for the first time. That work demonstrated a decrease in bicarbonate concentration and pCO2 in blood within a CVVH circuit after the infusion of predilution substitution fluids that did not contain bicarbonate (citrate was the buffer base in this instance). Such findings are not comparable to those studied in the current work, although the current model can apply to those conditions (results not shown). Empirical data to directly confirm the model predictions reported in this study are not currently available in the literature.
The mathematical model described herein predicts that the administration of bicarbonate via diffusion or convection results in only a modest increase in plasma bicarbonate concentration and pCO2 without a significant change in plasma pH in the blood exiting the extracorporeal circuit. This model prediction results from a conversion of bicarbonate to dissolved carbon dioxide and similar relative increases in bicarbonate concentration and pCO2 in plasma. The addition of bicarbonate to blood during CKRT is akin to adding bicarbonate to blood in a so-called “closed system”, where it has been previously suggested from in vitro experiments [23] and theoretical arguments [24] that such bicarbonate additions to blood do not produce large changes in plasma pH. Although the current model predictions appear counter intuitive since administration of solutions containing bicarbonate to patients will, in time, result in an increase in plasma pH, they are consistent with expectations in a so-called “closed system” when bicarbonate is added to blood. It should be noted that the plasma pH in blood exiting an extracorporeal circuit during maintenance hemodialysis [25, 26] is reduced when those patients are chronically treated with high dialysis fluid concentrations of bicarbonate and high dialysis fluid flow rates; such conditions result in substantially larger transfer rates of bicarbonate into blood. The current model of acid–base chemistry of blood does result in similar predictions of changes during maintenance hemodialysis as in those latter studies (Pietribiasi and Leypoldt, manuscript in review). The increase in whole body plasma pH after administration of bicarbonate solutions to patients is due to the “open system” conditions when accounting for the loss of dissolved CO2 in the patient’s lungs [24].
Differences in acid–base balance during CVVHDF versus CVVH therapies have not been directly evaluated in previous clinical studies although it has been proposed that metabolic acidosis would be more prevalent during CVVHDF than CVVH [27]; this is consistent with the model predictions in the current work. The only empirical comparison of acid–base balance during CVVH and CVVHDF in the literature is difficult to evaluate critically since the content of the substitution and dialysis solutions were different [28]. Although the current model predictions show differences between CVVHDF and CVVH therapies regarding acid–base balance, these differences are small and may not be clinically significant.
The mathematical model of acid–base chemistry used in this work is complex, but it cannot describe all chemical changes within blood during CKRT. For that purpose, more complex models would likely be required [29, 30]. It is noteworthy however that the model of Rees and Andreassen [9] has been shown to be consistent with several physiological systems, in particular the mixing of blood with different compositions [31]. The mixing of different fluid streams in the extracorporeal CKRT circuit examined in this study is essentially akin to that of mixing two different types of blood, although in this instance blood is mixed with a cell-free, protein-free crystalloid solution.
Although this work makes novel predictions regarding acid–base chemistry in a CKRT extracorporeal circuit, there are several limitations that deserve mention, especially regarding the assumptions in the mathematical model that were necessary to make the calculations practical. For example, it has been assumed that the overall sieving coefficients of bicarbonate and dissolved carbon dioxide are equal to unity, that is the total carbon dioxide concentration in plasma and ultrafiltrate are equal. Although measured true sieving coefficients for all small solutes are approximately equal to one [32], ultrafiltration occurs during CKRT only with the plasma water fraction of blood, not plasma itself. On the other hand, true sieving coefficients likely depend on the hemofilter employed; thus, further consideration of the effect of the true sieving coefficient and the effect of the volume of plasma proteins on model predictions using the current approach will require consideration of a hemofilter with specific sieving properties. Second, it has been assumed that the dialysance of bicarbonate transfer during CVHDF is equal to the inlet dialysate flow rate; this assumption provides an upper bound for the dialysance; thus, our contention that acid–base balance during CVVHDF may be less effective than during CVVH is likely correct. A third limitation is the assumption that the non-bicarbonate buffer in plasma is composed solely of albumin. It is known however that plasma phosphate levels contribute to the buffering capacity of blood and that the phosphate level of patients treated by CKRT can have both higher or lower than normal levels of plasma phosphate; the effects of altered plasma phosphate concentration on the reported results require further study. Finally, we note that no consideration in the current work addressed the anticoagulant used during CKRT that could potentially influence acid–base balance such as when using regional citrate anticoagulation. Thus, the current model predictions are only valid when using other anticoagulant strategies, such as when using heparin, the most widely used anticoagulant during CKRT according to Karkar and Ronco [33].
