Abstract
This study aimed to compare the prognostic performance of the ratio of mixed and central venous–arterial CO2 tension difference to arterial–venous O2 content difference (Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2, respectively) with that of the mixed and central venous-to-arterial carbon dioxide gradient (Pv-aCO2 and Pcv-aCO2, respectively) for adverse events after cardiac surgery. One hundred and ten patients undergoing cardiac surgery with cardiopulmonary bypass were enrolled. After catheter insertion, three blood samples were withdrawn simultaneously through arterial pressure, central venous, and pulmonary artery catheters, before and at the end of the operation, and preoperative and postoperative values were determined. The primary end-point was set as the incidence of postoperative major organ morbidity and mortality (MOMM). Receiver operating characteristic (ROC) curve and multivariate logistic regression analyses were performed to evaluate the prognostic reliability of Pv-aCO2, Pcv-aCO2, Pv-aCO2/Ca-vO2, and Pcv-aCO2/Ca-cvO2 for MOMM. MOMM events occurred in 25 patients (22.7%). ROC curve analysis revealed that both postoperative Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 were significant predictors of MOMM. However, postoperative Pv-aCO2 was the best predictor of MOMM (area under the curve [AUC]: 0.804; 95% confidence interval [CI] 0.688–0.921), at a 5.1-mmHg cut-off, sensitivity was 76.0%, and specificity was 74.1%. Multivariate analysis revealed that postoperative Pv-aCO2 was an independent predictor of MOMM (odds ratio [OR]: 1.42, 95% CI 1.01–2.00, p = 0.046) and prolonged ICU stay (OR: 1.45, 95% CI 1.05–2.01, p = 0.024). Pv-aCO2 at the end of cardiac surgery was a better predictor of postoperative complications than Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With advances in terms of the perioperative environment, including patient care and surgical techniques, the number of high-risk surgical patients has markedly increased [1, 2]. In these patients, maintaining adequate tissue perfusion and oxygenation can decrease postoperative adverse outcomes [3]. Cardiovascular surgery using cardiopulmonary bypass (CPB) can induce ischemic–reperfusion injury, due to decreased cardiac output (CO) and inadequate tissue perfusion. Accordingly, early identification of tissue hypo-perfusion is crucial, as it may improve postoperative outcomes in cardiac surgical patients [4].
Central venous oxygen saturation (ScvO2) and mixed venous oxygen saturation (SvO2) have traditionally been used to predict the systemic oxygen supply–demand balance [5, 6]. Low ScvO2 or SvO2 may indicate inadequate tissue oxygen delivery (DO2); however, these indices do not ensure adequate tissue perfusion in cases with normal or supra-normal values [7]. Additionally, normalization of these markers does not guarantee adequate tissue perfusion and may not lead to decreased organ dysfunction [8]. Therefore, additional markers of decreased DO2 are needed. Recently, the mixed venous-to-arterial carbon dioxide gradient (Pv-aCO2) and central venous-to-arterial carbon dioxide gradient (Pcv-aCO2) have been suggested as complementary markers for identifying septic patients with inadequate DO2 [9, 10]. Both of these variables have proven prognostic ability in high-risk surgical patients [11]. However, Pv-aCO2 and Pcv-aCO2 may increase without a decrease in DO2, because of the Haldane effect [12]. Therefore, in addition to the CO2 gradient, O2 changes should be considered.
The ratio of carbon dioxide elimination (VCO2) to oxygen consumption (VO2) is a reliable index for predicting global anaerobic metabolism. Under anaerobic metabolic conditions, VCO2 may exceed VO2 [13]. According to the Fick principle, the ratio of the venous–arterial carbon dioxide content difference to the arterial–venous oxygen content difference (Cv-aCO2/Ca-vO2) is equal to VCO2/VO2. Within the physiological range, the correlation between the partial pressure of CO2 (PCO2) and CO2 content is almost linear; thus, PCO2 could be utilized as a substitute for CO2 content [14]. The Pv-aCO2 to arterial-to-mixed venous O2 content difference ratio (Pv-aCO2/Ca-vO2) can be a reliable index for identifying inadequate DO2 [13]. Recently, the utility of this ratio as a marker of resuscitation has been demonstrated, particularly in patients with septic shock [15]. Additionally, in septic patients, Mallat et al. [16] have shown that the ratio of the central venous–arterial CO2 tension difference to arterial–central-venous O2 content difference (Pcv-aCO2/Ca-cvO2) is a more reliable index of inadequate systemic tissue hypoxia than ScvO2 and serum lactate level. However, in cardiac surgery patients, the prognostic power of Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 for postoperative outcomes is unclear.
