Abstract
Objective
To evaluate the effect of changing the amount of pre-dilution replacement fluid on the sieving coefficient (SC) and mass transfer of small solutes during isovolaemic high-volume haemofiltration (HVHF).
Design and setting
Prospective interventional study in the intensive care unit of a tertiary university hospital.
Patients
Eight patients with septic shock.
Interventions
Isovolaemic HVHF (6 l/h of replacement fluid) was performed. The proportion of replacement fluid delivered in pre-filter was altered to progressively decrease it from 6 to 0 l/h. Samples were simultaneously taken from the "pre-filter", "post-filter" and ultrafiltrate (UF) sampling ports.
Measurements and results
Sodium, potassium, chloride, total calcium, total magnesium, phosphate, total CO2, urea, creatinine and glucose concentrations were measured in each sample. The sieving coefficients of chloride, total CO2, phosphate, urea and glucose were higher than 1 in most pre-dilution states. The sieving coefficients of sodium, potassium, calcium, magnesium, total CO2 and urea decreased significantly with decreasing pre-dilution fluid rate. The sieving coefficients of chloride and glucose increased with decreasing pre-dilution fluid rate. There was a significant mass gain of sodium and glucose under all pre-dilution conditions. Mass chloride gains decreased with decreasing pre-dilution rates and changed into chloride loss during 6 l/h of post-dilution. Decreasing pre-dilution improved urea and creatinine mass removal.
Conclusions
Small solute SC and mass transfer during isovolaemic HVHF are significantly affected by the proportion of replacement fluid administered pre-filter. Isovolaemic HVHF is neither isonatraemic nor isochloraemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nomenclature workgroup of the Acute Dialysis Quality Initiative defined high-volume continuous veno-venous haemofiltration (HVHF) as an ultrafiltration rate higher than 35 ml/kg per hour [1]. With such aggressive fluid exchange, inflammatory mediators such as cytokines can be removed from the circulation. Some investigators have recently applied this new technique to the treatment of critically ill patients. They have found that HVHF can improve haemodynamics [2] and clinical outcomes [3, 4] in patients with septic shock and/or acute renal failure.
However, because of such aggressive fluid exchange rates (up to 100–150 ml/min of plasma water is lost and replaced with commercially available replacement fluids), there are some potential problems with HVHF. These problems might include hypothermia, the loss of useful substances such as vitamins and amino acids, difficulties in correct drug dosing and electrolyte abnormalities. Even when no ultrafiltrate (UF) is removed in excess of replacement fluid (so-called isovolaemic HVHF), significant amounts of solutes can be gained or lost. Furthermore, small solute clearance during HVHF is likely to be significantly affected by the proportion of replacement fluid administered pre-filter because replacement fluid infused pre-filter changes serum concentrations of such small solutes. Such small solute removal can be assessed in terms of sieving coefficient (the ratio between the UF and intra-filter plasma concentration for a given solute) which, multiplied by the UF rate, allows direct calculation of solute clearance and in terms of mass transfer (the difference between the mass administered and removed for a given solute over a unit of time during renal replacement therapy).
Accordingly, we prospectively evaluated the sieving coefficient (SC) and mass transfer of small solutes during isovolaemic HVHF, while systematically altering the proportion of replacement fluid delivered in the pre-filter position.
Materials and methods
Institutional approval was granted by the hospital Ethics Committee. Informed consent was obtained from each patient's next of kin. This study was conducted as a component of a cross-over trial of isovolaemic HVHF in human septic shock [2]. In summary, patients were included in this study if they had established acute renal failure (requiring renal replacement therapy) and fulfilled Bone's [5] criteria for septic shock: tachypnoea (respiratory rate >20 breaths/min;, if mechanically ventilated, >10 l/min), tachycardia (heart rate >90 beats/min), and hyperthermia or hypothermia (core or rectal temperature >38.3°C or <35.6°C). Patients were excluded if they had end-stage renal failure, acquired immunodeficiency syndrome, a life expectancy less than 6 months, or if withdrawal of therapy was a possibility. Eight patients were studied; their demographic characteristics are summarized in Table 1. Solute concentrations in patient serum and replacement fluid are presented in Table 2. Patients were randomized to receive an 8-h session of either isovolaemic continuous veno-venous haemofiltration (CVVH) or of isovolaemic HVHF. The order in which CVVH or HVHF was applied was random (sealed opaque envelopes). The first session was followed by an overnight wash out period. On the second day 8 h of the alternative therapy was applied (cross-over design). The current study was conducted during the HVHF period.