In conclusion, a mathematical model of acid–base chemistry of blood can predict the effect of operating conditions on acid–base balance within the extracorporeal circuit during CKRT. Additional empirical measurements during CKRT are necessary to validate and potentially improve these model predictions.
Data availability
Portions of the computer code used in making model calculations are available if requested.
References
Park S, Lee S, Jo HA, Han K, Kim Y, An JN, Joo KW, Lim CS, Kim YS, Kim H, Kim DK. Epidemiology of continuous renal replacement therapy in Korea: results from the National Health Insurance service claims database from 2005 to 2016. Kidney Res Clin Pract. 2018;37(2):119–29. https://doi.org/10.23876/j.krcp.2018.37.2.119.
Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten H, Ronco C, Kellum JA. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33(9):1563–70. https://doi.org/10.1007/s00134-007-0754-4.
Kashani K, Thongprayoon C, Cheungpasitporn W, Iacovella GM, Akhoundi A, Albright RC Jr. Association between mortality and replacement solution bicarbonate concentration in continuous renal replacement therapy: a propensity-matched cohort study. PLoS ONE. 2017;12(9):e0185064. https://doi.org/10.1371/journal.pone.0185064.
Shawwa K, Kompotiatis P, Jentzer JC, Wiley BM, Williams AW, Dillon JJ, Albright RC, Kashani KB. Hypotension within one-hour from starting CRRT is associated with in-hospital mortality. J Crit Care. 2019;54:7–13. https://doi.org/10.1016/j.jcrc.2019.07.004.
Allegretti AS, Flythe JE, Benda V, Robinson ES, Charytan DM. The effect of bicarbonate administration via continuous venovenous hemofiltration on acid-base parameters in ventilated patients. Biomed Res Int. 2015;2015:901590. https://doi.org/10.1155/2015/901590.
Darmon M, Clec’h C, Adrie C, Argaud L, Allaouchiche B, Azoulay E, Bouadma L, Garrouste-Orgeas M, Haouache H, Schwebel C, Goldgran-Toledano D, Khallel H, Dumenil AS, Jamali S, Souweine B, Zeni F, Cohen Y, Timsit JF. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol. 2014;9(8):1347–53. https://doi.org/10.2215/CJN.08300813.
van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013;17(3):R98. https://doi.org/10.1186/cc12743.
Nentwich J, Wichmann D, Kluge S, Lindau S, Mutlak H, John S. Low-flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: a pilot study. Ann Intensive Care. 2019;9(1):3. https://doi.org/10.1186/s13613-019-0480-4.
Rees SE, Andreassen S. Mathematical models of oxygen and carbon dioxide storage and transport: the acid-base chemistry of blood. Crit Rev Biomed Eng. 2005;33(3):209–64. https://doi.org/10.1615/critrevbiomedeng.v33.i3.10.
Rees SE, Andreassen S. ERRATUM: Clarification of and correction to "Mathematical models of oxygen and carbon dioxide storage and transport: the acid-base chemistry of blood”. Crit Rev Biomed Eng. 2007;33(3):209–64.
Sargent JA, Gotch FA. Principles and biophysics of dialysis. In: Jacobs C, Kjellstrand CM, Koch KM, Winchester JF, editors. Replacement of renal function by dialysis. Dordrecht: Kluwer Academic; 1996. p. 34–102.
Sargent JA, Marano M, Marano S, Gennari FJ. Acid-base homeostasis during hemodialysis: new insights into the mystery of bicarbonate disappearance during treatment. Semin Dial. 2018;31(5):468–78. https://doi.org/10.1111/sdi.12714.
Story DA. Stewart acid-base: a simplified bedside approach. Anesth Analg. 2016;123(2):511–5. https://doi.org/10.1213/ANE.0000000000001261.
Morgan TJ. The Stewart approach–one clinician’s perspective. Clin Biochem Rev. 2009;30(2):41–54.