We hypothesized that, in cardiac surgery, Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 may be better markers of global anaerobic metabolism than CO2 gradient variables. To test our hypothesis, we compared the prognostic performance of Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 with that of Pv-aCO2 and Pcv-aCO2 for predicting adverse events after cardiac surgery.
2 Methods
2.1 Patients and anesthetic management
The current study protocol was approved by the institutional review board of our hospital. Written informed consent was obtained from all patients. Patients undergoing elective cardiac surgery using CPB were included in this study. The exclusion criteria were as follows: intra-cardiac shunts and renal failure requiring hemodialysis.
No premedication was given, and all enrolled patients could take clear liquids until 3 h preoperatively. Anesthesia induction was accomplished by bolus administration of midazolam (0.04–0.18 mg/kg), propofol (0.5–2 mg/kg), fentanyl (2–10 µg/kg), rocuronium (0.6–1.2 mg/kg), and inhalation agents (sevoflurane [0.5–3.0%] or desflurane [2.0–6.0%]). Following tracheal intubation, the arterial pressure catheter was inserted into the radial artery, and a central venous catheter (CVC), and pulmonary artery catheter (PAC) were placed into the right internal jugular vein. The positions of the CVC and PAC were confirmed by the pressure waveform and transesophageal echocardiography. Cardiac output and stroke volume were continuously measured by the PAC. Patients were ventilated with a tidal volume of 6–7 mL/kg (ideal body weight). The fraction of inspiratory oxygen was adjusted to maintain PaO2 from 100 to 150 mmHg. Respiratory rate (normally 10–12 times/min) was controlled to keep PaCO2 in the range of 35–40 mmHg. Anesthetic maintenance was performed using inhalation agents (sevoflurane [1.0–2.0%]) or (desflurane [3.0–6.0%]), remifentanil (0.1–0.4 µg/kg/min), fentanyl (10–40 µg/kg for the total dose). Rocuronium or vecuronium was administered for muscle relaxation, as appropriate. The depth of general anesthesia was adjusted by maintaining the bi-spectral index value (BIS monitor v4.0; Medtronic Inc, Minneapolis, MN, USA) in the range of 45–60.
2.2 Cardiopulmonary bypass management and postoperative course
During the CPB procedure, propofol (2–6 mg/kg/h) was continuously infused for general anesthesia maintenance. Standard CPB management was provided with a flow rate around 2.5 L/min/m2 and mean arterial pressure between 50 and 75 mmHg. The PaCO2 value was adjusted from 40 to 45 mmHg by alpha-stat management. Hematocrit level was maintained at around 20%. For cardiac arrest, mild hypothermia (rectal temperature 32 °C) was induced, and standard antegrade and retrograde crystalloid was administered for myocardial protection. When performing circulation arrest, deep hypothermia was performed (rectal temperature around 26 °C), and selective cerebral perfusion was provided (flow rate: 500 mL/min, mean arterial pressure in the right radial artery: 40–50 mmHg). Intra-aortic balloon pumping (IABP) was used when circulatory failure (cardiac index less than 1.5 L/min/m2 and systolic blood pressure less than 80 mmHg) continued even after massive infusion of inotropic drugs (the doses of dopamine + dobutamine > 10 µg/kg/min and norepinephrine > 0.1 µg/kg/min).
After surgery, all patients were transferred to the intensive care unit (ICU) while remaining intubated. Postoperative management was performed by cardiac surgeons blinded to the study protocol. All patients remained in the ICU at least until the first postoperative day and were discharged from the ICU when patient’s state was stable. The criteria for ICU discharge were as follows: (1) hemodynamic stability was defined as the absence of arrhythmias requiring drug treatment, systolic blood pressure more than 100 mmHg, with less than two inotropic drugs, and urine output more than 1 mL/kg/h; (2) respiratory stability was defined as blood oxygen saturation more than 94% with an oxygen mask (< 5 L/min).
