Abstract
Objective: The mixed venous-arterial (v-a) pCO2 difference has been shown to be inversely correlated with the cardiac index (CI). A central venous pCO2, which is easier to obtain, may provide similar information. The purpose of this study was to examine the correlation between the central venous-arterial pCO2 difference and CI. Design: Prospective, cohort study. Setting: Intensive care unit of an urban tertiary care hospital. Patients and participants: Eighty-three consecutive intensive care unit patients. Measurements: Simultaneous blood gases from the arterial, pulmonary artery (PA), and central venous (CV) catheters were obtained. At the same time point, cardiac indices were measured by the thermodilution technique (an average of three measurements). The cardiac indices obtained by the venous-arterial differences were compared with those determined by thermodilution. Results: The correlation (R2) between the mixed venous-arterial pCO2 difference and cardiac index was 0.903 ( p <0.0001), and the correlation between the central venous-arterial pCO2 difference and cardiac index was 0.892 ( p <0.0001). The regression equations for these relationships were natural log (CI)=1.837−0.159 (v-a) CO2 for the PA and natural log (CI)=1.787−0.151 (v-a) CO2 for the CV ( p <0.0001 for both). The root-mean-squared error for the PA and CV regression equations were 0.095 and 0.101, respectively. Conclusion: Venous-arterial pCO2 differences obtained from both the PA and CV circulations inversely correlate with the cardiac index. Substitution of a central for a mixed venous-arterial pCO2 difference provides an accurate alternative method for calculation of cardiac output.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac output is a fundamental measurement in the hemodynamic assessment of critically ill patients. The thermodilution technique, using a flow-directed pulmonary artery catheter (PAC), has traditionally been the most frequently utilized method to measure flow [1]. However, this technology is not readily available in all clinical settings such as the emergency department, the intermediate care unit, or the general practice unit. A number of alternative techniques have been proposed to measure cardiac output with varying degrees of accuracy, accessibility, and ease of use [1]. One such method consists of calculating the cardiac output based upon the measurement of the mixed venous-arterial partial pressure of carbon dioxide (pCO2) difference [2].
The mixed venous-arterial pCO2 difference is determined by subtracting the peripheral arterial pCO2 from the mixed venous pCO2 (pulmonary artery, PA). Obtaining a mixed venous-arterial (v-a) pCO2 difference requires access to the pulmonary arterial circulation and thus has limitations similar to the PAC. Furthermore, if a PAC is in place, cardiac output can be obtained by thermodilution, and the clinical utility of an alternative method of measurement is diminished. In contrast, central venous (CV) access is more frequently and easily obtained, yet no direct measure of cardiac output can be calculated. The substitution of a central venous pCO2 for a mixed venous pCO2 may yield a similar inverse relationship to cardiac output [3]. If this relationship could be obtained by substitution of central venous pCO2, cardiac output could be calculated in a more feasible and rapid fashion. The purpose of this study was to examine the correlation between the central venous-arterial pCO2 difference and the cardiac index (CI).
Materials and methods
A prospective cohort study of 83 consecutive critically ill patients was performed in a large inner-city tertiary care intensive care unit. Patients were eligible for enrollment if they had pulmonary and radial or femoral artery catheterization. The study was approved by the Henry Ford Hospital Review Board for Human Research.
Arterial, central venous, and mixed venous blood gases (Radiometer, Waltham, MA, USA) were obtained from all 83 patients. Simultaneously, thermodilution cardiac output measurements were determined from the pulmonary artery catheter (Baxter-Edwards, Irvine, CA, USA). Thermodilution cardiac output was measured at end expiration by injecting 10 ml of isotonic saline at 24–26.5°C through the proximal port of the catheter. Three cardiac outputs, which were within 10% of each other, were obtained and averaged. The venous-arterial pCO2 differences were calculated from the pCO2 from both the central venous system and the pulmonary artery. Cardiac index was calculated by dividing the cardiac output by the body surface area.
Statistical analysis
The agreement between the mixed and central venous pCO2 differences was graphically described using a Bland/Altman plot. An intra-class correlation coefficient was obtained to measure the strength of the agreement between the mixed and central readings.
Linear regression equations were derived to depict the relationships between the natural log of the cardiac index (CI) and the venous-arterial pCO2 differences from both the central and mixed venous circulations. Pearson correlation coefficients and root-mean-squared errors were obtained to evaluate the strength of the relationships.
