Abstract
Spectroscopic investigation supported by molecular modeling methods has been used to describe the inclusion complex of β-cyclodextrin (β-CD) with 1-Methyl-1-({2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-4-yl}methyl) piperidinium chloride (1MPTMPC) in solution and in solid state. The formation of inclusion complex between the β-CD and the 1MPTMPC has been investigated both in solution and in the solid state. Solution-state complexation between the 1MPTMPC and β-CD was established using 1H NMR spectroscopy and isothermal titration calorimetry (ITC). From the 1H NMR spectroscopic studies, 1:1 complex stoichiometry was deduced with an association constant (K) of 925 M−1. Using an independent binding model, the ITC technique provides a K value of the same order with the one determined by NMR and the thermodynamic parameters ΔH, ΔS and ΔG which reveals driving forces involved during complex formation. The formation of the solid inclusion compound was confirmed by X-ray powder diffraction and differential scanning calorimetry. The most probable conformation of the inclusion complex obtained through a molecular docking investigation corroborates well to ROESY experiment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anticholinergic medicines are a heterogeneous group of substance both from the structural and the therapeutic indications point of view. Pharmacologically, most of these compounds act as nonselective muscarinic receptor antagonists. The wide and varied distribution of these receptors throughout the body means that a nonselective antagonism of all these receptors leads to a diverse range of simultaneous effects. As a result, anticholinergic medicines have a vast array of therapeutic applications as they can be used in the treatment of many disorders: asthma, gastro-duodenal ulcers, renal/biliary colics, urinary incontinence [1, 2].
In order to limit these side-effects, selective acting compounds have been devised. They act selectively just on a subtype of muscarinic receptor, for example solifenacine and darifenacine are selective towards the M3 receptors from the urinary bladder [2].
Moreover, side-effects can be reduced by locally administering the substances or by using compounds with a specific tropism, that are capable of achieving high concentrations in specific target organs like the gastro-intestinal tract, urinary bladder.
Quaternary ammonium compounds are considered among the substances which can influence the contractility of smooth muscles from the digestive tract, acting via cholinergic mechanisms.
In this context, new molecules that contain quaternary ammonium compounds with improved pharmacokinetic and pharmaco-toxicological profile were synthesized [3,4,5]. Therefore, encapsulation process of these new compounds synthesized into cyclodextrin could offer the possibility of controlling drug delivery in biological systems, extending the antispasmodic effect and reducing its toxicity and also the increase of the solubility.
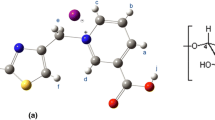
This study presents an account of β-CD complexation with 1MPTMPC (Fig. 1), illustrating how complementary analytical techniques enhance insight into the process of host–guest complexation.
Experimental and theoretical methods
Materials
β-Cyclodextrin (water content 8 mol/mol) was purchased from Sigma Chemie GmbH. Germany and 1MPTMPC compound was synthesized by the Iuliu Haţieganu University, Pharmacy Faculty Cluj-Napoca [5]. Both chemicals were used without any further purification. Deuterium oxide (99.7% D) was obtained from from Heavy Water Plant Romag-Prod, Romania.
Sample preparation
The solid-state inclusion compound was prepared in 1:1 molar ratio of the 1MPTMPC and β-CD by co-precipitation. An amount of 3 × 10−5 mol 1MPTMPC was dissolved in 2 mL of saturated aqueous solution of 3 × 10−5 mol β-CD. The mixture was stirred for 24 h at room temperature, succeeded by evaporation of solvent and drying into an oven at 27 °C.
Isothermal titration calorimetry (ITC)
ITC experiments were performed using a Nano ITC2G Calorimeter (TA Instruments, New Castle, Delaware, USA). Before each titration, all solutions were degassed prior to loading into the ITC cell. Titration were performed at 25 °C by injecting consecutive aliquots (10 µL) of 12 Mm β-CD aqueous solution into the ITC cell containing 0.6 mM 1MPTMPC, keeping the stirring speed fixed at 350 rpm. Intervals between injections were set to be 600 s in order to allow the heat signal after each injection to return to the baseline. During β-CD addition, the molecules interact and the observed released heat is directly proportional to the amount of binding compound added with the solution aliquot. As the population of interacting molecules in the cell becomes more saturated with β-CD, the heat signal diminishes until only the background heat of dilution is observed.