2.3 Blood gas measurements
After insertion of the pulmonary catheter, three blood samples were withdrawn simultaneously through the arterial pressure, central venous, and pulmonary artery catheters, and the samples were analyzed using an ABL800 (Radiometer Medical. Co., Ltd., Copenhagen, Denmark) to determine the following variables: arterial oxygen tension (PaO2), arterial oxygen saturation (SaO2), arterial carbon dioxide tension (PaCO2), central venous oxygen tension (PcvO2), central venous oxygen saturation (ScvO2), central venous carbon dioxide tension (PcvCO2), mixed venous oxygen tension (PvO2), mixed venous oxygen saturation (SvO2), mixed venous carbon dioxide tension (PvCO2), and hemoglobin concentration (Hb). Pv-aCO2, Pcv-aCO2, Pv-aCO2/Ca-vO2, and Pcv-aCO2/Ca-cvO2 were calculated as follows:
Blood gas analysis was performed twice (before and at the end of the operation), in the same way, and Pv-aCO2, Pcv-aCO2, Pv-aCO2/Ca-vO2, and Pcv-aCO2/Ca-cvO2 were also calculated. The values before and at the end of the operation were recorded as the “preoperative” and “postoperative” values, respectively.
2.4 Statistical analysis
The primary end-point in the current study was set as the incidence of postoperative severe adverse events (major organ morbidity and mortality: MOMM), as previously described [17]. MOMM was determined as major complications (either life threatening or potentially resulting in permanent functional disability) from the Society of Thoracic Surgeons 30-day operative mortality and morbidity risk model. MOMM events were defined as follows: death, stroke requiring drug treatment, renal failure requiring dialysis, prolonged mechanical ventilation (more than 48 h postoperatively), re-operation, and deep sternal infection. We investigated the prognostic ability of Pv-aCO2, Pcv-aCO2, Pv-aCO2/Ca-vO2, and Pcv-aCO2/Ca-cvO2 for postoperative outcomes. The threshold value of 1.4 mmHg·dL/mL reported by Mekontso-Dessap et al. [13], was used to predict the presence of hyperlactatemia in critically ill patients, while the current study was conducted to predict postoperative adverse outcomes in cardiac surgery. To the best of our knowledge, there is no study to evaluate the threshold value of Pv-aCO2/Ca-vO2 for predicting postoperative adverse outcomes in cardiac surgery. Therefore, we used the threshold value of 1.0 mmHg·dL/mL for the calculation of sample size, because, generally, VCO2/VO2 > 1.0 suggests the presence of tissue hypoperfusion [14]. In a preliminary study (the data were personal and unpublished), Pv-aCO2/Ca-vO2 was higher than 1.0 mmHg·dL/mL in 3 of 15 patients evaluated (20.0%). Postoperative MOMM occurred in 33.3% of patients with Pv-aCO2/Ca-vO2 above the threshold of 1.0, and in 16.7% of patients below this threshold. A power analysis using this preliminary data indicated that a sample size of 100 subjects would be sufficient to detect a difference of 16.6% in the incidence of MOMM events between patients with higher or lower values of Pv-aCO2/Ca-vO2, with a power of 0.80 and alpha of 0.05. Considering a drop-out rate of 10%, we therefore enrolled 110 patients.
Receiver operating characteristic (ROC) curves were constructed to evaluate the prognostic reliability of Pv-aCO2, Pcv-aCO2, Pv-aCO2/Ca-vO2., and Pcv-aCO2/Ca-cvO2 for predicting the incidence of postoperative MOMM, the results of which were expressed as the area under the curve (AUC) with 95% confidence intervals (95% CIs), as well as sensitivity and specificity for the optimal threshold. The method shown by Hanley and McNeil [18] was used to compare the AUCs in the ROC analysis. Multivariate logistic regression analyses were performed to investigate the independent effects of perioperative factors on the risk of developing postoperative MOMM and prolonged ICU length of stay, and the results were expressed as odds ratio (OR) with 95% CI. In the multivariate analysis, we included the perioperative factors with the p value less than 0.10 in the univariate analyses comparing patients “with and without postoperative MOMM” and “with and without ICU length of stay ≥ 3”. Student’s t-test and the Mann–Whitney U-test were used to compare perioperative continuous variables between patients with and without MOMM events. Categorical variables were compared with the χ2 test or Fisher’s exact test. Paired t-test and Wilcoxon’s signed-rank test were used to compare metabolic status, body temperature, hemodynamic data, as well as CO2 and O2 derived parameters, between preoperative and postoperative periods. All results were expressed as mean ± standard deviation unless otherwise indicated. For all analyses, p-values < 0.05 were considered to indicate statistical significance. Statistical analyses were performed using StatFlex version 6.0 (Artech. Co., Ltd., Osaka, Japan).