Agarwal B, Kovari F, Saha R, Shaw S, Davenport A. Do bicarbonate-based solutions for continuous renal replacement therapy offer better control of metabolic acidosis than lactate-containing fluids? Nephron Clin Pract. 2011;118(4):c392-398. https://doi.org/10.1159/000324157.
Barenbrock M, Hausberg M, Matzkies F, de la Motte S, Schaefer RM. Effects of bicarbonate- and lactate-buffered replacement fluids on cardiovascular outcome in CVVH patients. Kidney Int. 2000;58(4):1751–7. https://doi.org/10.1046/j.1523-1755.2000.00336.x.
Davenport A, Bouman C, Kirpalani A, Skippen P, Tolwani A, Mehta RL, Palevsky PM. Delivery of renal replacement therapy in acute kidney injury: what are the key issues? Clin J Am Soc Nephrol. 2008;3(3):869–75. https://doi.org/10.2215/CJN.04821107.
Kierdorf HP, Leue C, Arns S. Lactate- or bicarbonate-buffered solutions in continuous extracorporeal renal replacement therapies. Kidney Int Suppl. 1999;72:S32-36.
McLean AG, Davenport A, Cox D, Sweny P. Effects of lactate-buffered and lactate-free dialysate in CAVHD patients with and without liver dysfunction. Kidney Int. 2000;58(4):1765–72. https://doi.org/10.1046/j.1523-1755.2000.00338.x.
Tan HK, Uchino S, Bellomo R. The acid-base effects of continuous hemofiltration with lactate or bicarbonate buffered replacement fluids. Int J Artif Organs. 2003;26(6):477–83. https://doi.org/10.1177/039139880302600605.
Zimmerman D, Cotman P, Ting R, Karanicolas S, Tobe SW. Continuous veno-venous haemodialysis with a novel bicarbonate dialysis solution: prospective cross-over comparison with a lactate buffered solution. Nephrol Dial Transplant. 1999;14(10):2387–91. https://doi.org/10.1093/ndt/14.10.2387.
Jonckheer J, Spapen H, Debain A, Demol J, Diltoer M, Costa O, Lanckmans K, Oshima T, Honore PM, Malbrain M, De Waele E. CO2 and O2 removal during continuous veno-venous hemofiltration: a pilot study. BMC Nephrol. 2019;20(1):222. https://doi.org/10.1186/s12882-019-1378-y.
Ostrea EM Jr, Odell GB. The influence of bicarbonate administration on blood pH in a “closed system”: clinical implications. J Pediatr. 1972;80(4):671–80. https://doi.org/10.1016/s0022-3476(72)80073-8.
Gattinoni L, Taccone P, Carlesso E. Respiratory acidosis: is the correction with bicarbonate worth? Minerva Anestesiol. 2006;72(6):551–7.
Sombolos KI, Bamichas GI, Christidou FN, Gionanlis LD, Karagianni AC, Anagnostopoulos TC, Natse TA. pO2 and pCO2 increment in post-dialyzer blood: the role of dialysate. Artif Organs. 2005;29(11):892–8. https://doi.org/10.1111/j.1525-1594.2005.00126.x.
Symreng T, Flanigan MJ, Lim VS. Ventilatory and metabolic changes during high efficiency hemodialysis. Kidney Int. 1992;41(4):1064–9. https://doi.org/10.1038/ki.1992.162.
Bouchard J, Mehta RL. Acid-base disturbances in the intensive care unit: current issues and the use of continuous renal replacement therapy as a customized treatment tool. Int J Artif Organs. 2008;31(1):6–14. https://doi.org/10.1177/039139880803100103.
Morimatsu H, Uchino S, Bellomo R, Ronco C. Continuous renal replacement therapy: does technique influence electrolyte and bicarbonate control? Int J Artif Organs. 2003;26(4):289–96. https://doi.org/10.1177/039139880302600403.
Jezek F, Kofranek J. Modern and traditional approaches combined into an effective gray-box mathematical model of full-blood acid-base. Theor Biol Med Model. 2018;15(1):14. https://doi.org/10.1186/s12976-018-0086-9.
Wolf MB. Physicochemical models of acid-base. Semin Nephrol. 2019;39(4):328–39. https://doi.org/10.1016/j.semnephrol.2019.04.003.