HVHF technique
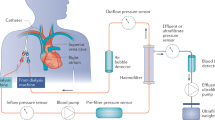
Vascular access was obtained with 13.5-FG dual lumen catheters (Niagara, Bard, Ontario, Canada). A BM 11/14 machine (Edwards Lifescience, Sydney, Australia) was used for HVHF together with a 1.6 m2 AN69 filter (Filtral 16, Hospal, Lyon, France). Blood flow was set at 300 ml/min and total replacement flow at 6 l/h (100 ml/min). Lactate-buffered replacement fluid (Haemofiltration Replacement Fluid, Baxter Healthcare, Sydney, Australia) was used. Additional potassium and phosphate ions were added to replacement fluid (KH2PO4, David Bull Laboratories, Sydney, Australia). Heparin sodium (David Bull Laboratories, Melbourne, Australia) was administered pre-filter at a rate of 1000 IU/h, while protamine sulfate (Fisons Pharmaceuticals, Sydney, Australia) was administered post-filter at a rate of 10 mg/h (regional anticoagulation). The circuit set-up is shown in Fig. 1.
Study protocol and measurements
With the assistance of an additional volumetric pump (Gemini PC-2, IMED Corporation, San Diego, Calif., USA), replacement fluid was infused both pre-filter and post filter simultaneously. Initially, all replacement fluid was infused pre-filter (6 l/h pre-dilution). Samples were taken from the pre-filter sampling port, post-filter sampling port and ultrafiltrate sampling port 5–10 min after commencing haemofiltration. Sampling was then repeated at 5 l/h pre-dilution with 1 l/h post-dilution, 4 l/h pre-dilution with 2 l/h post-dilution, 2 l/h pre-dilution with 4 l/h post-dilution, 1 l/h pre-dilution with 5 l/h post-dilution and no pre-dilution with 6 l/h post-dilution. At each time the sampling occurred after 5–10 min of steady-state of operation. Samples were drawn into plain tubes and sodium, potassium, chloride, total calcium, total magnesium, phosphate, urea, creatinine, total CO2, and glucose concentrations were measured (Hitachi 747 Analyser, Hitachi). Haematocrit and albumin concentrations were also measured on the morning of the study prior to HVHF (Hitachi 747 Analyser or Sysmex SE 9000 Analyser, Roche).
Calculations
Pre-dilution fluid in the BM11/14 machine is infused pre-blood pump. Therefore plasma flow (Qp, ml/min) can be calculated as:
Qp=(Qb−Qpre)×(1−Ht/100)
Where Qb is blood pump flow (300 ml/min), Qpre is pre-dilution fluid rate (0–100 ml/min), and Ht is haematocrit in the venous blood (%). The pre-filter sampling port is proximal to the pre-dilution fluid infusion site. Therefore pre-filter solute concentration (Cpre, mmol/l) is calculated as:
Cpre=(Ca×Qp+Cr×Qpre)/(Qp/Qpre)
Where Ca is solute concentration in the blood from the pre-filter ("arterial") sampling port (mmol/l), and Cr is that in the replacement fluid. Pre- and post-filter albumin concentration (Apre and Apost, g/l) were calculated as:
Apre=A×Qp/(Qp+Qpre)
Apost=A×Qp/(Qp+Qpre−Quf)
Where A is the venous albumin concentration (g/l) and Quf is the ultrafiltrate rate (100 ml/min). The SC of a solute was calculated as:
SC=2×Cuf/{Cpre/[1−0.00107×Apre/0.6]+[Cpost/1−(0.00107×Apost/0.6)]}
Where Cuf is solute concentration in ultrafiltrate (mmol/l) and Cpost is post-filter solute concentration (mmol/l). The term "0.00107" is the constant to convert total plasma concentration to plasma water concentration [6]. Because we did not measure total protein concentration, this value was estimated (albumin divided by 0.6) [7]. Mass transfer (Mt, mmol/day) was calculated as:
Mt=(Cr×Qr−Cuf×Quf)×60×24/1000
Where Cr is solute concentration in the replacement fluid (mmol/l), Qr is total replacement fluid rate (pre-dilution and post-dilution), and Quf is ultrafiltrate rate. Because of isovolaemic fashion both Qr and Quf were 100 ml/min. The 60 refers to minutes, 24 to hours, and 1000 is correction factor for concentration in liters. A positive value of Mt suggests that a solute excess is infused into the patient.