3 Results
Of the 110 patients enrolled in this study, no patient was excluded. MOMM events occurred in 25 patients (22.7%) postoperatively. Tables 1 and 2 show the Baseline characteristics in patients with and without MOMM events and ICU length of stay ≥ 3 days. Patients with postoperative MOMM had higher New York Heart Association (NYHA) classifications and EuroScore II than those without MOMM (p = 0.016). Perioperative data in patients with and without MOMM and ICU length of stay ≥ 3 days are shown in Tables 7 and 3. Patients with postoperative MOMM had significantly longer operation time, and larger transfusion volume and blood loss than those without MOMM (p < 0.05). SvO2 and ScvO2 were not significantly different between the two groups. Although the preoperative lactate concentration was not significantly different, the postoperative value in the patients with MOMM was significantly higher than those without MOMM (p = 0.030). Patients with postoperative MOMM had significantly lower postoperative mean arterial pressure (p = 0.012). Cardiac index and stroke volume were not significantly different between the patients with and without MOMM. However, the patients with MOMM were more likely to receive the inotropic support using epinephrine, norepinephrine and milrinone compared to those without MOMM. In terms of CO2- and O2-derived parameters, postoperative Pv-aCO2, Pcv-aCO2, Pv-aCO2/Ca-vO2, and Pcv-aCO2/Ca-cvO2 values were significantly higher in patients with MOMM than in those without MOMM, while no significant difference was noted regarding preoperative values.
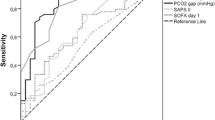
Figure 1a, b reveal ROC curve analyses for the prognostic ability for MOMM events of Pv-aCO2- and Pcv-aCO2-related variables, respectively. Both postoperative Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 were significant predictors of the incidence of postoperative MOMM (AUC: 0.780 [95% CI 0.663–0.897] and AUC: 0.688 [95% CI 0.543–0.834]) (Table 4). The AUCs of postoperative Pv-aCO2 and Pv-aCO2/Ca-vO2 were significantly higher than the preoperative values, respectively (Pv-aCO2: p = 0.003, Pv-aCO2/Ca-vO2: p = 0.009) (Fig. 1a). Postoperative Pcv-aCO2 and Pcv-aCO2/Ca-cvO2 had a higher (but not significant) AUC than preoperative values, respectively (Pcv-aCO2: p = 0.084, Pcv-aCO2/Ca-cvO2: p = 0.079) (Fig. 1b). Additionally, postoperative Pv-aCO2 was the best predictor for the incidence of postoperative MOMM (AUC: 0.804 [95% CI 0.688–0.921], cut-off value: 5.1 mmHg, sensitivity: 76.0%, specificity: 74.1%).
Receiver operating characteristic (ROC) curve analyses for evaluating the prognostic reliability of the mixed and central venous–arterial CO2 tension difference to arterial–venous O2 content difference ratio (Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2, respectively) and the mixed and central venous-to-arterial carbon dioxide gradient (Pv-aCO2 and Pcv-aCO2, respectively) for predicting the incidence of postoperative major organ morbidity and mortality. a Mixed venous-related variables (Pv-aCO2 and Pv-aCO2/Ca-vO2), b central venous-related variables (Pcv-aCO2 and Pcv-aCO2/Ca-cvO2). Filled circle: preoperative Pv-aCO2 and Pcv-aCO2, open circle: postoperative Pv-aCO2 and Pcv-aCO2, filled box: preoperative Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2, open box: postoperative Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2
Tables 5 and 6 reveal the results of multivariate analysis for postoperative MOMM and prolonged ICU length of stay (more than or equal to 3 days). Postoperative Pv-aCO2 was an independent predictor of both MOMM (OR: 1.42, 95% CI 1.01–2.00, p = 0.046) (Table 5) and prolonged ICU length of stay (OR: 1.45, 95% CI 1.05–2.01, p = 0.024) (Table 6), whereas the postoperative lactate concentration was not.