Dilution heat of the host was measured under identical experimental conditions by injecting the β-CD into water. In general, heats of dilution were very small compared to the heats of complexation. The blank effects were subtracted in order to correct for dilution, mixing and injection effects.
The data were fitted with independent binding model in the Nano Analyze software. The binding constant (K), the binding stoichiometry (n), change in enthalpy (ΔH), change in entropy (ΔS) were thus obtained.
1H NMR
All 1H NMR measurements were carried out on a Bruker AVANCE III spectrometer operating at 500.13 MHz for protons and equipped with a broad-band observation probe. The NMR spectra were recorded in D2O solutions at 298 ± 0.1 K and all chemical shifts were measured relative to TMS salt (3-(Trimethylsilyl)-1-propanesulfonic acid sodium salt).
In order to study the stoichiometry between 1MPTMPC and β-CD in solution by NMR spectroscopy, two stack solutions in D2O, both having 1 mM were prepared. Based on these two equimolar solutions, a series of eleven samples (i = 1–11) containing both the 1MPTMPC and β-CD molecules were prepared. This was accomplished by mixing the two solutions to constant volume at varying proportions, so that a complete range (0 < r < 1) of the ratio r = [X]/([H]+[G]) was sampled. X = H or G and [H] and [G] are the total concentrations of the host (β-CD) and guest (1MPTMPC), respectively. Thus, the total concentration [H]t + [G]t = 1 mM was kept constant for each solution. In order to determine the association constant we used a second set of samples with [G] = ct = 1 mM and [H] varying between 0 and 14 mM.
For each 1H NMR experiment, between 64 and 256 transients were collected into 65 K data points over a 5000 Hz spectral window, using a 3 s relaxation delay.
The 2D ROESY spectrum was acquired in the phase sensitive mode with residual water suppression, using Bruker standard parameters (pulse program roesyphpr). The spectrum consisted of a matrix of 8192 × 1048 data points covering a spectral width of 4.500 Hz. The spectrum was obtained with a 500 ms spin-lock mixing time, relaxation delay 4.3 s and 32 scans.
X-ray powder diffraction (XRD)
X-ray patterns were collected at room temperature on a Rigaku SmartLab multipurpose difractometer using Cu Kα1 radiation (λ = 1.54056 Å), equipped with a 9 kW rotating anode. The sample was ground to a fine homogeneous powder using an agate pestle and mortar and mounted in a sample holder. The measurements were performed in the 3–40° 2θ range in steps of 0.01° at a rate of 6 steps/s.
Differential scanning calorimetry (DSC)
The DSC thermograms were obtained with a DSC-60 Shimadzu differential scanning calorimeter, using Shimadzu TA-WS60 and TA60 2.1 version system software for data acquisition. The heating of the samples was done with a rate of 10 °C/min in the 20–350 °C temperature range using sealed aluminum cells under nitrogen atmosphere. A crimped cell containing alumina was taken as reference.
Computational methods
Computational molecular investigation aiming to obtain the most probable conformation of the inclusion compound was performed using Autodock [6] and AutoDock Vina [7]. The initial 3-dimensional molecular structure of 1MPTMPC was optimized using M06-2X functional and 6-311++G(d,p) basis set using Gaussian 09 software [8] and no imaginary frequencies were obtained. The β-CD structure was extracted from the crystallographic inclusion complex of β-CD with Mg2+ and Ca2+ salts of meclofenamic acid determined by Caira et al. [9] (CSD entry: WERGUW). AutoDockTools v 1.5.6 [6] was used to prepare the pdbqt files, which gather the atomic coordinates (PDB), partial charges (Q) and atom types (T) in one file. The flexibility of the ligand has been taken into account by setting up four torsion angles around the rotatable bonds.
Results and discussions
Determination of the association constant by ITC
ITC has been developed to determine thermodynamic characterization of non-covalent, equilibrium interactions involving small molecules with macromolecule and seems to be the most sensitive method for measuring the total heat changes during the complexation process.
A typical ITC titration curve corresponding to the binding interaction of β-CD and 1MPTMPC in presented on Fig. 2. During β-CD addition into 1MPTMPC solution, heat release was observed indicating an interaction between these two compounds. The exothermic heat was directly proportional to the amount of added β-CD and as the 1MPTMPC in the cell become saturated with macromolecule, the heat signal diminished progressively (Fig. 2a). Heat flow which was released after the successive injection of 10 µL aliquots of β-CD into the solution of 1MPTMPC were integrated and expressed as a function of the molar ratio between the two reactants (Fig. 2b).