Statistical analysis
A commercially available statistical package was used for data analysis (Staview, Abacus, Berkeley, Calif., USA). Data are presented as means ±standard deviation. The changes in SC and mass transfer with different proportion of pre-dilution were compared using non-parametric analysis of variance (Friedman's Test). A p value less than 0.05 was considered significant.
Results
The SCs of cations, anions and other solutes, were calculated at variable pre-dilution fluid rates (Fig. 2). These calculations showed that the SCs of chloride, total CO2, phosphate, urea and glucose were higher than 1 in most pre-dilution states. The SCs of sodium, potassium, calcium, magnesium, total CO2 and urea decreased significantly with decreasing pre-dilution fluid rate (sodium: p=0.0004, potassium: p=0.02, calcium: p<0.0001, magnesium: p<0.0001, total CO2: p=0.02, urea: p=0.033). On the other hand, the SCs of chloride and glucose increased with decreasing pre-dilution fluid rate (chloride: p=0.001, glucose: p=0.0015).
Means and standard deviations of sieving coefficients (SC) in sodium (Friedman's test, p=0.0004), potassium (p=0.02), total calcium (p<0.0001) and total magnesium (p<0.0001). B Means and standard deviations of SC in chloride (p=0.001), total CO2 (p=0.02) and phosphate (p=0.72). C Means and standard deviations of SC in urea (p=0.033), creatinine (p=0.47) and glucose (p=0.0015)
Mass transfer calculations showed significant mass gains or losses of some small solutes despite isovolaemic therapy. The mass transfer values for electrolytes and glucose are shown in Table 3. They demonstrate a significant gain of sodium and glucose under all pre-dilution conditions. Mass chloride gains decreased with decreasing pre-dilution rates and changed into chloride loss during 6 l/h of post-dilution (p<0.0001). Decreasing pre-dilution improved urea and creatinine mass removal (urea: p<0.0001, creatinine: p=0.0003). However, it also increased total CO2 loss (p<0.0001).
Discussion
This investigation of the effect of changing the proportion of replacement fluid administered pre-filter on SC and mass transfer for small solutes during HVHF revealed several important findings.
First, the SC of sodium, potassium, calcium, magnesium, total CO2 and urea decreased as the proportion of replacement fluid administered pre-filter decreased. This phenomenon has several possible explanations. One may be related to the effect of the phenomenon called "concentration polarization" [8] on SC. With increasing blood viscosity the thickness of the protein layer at the blood-membrane interface can be expected to increase. This increase should reduce effective membrane permeability and thereby SC. For cations, such as sodium, potassium, calcium and magnesium, decreased SC might also be explained by changes in the Gibbs-Donnan effect. Pre-dilution replacement fluid in fact should decrease the concentration of negatively charged proteins in blood thus decreasing their capacity to electrically hinder cation convection. This Gibbs-Donnan effect might also explain the increase in the SC of chloride with decreasing pre-dilution.
Mass transfer for sodium and calcium increased, that of potassium and chloride decreased, and that of magnesium, phosphate and glucose did not change with decreasing pre-dilution. The combined effect of pre-filter concentration and SC determines the mass transfer of a given solute. For example, because replacement fluid contains electrolytes and glucose, pre-filter fluid administration changes the pre-filter concentration of these molecules. Such changes, in addition to changes in SC, make the prediction of the final mass transfer balance difficult. Clinicians need to be aware of how changing the site of administration of the replacement fluid can powerfully affect solute mass balance even during isovolaemic therapy.