4 Discussion
To the best of our knowledge, this is the first study to evaluate the prognostic ability of Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 for postoperative adverse outcomes in cardiac surgery. Patients with MOMM had a larger increase in postoperative Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 values. Additionally, ROC analysis revealed that postoperative Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 had adequate power to predict postoperative severe complications. Postoperative Pv-aCO2 demonstrated better predictive ability than Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2. Although the sensitivity and specificity derived from the ROC analysis were not so good, multivariate analyses revealed that postoperative Pv-aCO2 was the best predictor of postoperative outcomes.
The values of Pv-aCO2/Ca-vCa-vO2 and Pcv-aCO2/Ca-cvO2 can be used as reliable markers of global anaerobic metabolism, which is based on the ratio of CO2 production to VO2 in the whole body. Pv-aCO2 and Pcv-aCO2 are accepted complementary markers for identifying patients with inadequate DO2 [9, 10]. Both of these variables have demonstrated prognostic ability in patients with shock status [11, 19]. Furthermore, by also considering O2 changes, given by the arterial–venous O2 gradient, the accuracy and precision of this concept can be increased. The ratio of VCO2/VO2 is a reliable index for assessing global anaerobic metabolism. Recent studies have shown that Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 could be a surrogate for VCO2/VO2 and may be useful for evaluating global anaerobic metabolism [13, 16, 20,21,22]. Furthermore, some previous studies have suggested that these ratios respond to the changes in global tissue oxygenation faster than blood lactate concentration [15, 16]. Lactate concentration may not be able to track the changes in tissue perfusion rapidly, which could be the reason why the blood lactate concentration was not an independent predictor of postoperative outcomes in the present study. In our study, the ROC analysis showed that postoperative Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 can predict poor outcomes after cardiac surgery, with AUCs of 0.780 and 0.688, respectively. The cut-off values of Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 (1.3 and 1.8 mmHg·dL/mL, respectively) for predicting postoperative MOMM in our study was highly similar to that in previous studies. However, postoperative Pv-aCO2 demonstrated better predictive ability than Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2. Additionally, multivariate analysis revealed that postoperative Pv-aCO2, but not Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2, was an independent predictor of both MOMM and prolonged ICU length of stay.
The possible reasons for the superior ability of Pv-aCO2 for predicting postoperative outcomes as compared to Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2 may be as follows. First, Pv-aCO2/Ca-vO2 is not equivalent to Cv-aCO2/Ca-vO2. Calculation of Cv-aCO2 is complicated, whereas Pv-aCO2 is easily calculated. The relationship between Cv-aCO2 and Pv-aCO2 is almost linear under normal conditions, but becomes non-linear when Pv-aCO2 is abnormal [23]. Haldane effect facilitates the binding of CO2 to hemoglobin at lower O2 saturation, and the relationship between CO2 content and CO2 tension is affected by O2 saturation and acidosis [24]. In anaerobic conditions, tissue acidosis and hypoxia frequently occur, and the discrepancy between Cv-aCO2 and Pv-aCO2 increases due to the Haldane effect. Similarly, the value of Pv-aCO2/Ca-vO2 should be equal to Cv-aCO2/Ca-vO2 under normal conditions, but is greatly affected by the Haldane effect, and thus, its interchangeability to Cv-aCO2/Ca-vO2 may be doubtful under anaerobic conditions [25]. Although the physiology of Cv-aCO2/Ca-vO2 is robust, Pv-aCO2/Ca-vO2 may not detect anaerobic conditions in some cases. Ospina-Tascon et al. found that Cv-aCO2/Ca-vO2 was significantly associated with mortality in septic shock patients, whereas Pv-aCO2/Ca-vO2 was not [15]. Second, calculating VCO2 according to Fick’s principle is valid under stable conditions. However, the recovery of blood-flow after tissue ischemia can lead to overestimation of VCO2 and increases in VCO2/VO2. In such cases, the value of Pv-aCO2/Ca-vO2 may not be valid for predicting patients’ outcomes. Third, pseudo-normalization of Pv-aCO2/Ca-vO2 could occur under conditions of high oxygen consumption. A CO increase can cause a decrease in Ca-vO2 if VO2 is maintained constant under anaerobic conditions. Therefore, the pseudo-normalization of Pv-aCO2/Ca-vO2 may not occur under high CO conditions combined with reduced or constant VO2 [26]. However, pseudo-normalization of Pv-aCO2/Ca-vO2 could occur under conditions of high VO2 and high CO. The results revealed that the values of continuous CO and VO2 (both Ca-vO2 × CO and Ca-cvO2 × CO) were not high in the current study (Tables 7, 3). Therefore, the third possible reason may not be applicable in the current study.