Results
Samples from 83 individual critically ill patients were analyzed in the study group. All patients enrolled were mechanically ventilated. The mean age of the patients was 62.6±7 years, with a range of 36–98 years. The mean temperature was 37.7±1.0°C with a range of 35.5–41.1°C. The spectrum of disease states is represented in Table 1. The cardiac indices ranged from 1.6 l/min/m2 to 9.2 l/min/m2,with a mean cardiac index of 3.6±1.2 l/min/m2. The mean venous-arterial pCO2 gradients were 3.8±1.8 mmHg and 3.7±1.3 mmHg in the CV and PA, respectively ( p ≥0.05). Using a cardiac index of 2.5 l/min/m2 to distinguish between a normal and low flow state, 12 of 83 patients had a cardiac index of less than 2.6 l/min/m2. The mean venous-arterial pCO2 differences for those in a low flow state were 6.9±0.9 mmHg and 6.8±0.7 mmHg for the CV and PA gases, respectively ( p =0.1). For patients with a normal flow state, the venous-arterial pCO2 differences were 3.1±1.4 mmHg and 3.3±1.4 mmHg for the CV and PA circulation, respectively ( p <0.05).
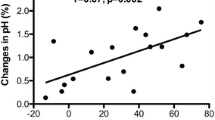
To secondarily evaluate the agreement between the mixed and central pCO2 difference data, a Bland/Altman plot of each patient’s pCO2 difference delta (mixed minus central) versus the corresponding mixed and central venous pCO2 average is given in Fig. 1. Regarding the pCO2 difference delta (mixed minus central), the mean is 0.14; the standard deviation is 0.39; the minimum is −0.8, and the maximum is 1.0. The mean delta of 0.14 is significantly different from 0 (paired t -test p value<0.05), suggesting that the mixed pCO2 difference readings tend to be very slightly higher than central pCO2 differences. However, the data points in the Bland/Altman plot appear to be well scattered, and the limits-of-agreement band (mean±SD) of −0.64 to 0.92 appears to be relatively narrow, suggesting good overall agreement between the mixed and central pCO2 differences. Furthermore, the intra-class correlation coefficient between the mixed and central pCO2 differences is 0.978, indicating extremely good agreement.
The relationship between the cardiac index and the venous-arterial pCO2 difference was evaluated for both circulations using linear regression analysis. Linear regression models with the best fit were obtained using the natural log of the cardiac index. The resulting regression lines and 95% individual prediction interval bands are given in Figs. 2 and 3. The regression equations for these relationships are natural log (CI)=1.837−0.159 (v-a) CO2 for the PA and natural log (CI)=1.787−0.151 (v-a) CO2 for the CV ( p <0.0001 for both). The resulting (R2) (correlation coefficient squared) for the model involving the mixed venous-arterial pCO2 difference and cardiac index is 0.903 ( p <0.0001), which is very similar to the resulting R2 of 0.892 ( p <0.0001) for the model involving the central venous-arterial pCO2 difference data. The root-mean-squared error for the PA and CV regression equations are also very similar (0.095 and 0.101, respectively).
Discussion
Circulatory failure secondary to hypovolemia, sepsis, and primary cardiac dysfunction is associated with increased tissue (mixed venous) hypercapnia [4]. Mixed venous hypercapnia results when cellular oxidation overwhelms existing buffering systems. While arterial pCO2 is variable and dependent on pulmonary gas exchange, mixed venous pCO2 is dependent on circulatory flow. Thus, an increased difference reflects decreased flow [2,5–12]. The Fick principle states that the mass flux of a diffusing substance is proportional to the concentration gradient in that direction. Applying the Fick principle to cardiac output, the cardiac output is calculated from the ratio between alveolar oxygen uptake and arteriovenous oxygen content difference (cardiac output=VO2/CaO2−CvO2), or from the ratio of carbon dioxide production and arteriovenous carbon dioxide content difference (cardiac output=VCO2/(CaCO2−CvCO2). By substituting CO2 pressure for content and rearranging the equation, the inverse relationship between cardiac output and the mixed venous-arterial pCO2 difference can be seen [13]. An increase in the venous-arterial pCO2 difference occurs in states of decreased flow, regardless of the reason for the circulatory failure, and this has been demonstrated in clinical trials to be inversely related to cardiac output [2, 5, 7, 8, 10,14].
Clinical assessment of cardiac output is largely limited to the use of a pulmonary artery catheter. Unfortunately, the PAC cannot be utilized when the technology is not available (i.e., emergency department, intermediate care unit, or general practice unit) or the risks for placement are unjustifiably high [1, 15,16]. A reliable substitution, such as a central venous-arterial pCO2 difference, is practical and can provide immediate information until a more definitive procedure can be performed. The increased implementation of “early goal-directed therapy” provides one example where central and arterial access would be easily accessible in the absence of other technologies such as a PAC [17].