Rees SE, Klaestrup E, Handy J, Andreassen S, Kristensen SR. Mathematical modelling of the acid-base chemistry and oxygenation of blood: a mass balance, mass action approach including plasma and red blood cells. Eur J Appl Physiol. 2010;108(3):483–94. https://doi.org/10.1007/s00421-009-1244-x.
Uchino S, Cole L, Morimatsu H, Goldsmith D, Ronco C, Bellomo R. Solute mass balance during isovolaemic high volume haemofiltration. Intensive Care Med. 2003;29(9):1541–6. https://doi.org/10.1007/s00134-003-1857-1.
Karkar A, Ronco C. Prescription of CRRT: a pathway to optimize therapy. Ann Intensive Care. 2020;10(1):32. https://doi.org/10.1186/s13613-020-0648-y.
Acknowledgements
The authors gratefully acknowledge the careful review of our work by the anonymous reviewers. Their comments corrected certain errors in the original submission.
Funding
This work was supported by the Polish National Science Center (grant number 2017/27/B/ST7/03029) and Baxter International.
Author information
Authors and Affiliations
Contributions
JKL designed the study, wrote the original computer programs, and wrote the first draft of the manuscript. MP assisted in writing the computer program; JE and KH assisted in designing the study and provided clinical input. All authors reviewed the manuscript, provided feedback on the first draft, and approved the submission of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
JKL is a consultant to Baxter International. JE and KH are full-time employees of Baxter International with ownership interests.
Ethical approval
No studies involving patients were reported in this manuscript, thus, no ethics approval was necessary.
Research involving human and animal rights statements
This manuscript does not involve research requiring human and animal rights statements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix
Appendix
Equations (1) and (2) in Methods can be derived from mass balance considerations before and after the infusion of substitution fluids during CVVH. First, the mass flow rate of total carbon dioxide after predilution infusion of substitution fluid and entering the hemofilter (total carbon dioxide concentration times fluid flow rate or Ci × (Qbi + fQs)) must equal the mass flow rate of total carbon dioxide in blood entering the extracorporeal CVVH circuit or Cbi × Qbi plus the mass flow rate of total carbon dioxide with infusion of substitution fluid or Cs × fQs. Stated in mathematics, this requires
After some algebra, this equation can be rearranged to Eq. (1) in Methods. Second, the mass flow rate of total carbon dioxide exiting the hemofilter or Co × {Qbi + (f-1)Qs} plus the mass flow rate of total carbon dioxide via the postdilution infusion of substitution fluid or Cs × (1-f)Qs must equal the mass flow rate of total carbon dioxide exiting the extracorporeal circuit or Cbo × Qbi. Stated as an equation, this requires
This equation can be rearranged to Eq. (2) in Methods.
To assess mass or solute transport from blood to dialysate during hemodialysis with both diffusive and convective solute transport, the following equation (in the terminology of the current work) was defined by Sargent and Gotch [11] as
where D denotes the dialysance and Qi denotes the blood flow rate into the hemofilter. In this equation both J, the overall solute transport rate, and Qs, the ultrafiltration rate, were defined as positive in the blood-to-dialysate direction. As in Methods, Ci and Cs denote total carbon dioxide concentrations in blood and dialysis fluid, respectively. When it is noted that J in Methods is defined as positive in the opposite or dialysate-to-blood direction, Eq. (3) is the result after some algebra if the dialysis fluid concentration is modified by the Donnan effect as suggested by Sargent et al. [12]. Mass balance during CVVHDF requires that the mass flow rate of total carbon dioxide exiting the hemofilter or Cbo × Qbi must equal the mass flow rate into the hemofilter or Ci × Qi plus the mass transport rate of total carbon dioxide from dialysis fluid to blood or J. Stated as an equation, this requires
Algebraic rearrangement of this equation in conjunction with Eqs. (1) and (4) yields Eq. (5) in Methods.
Rights and permissions
About this article
Cite this article
Leypoldt, J.K., Pietribiasi, M., Echeverri, J. et al. Modeling acid–base balance during continuous kidney replacement therapy. J Clin Monit Comput 36, 179–189 (2022). https://doi.org/10.1007/s10877-020-00635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-020-00635-3