In the current study, mixed venous-related variables (Pv-aCO2 and Pv-aCO2/Ca-vO2) had better predictive ability than central venous-related variables (Pcv-aCO2 and Pcv-aCO2/Ca-cvO2). The equivalence between mixed venous-related variables and central venous-related variables has not been proven, although relatively good agreement between Pv-aCO2 and Pcv-aCO2 has been demonstrated [27]. However, as indicated in a previous study, the CO distribution changes in patients with hemodynamic collapse [28]. During hemodynamic instability, blood-flow to the abdominal organs is reduced while that to vital organs, including brain and heart, is maintained [28]. Therefore, the discrepancy between Pv-aCO2 and Pcv-aCO2 may increase during periods of hemodynamic instability. In previous studies [29, 30], it has been shown that the venous–arterial difference in CO2 tension could not predict postoperative complications. However, in these studies, venous blood gas measurement was performed from the central venous catheter. As shown in the current study, mixed venous-related variables should be used to predict postoperative outcomes in cardiac surgery patients.
The current study had some methodological limitations. First, blood gas measurement was not performed during the postoperative period. The Pv-aCO2 value in the postoperative period may have more reliability for detecting postoperative complications than that during surgery. Second, patients who underwent circulatory arrest and cerebral perfusion were enrolled in the current study. This may have affected the results of blood gas measurement, and some postoperative complications, including infectious disease and stroke. Third, postoperative body temperature was significantly lower than preoperative value, which can affect blood/gas solubility coefficient. However, postoperative temperature was in almost normal range both for the patients with and without MOMM, and did not significantly differ between these two groups. Therefore, the difference between pre- and postoperative temperature did not affect the results. Even with these limitations, the results of the present study suggest that clinicians may be able to predict postoperative complications in cardiac surgery patients by measuring Pv-aCO2 values at the end of surgery.
5 Conclusions
In the current study, we demonstrated that Pv-aCO2 at the end of surgery had superior ability for predicting postoperative complications than Pv-aCO2/Ca-vO2 and Pcv-aCO2/Ca-cvO2. Pv-aCO2 at the end of surgery is an independent risk factor for postoperative complications, such as prolonged ICU length of stay and MOMM. Thus, it would be possible that the incidence and severity of postoperative complications can be predicted by measuring the value of Pv-aCO2 at the end of surgery.
References
Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg. 2003;238:170–7.
Hallqvist L, Martensson J, Granath F, Sahlen A, Bell M. Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: an observational study. Eur J Anaesthesiol. 2016;33:450–6.
Shoemaker WC, Appel PL, Kram HB. Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest. 1992;102:208–15.
Aya HD, Cecconi M, Hamilton M, Rhodes A. Goal-directed therapy in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2013;110:510–7.
Holm J, Hakanson E, Vanky F, Svedjeholm R. Mixed venous oxygen saturation predicts short- and long-term outcome after coronary artery bypass grafting surgery: a retrospective cohort analysis. Br J Anaesth. 2011;107:344–50.
Reinhart K, Kuhn HJ, Hartog C, Bredle DL. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med. 2004;30:1572–8.
Perz S, Uhlig T, Kohl M, Bredle DL, Reinhart K, Bauer M, Kortgen A. Low and “supranormal” central venous oxygen saturation and markers of tissue hypoxia in cardiac surgery patients: a prospective observational study. Intensive Care Med. 2011;37:52–9.
Puskarich MA, Trzeciak S, Shapiro NI, Heffner AC, Kline JA, Jones AE. Outcomes of patients undergoing early sepsis resuscitation for cryptic shock compared with overt shock. Resuscitation. 2011;82:1289–93.
van Beest PA, Lont MC, Holman ND, Loef B, Kuiper MA, Boerma EC. Central venous-arterial pCO(2) difference as a tool in resuscitation of septic patients. Intensive Care Med. 2013;39:1034–9.
Vallet B, Pinsky MR, Cecconi M. Resuscitation of patients with septic shock: please “mind the gap”! Intensive Care Med. 2013;39:1653–5.
Futier E, Robin E, Jabaudon M, Guerin R, Petit A, Bazin JE, Constantin JM, Vallet B. Central venous O(2) saturation and venous-to-arterial CO(2) difference as complementary tools for goal-directed therapy during high-risk surgery. Crit Care. 2010;14:R193.