The current study illustrates that the venous-arterial pCO2 difference is significantly correlated with the cardiac index when derived from both the pulmonary arterial and central venous circulations across different flow states. The linear regression curves and equations demonstrate the close relation between the venous-arterial pCO2 difference and cardiac index for both the central and mixed circulations (Figs. 2 and 3). The Bland/Altman plot revealed a very strong agreement between the mixed venous and central venous pCO2 differences (Fig. 1). The mean delta between the mixed and central venous differences was 0.14 (very slightly higher for the mixed circulation). This difference of 0.14 is very small and does not appear to be clinically significant. Finally, the large intra-class correlation coefficient (0.978) indicates very good agreement between circulations. Thus, the substitution of a central venous pCO2 is valid and can be utilized in a clinical setting. In addition to the potential usage of venous-arterial pCO2 differences to assess cardiac output, the combination of venous-arterial pCO2 differences with oxygen-content differences may provide assessment of global tissue hypoxia. Mekontso-Dessap et al. have demonstrated that the ratio of the mixed venous-arterial pCO2 difference to the arteriovenous oxygen content difference is correlated with lactic acid elevation and thus may reflect global tissue hypoxia [18]. As further research into this potential utilization is pursued, perhaps the central venous circulation could again be substituted for the mixed venous circulation [13].
Central venous oxygen derived parameters have been shown to be correlated with those traditionally determined in the pulmonary circulation [19]. In the current trial, central venous pCO2 has been shown to be of equal utility as the pulmonary-derived parameters for venous-arterial pCO2 differences. This finding offers potential clinical utilization (i.e., cardiac output determination) and provides a base for future research.
Limitations
A potential confounding variable of venous-arterial differences is hypothermia, which may decrease cellular respiration and therefore CO2 generation. A larger sample size with control of temperature may help delineate if this has any significant clinical implications, especially at very low temperatures.
Conclusion
The venous-arterial pCO2 difference of the mixed venous and central venous circulation were both inversely correlated with cardiac index. In the absence of a pulmonary artery catheter, a central venous-arterial pCO2 difference could potentially be used to determine cardiac output. This substitution provides an easily accessible and feasible alternative method to determine cardiac output in critically ill patients.
References
Pittman J, Gupta KJ (2003) Cardiac output monitoring: Will new technologies replace the pulmonary artery catheter. Yearbook of intensive care and emergency medicine, pp 481–498
Durkin R, Gergits MA, Reed JF 3rd, Fitzgibbons J (1993) The relationship between the arteriovenous carbon dioxide gradient and cardiac index. J Crit Care 8:217–21
Cuschieri J, Hays G, Rivers EP (1998) Arterial-venous carbon dioxide gradients as an indicator of cardiac index: A comparison between the mixed and central circulation. Crit Care Med 26:A56
Johnson BA, Weil MH (1991) Redefining ischemia due to circulatory failure as dual defects of oxygen deficits and of carbon dioxide excesses. Crit Care Med 19:1432–438
Bakker J, Vincent JL, Gris P, Leon M, Coffernils M, Kahn RJ (1992) Veno-arterial carbon dioxide gradient in human septic shock. Chest 101:509–515
Cavaliere F, Martinelli L, Guarneri S, Varano C, Rossi M, Schiavello R (1996) Arterial-venous PCO2 gradient in early postoperative hours following myocardial revascularization. J Cardiovasc Surg (Torino) 37:499–503
Ducey JP, Lamiell JM, Gueller GE (1992) Arterial-venous carbon dioxide tension difference during severe hemorrhage and resuscitation. Crit Care Med 20:518–22
Halmagyi DF, Kennedy M, Varga D (1970) Hidden hypercapnia in hemorrhagic hypotension. Anesthesiology 33: 594–601
Jacob SV, Hornby L, Lands LC (1997) Estimation of mixed venous PCO2 for determination of cardiac output in children. Chest 111:474–480
Sato S, Okubo N, Satsumae T, Kumagai M, Yamamoto S, Nakayama H, Taguchi N (1994) Arteriovenous differences in PCO2 and cardiac output during CPR in the dog. Resuscitation 27:255–259
Sato Y, Weil MH, Tang W (1998) Tissue hypercarbic acidosis as a marker of acute circulatory failure (shock). Chest 114:263–274
Weil MH, Rackow EC, Trevino R, Grundler W, Falk JL, Griffel MI (1986) Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med 315:153–156
Teboul JL , Monnet X (2003) Clinical use of venoarterial pCO2 difference in septic shock. Yearbook of intensive care and emergency medicine, pp 574–582
Rackow EC, Astiz ME, Mecher CE, Weil MH (1994) Increased venous-arterial carbon dioxide tension difference during severe sepsis in rats. Crit Care Med 22:121–125
Sise MJ, Hollingsworth P, Brimm JE, Peters RM, Virgilio RW, Shackford SR (1981) Complications of the flow-directed pulmonary artery catheter: A prospective analysis in 219 patients. Crit Care Med 9:315–318
Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M (2003) A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 348: 5–14
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, Richard C, Teboul JL (2002) Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med 28:272–277
Reinhart K, Rudolph T, Bredle DL, Hannemann L, Cain SM (1989) Comparison of central-venous to mixed-venous oxygen saturation during changes in oxygen supply/demand. Chest 95:1216–1221
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cuschieri, J., Rivers, E.P., Donnino, M.W. et al. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Med 31, 818–822 (2005). https://doi.org/10.1007/s00134-005-2602-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2602-8