Sodium and potassium
During isovolaemic HVHF patients gained a significant amount of sodium. For example, 243 mmol sodium (equivalent to 14 g salt and to 1.6 l of normal saline) would be gained if HVHF were conducted for 24 h in pure post-dilution mode. This is despite a mean serum concentration of sodium (139.8 mmol/l), which was almost identical to the replacement fluid concentration (140 mmol/l). This gain occurs because the SC of sodium is less than 1, and because of the high volume of replacement fluid infused. The clinical effects of such positive sodium balance may be important (organ oedema).
Although the mean serum potassium concentration was in the normal range, more than 30 mmol/day potassium could be removed during HVHF. Replacement fluid often contains a low concentration of potassium to facilitate the treatment of hyperkalaemia. We added potassium to the replacement fluid to increase the potassium concentration from 1 to 3.5 mmol/l. If such additional potassium were not given, HVHF would of course cause severe hypokalaemia.
Calcium and magnesium
We found that calcium and magnesium had low SCs (from 0.6 to 0.8), and that these SCs decreased with decreasing pre-dilution more significantly than for other solutes. This can be explained by the measurement of total instead of ionized serum calcium and magnesium concentration. Protein-bound calcium and magnesium cannot move across the membrane. Pre-dilution replacement fluid dilutes serum protein concentration and should increase free calcium and magnesium concentration, facilitating their movement across the membrane. An important finding is that more than 10 mmol/day of calcium can be removed from a patient during isovolaemic HVHF, even though the replacement fluid contains 1.6 mmol/l of calcium. In support of our observation, Tan et al. [9] reported that hypocalcaemia was common during conventional continuous veno-venous haemodiafiltration (1 l/h dialysate and 700 ml/h replacement). HVHF might contribute to hypocalcaemia if used for more than 24 h.
Chloride and total CO2
The SCs of chloride and total CO2 were greater than 1 in this study, a finding consistent with previous investigations [10, 11]. This phenomenon is possibly due to a Gibbs-Donnan effect. An interesting finding is that despite a lower mean serum chloride concentration in our patients (94.4 mmol/l) than in the replacement fluid (100 mmol/l), more than 200 mmol/day of chloride could be removed from a patient during HVHF with pure post-dilution. Such chloride loss would likely increase the patient's strong ion difference and attenuate any metabolic acidosis [12], which is often seen in acute renal failure patients.
Phosphate
Tan et al. [13] reported that hypophosphataemia was common during continuous veno-venous haemodiafiltration. We added phosphate to the replacement fluid to prevent such hypophosphataemia. More than 200 mmol/day of phosphate would have been added to replacement fluid for HVHF. This would lead to a phosphate mass gain of only approximately 10 mmol/day. These observations suggest that serum phosphate concentration should be closely monitored during isovolaemic HVHF, and that phosphate administration is mandatory if one wishes to avoid severe hypophosphataemia and its consequences on organ function [14].
Urea and creatinine
The SC of urea was slightly greater than 1 with high pre-dilution fluid rates (from 6 to 4 l). This phenomenon can be explained by urea movement out of the erythrocyte in response to a dilution gradient. Cheung et al. [15] measured urea mass in plasma before and after replacement fluid infusion during pre-dilution haemofiltration. They found that there was approximately a 10% increase in urea mass in plasma after dilution. In our study, with 6 l/h of pre-dilution, plasma would have been diluted by almost 40%. Although we did not measure urea concentration after replacement fluid infusion, there could have been significant urea mass movement across the erythrocyte membrane, which would have then increased the urea concentration in plasma and thereby increased the calculated SC.
Glucose
The SC of glucose was always greater than 1. The cause of this phenomenon is unclear but the slight negative electric charge of glucose might have played a role. Mass transfer calculations showed that approximately 200 mmol/day glucose would have been infused into each patient, equal to 602 kJ energy intake. The average glucose concentration in our population was 7.5 mmol/l. If intensive insulin therapy were applied to keep blood glucose concentrations between 4.4 and 6.1 mmol/l [16], approximately 1000 kJ/day of additional energy would be administered during isovolaemic HVHF. This gain should be taken into account for the calculation of a correct caloric balance.