Jakob SM, Kosonen P, Ruokonen E, Parviainen I, Takala J. The Haldane effect—an alternative explanation for increasing gastric mucosal PCO2 gradients? Br J Anaesth. 1999;83:740–6.
Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, Richard C, Teboul JL. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 2002;28:272–7.
Ospina-Tascon GA, Hernandez G, Cecconi M. Understanding the venous-arterial CO2 to arterial-venous O2 content difference ratio. Intensive Care Med. 2016;42:1801–4.
Ospina-Tascon GA, Umana M, Bermudez W, Bautista-Rincon DF, Hernandez G, Bruhn A, Granados M, Salazar B, Arango-Davila C, De Backer D. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med. 2015;41:796–805.
Mallat J, Lemyze M, Meddour M, Pepy F, Gasan G, Barrailler S, Durville E, Temime J, Vangrunderbeeck N, Tronchon L, Vallet B, Thevenin D. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care. 2016;6:10.
Shroyer AL, Coombs LP, Peterson ED, Eiken MC, DeLong ER, Chen A, Ferguson TB Jr, Grover FL, Edwards FH. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. 2003;75:1856–64. discussion 1864 – 1855.
Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43.
Ospina-Tascon GA, Bautista-Rincon DF, Umana M, Tafur JD, Gutierrez A, Garcia AF, Bermudez W, Granados M, Arango-Davila C, Hernandez G. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care. 2013;17:R294.
Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, Persichini R, Anguel N, Richard C, Teboul JL. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41:1412–20.
He HW, Liu DW, Long Y, Wang XT. High central venous-to-arterial CO2 difference/arterial-central venous O2 difference ratio is associated with poor lactate clearance in septic patients after resuscitation. J Crit Care. 2016;31:76–81.
Mesquida J, Saludes P, Gruartmoner G, Espinal C, Torrents E, Baigorri F, Artigas A. Central venous-to-arterial carbon dioxide difference combined with arterial-to-venous oxygen content difference is associated with lactate evolution in the hemodynamic resuscitation process in early septic shock. Crit Care. 2015;19:126.
Ospina-Tascon GA, Umana M, Bermudez WF, Bautista-Rincon DF, Valencia JD, Madrinan HJ, Hernandez G, Bruhn A, Arango-Davila C, De Backer D. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med. 2016;42:211–21.
Teboul JL, Scheeren T. Understanding the Haldane effect. Intensive Care Med. 2017;43:91–3.
Ospina-Tascon GA, Hernandez G, Cecconi M. Understanding the venous-arterial CO to arterial-venous O content difference ratio. Intensive Care Med. 2016;42:1801–4
He H, Liu D. The pseudo-normalization of the ratio index of the venous-to-arterial CO2 tension difference to the arterial-central venous O2 difference in hypoxemia combined with a high oxygen consumption condition. J Crit Care. 2017;40:305–6.
Cuschieri J, Rivers EP, Donnino MW, Katilius M, Jacobsen G, Nguyen HB, Pamukov N, Horst HM. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Med. 2005;31:818–22.
Suehiro K, Tanaka K, Matsuura T, Funao T, Yamada T, Mori T, Nishikawa K. Discrepancy between superior vena cava oxygen saturation and mixed venous oxygen saturation can predict postoperative complications in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2014;28:528–33.
Morel J, Grand N, Axiotis G, Bouchet JB, Faure M, Auboyer C, Vola M, Molliex S. High veno-arterial carbon dioxide gradient is not predictive of worst outcome after an elective cardiac surgery: a retrospective cohort study. J Clin Monit Comput. 2016;30:783–9.
Guinot PG, Badoux L, Bernard E, Abou-Arab O, Lorne E, Dupont H. Central venous-to-arterial carbon dioxide partial pressure difference in patients undergoing cardiac surgery is not related to postoperative outcomes. J Cardiothorac Vasc Anesth. 2017;31:1190–6.
Funding
Only departmental funds were used for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mukai, A., Suehiro, K., Kimura, A. et al. Comparison of the venous–arterial CO2 to arterial–venous O2 content difference ratio with the venous–arterial CO2 gradient for the predictability of adverse outcomes after cardiac surgery. J Clin Monit Comput 34, 41–53 (2020). https://doi.org/10.1007/s10877-019-00286-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00286-z