This study has some limitations. First, only eight patients were studied. Some of the findings could have been influenced by individual variation, as mass transfer is dependent on the serum concentration of each solute in each patient. Nonetheless, the concentrations of solute were not markedly abnormal and were representative of the likely values in a similar larger population of patients with septic shock and acute renal failure. Second, we conducted measurements soon after HVHF was started and not after several hours of application. "Protein fouling" of the membrane due to high transmembrane pressures over time might significantly reduce the SC of solutes and their mass transfer. However, the effect of such "fouling" on small solute movement should be relatively small.
In summary, we evaluated the effect of changing the proportion of replacement fluid delivered in the pre-filter position on the SC and mass transfer of small molecules during HVHF. Beyond the expected and often desirable losses of urea, creatinine and potassium, isovolaemic HVHF can cause significant gains of sodium and glucose and significant losses of calcium, phosphate and chloride. The proportion of replacement fluid administered pre-filter has a marked effect on overall solute balance and mass removal. Isovolaemic HVHF is in fact neither isonatraemic nor isochloraemic. Physicians who apply HVHF to critically ill patients should be aware of these previously unreported effects.
References
Gibney RTN, Kimmel PL, Lazarus M (2003) ADQI, workgroup 1: definitions and nomenclature (www.ADQI.net)
Cole L, Bellomo R, Journois D, Davenport P, Baldwin I, Tipping P (2001) High-volume haemofiltration in human septic shock. Intensive Care Med 27:978–986
Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G (2000) Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomized trial. Lancet 355:26–30
Honore PM, Jamez J, Wauthier M, Lee PA, Dugernier T, Pirenne B, Hanique G, Matson JR (2000) Prospective evaluation of short-term, high volume isovolemic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med 28:3581–3587
Bone RC (1991) Sepsis, the sepsis syndrome, multiorgan failure: a plea for comparable definitions Ann Intern Med 114:332–333
Colton CK, Henderson LW, Ford CA, Lysaght MJ (1975) Kinetics of hemodiafiltration: I in vitro transport characteristics of a hollow fiber blood ultrafilter. J Lab Clin Med 85:355–371
Sun X, Iles M, Weissman C (1993) Physiologic variables and fluid resuscitation in the postoperative intensive care unit patient. Crit Care Med 21:555–561
Ronco C, Bellomo R (1998) Principles of solute clearance during continuous renal replacement therapy. In: Ronco C, Bellomo R (eds. Critical care nephrology. Kluwer, pp 1213–1223
Tan HK, Bellomo R, M'Pisi DA, Ronco C (2002) Ionised serum calcium levels during acute renal failure: intermittent hemodialysis vs. continuous hemodiafiltration. Ren Fail 24:19–27
Kohnle W, Heimsch E, Schmidt-Wiederkehr P, Franz HE (1977) Acid base status during treatment of chronic uremia with diafiltration. J Dial 1:419–430
Feriani M, Aquila RD (1998) Acid-base balance and replacement solutions in continuous renal replacement therapies. Kidney Int 53: S156–159
Stewart P (1983) Modern quantitative acid-base chemistry. Can J Physiol Pharmacol 61:1444–1461
Tan HK, Bellomo R, M'Pisi DA, Ronco C (2001) Phosphatemic control during acute renal failure: intermittent hemodialysis versus continuous hemodiafiltration. Int J Artif Organs 24:186–191
Weisinger JR, Bellorin-Font E (1998) Magnesium and phosphorus. Lancet 352:319–396
Cheung AK, Alford MF, Wilson MM, Leypoldt JK, Henderson LW (1983) Urea movement across erythrocyte membrane during artificial kidney treatment. Kidney Int 23:866–869
Van Den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P and Bouillon R (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchino, S., Cole, L., Morimatsu, H. et al. Solute mass balance during isovolaemic high volume haemofiltration. Intensive Care Med 29, 1541–1546 (2003). https://doi.org/10.1007/s00134-003-1857-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1857-1