Variation of heat-flow/electrical power as a function of time, titrant: β-CD; titrand: 1MPTMPC. a Heat rate released after injecting β-cyclodextrin into the reacting cell containing 1MPTMPC in solution. The volume per injection was 10 µL and the heat of dilution has been taken off. b The dependence of the heat released per injection on the molar ratio of reaction partners. The solid line is obtained by fitting the experimental data
The value of binding constant obtained from ITC measurement was K = 808 M−1 which correspond to a Gibbs free energy ΔG = − 3.964 Kcal/mol. The ΔG value is negative which suggest that the formation of the inclusion complex in aqueous solution is energetically favorable. The exothermic nature of the inclusion process of 1MPTMPC in β-CD is attributed to negative value of the formation enthalpy (ΔH = − 1.312 Kcal/mol) of this process. This negative enthalpy change arises due to association between small guest molecules and a polar cavity in water [10].
The entropy effect ΔS of the host–guest complex in 1:1 (n = 0.945) stoichiometry which is positive and make evidently larger contribution to the negative change of Gibbs energy that the heat effects do.
The negative sign of the enthalpy change ΔH < 0 combined with the positive sign of the entropy change ΔS > 0 confirm that both hydrophobic and electrostatic interactions contribute to the binding process. The analysis of the thermodynamic data reveals that the driving force of the binding process is a hydrophobic interaction with the release water molecule from the β-CD cavity which has positive contribution to entropy.
1H NMR experiments
Determination of the stoichiometry
NMR is a technique which provides the most evidence for the inclusion of a guest molecule into the hydrophobic CD cavity in solution. Inclusion of 1MPTMPC in β-CD cavity is evidenced by the change in chemical shifts of some of the guest and host protons, in comparison with the chemical shifts of the same protons in the free components. The 1H NMR spectrum of 1MPTMPC with the assignment of the signals is presented in Fig. 3. Partial 1H NMR spectra of pure components and 1MPTMPC:β-CD mixture in a 1:1 molar ratio are presented in Figs. 4 and 5.
Comparing the spectra from Fig. 4, a chemical shifts variations for some aromatic protons of 1MPTMPC can be observed, due to the presence of β-CD. As expected, the H3 and H5 protons located inside the cavity are appreciably upfield shifted (see Fig. 5), evidencing the existence of an interaction between the 1MPTMPC molecule and the interior of the β-CD cavity. This is a clear indication of the formation of the 1MPTMPC: β-CD inclusion complex.
Determination of the stoichiometry of the 1MPTMPC:βCD complex, by the continuous variation method was based on the induced chemical shift variation, Δδ, which is directly related to the concentration of the complex [11, 12]. The 1H NMR spectra were obtained using the first set of samples in which the total concentration was kept constant at 1 mM. The resulting continuous variation plots show a maximum at r = 0.5 and a highly symmetrical shape (Fig. 6) indicating the existence of a complex with a 1:1 stoichiometry.
ROESY experiments
While the 1D NMR provides unambiguous evidence on the formation of a complex, ROESY experiments provide information on the dynamics and the averaged relative inter- and intramolecular proton distances. In the present study, to gain further information on the inclusion complexation mode and additional insights into the dynamic structure, a 2D ROESY 1H NMR spectrum was acquired. Due to the rapid dynamics of the complexation process, the ROESY effects were only qualitatively used and no conclusions on intermolecular distances were extracted. An expansion of the ROESY spectrum of the 1MPTMPC: β-CD complex is reported in Fig. 7.
The 2D NMR spectrum shows several intermolecular cross-peaks between H3 and H5 protons of β-CD and protons of aromatic ring of 1MPTMPC, demonstrating the inclusion of this group in the β-CD cavity. As can be seen from the ROESY spectrum, the Hb, Hb′ protons interact more strongly with β-CD H3 protons and Ha, Ha′ with H5. Also, Hc proton of 1MPTMPC don’t interact with the inner protons of β-CD in the ROESY spectrum having only noise. Based on these findings, the geometrical structure of the 1MPTMPC:β-CD inclusion complex can be schematically presented as shown in Fig. 8.
Evaluation of the association constant
In order to determine the extent of the intermolecular binding between 1MPTMPC and β-CD, the association constant has been evaluated. The association constant, K for a 1:1 complex can be determined according to the following equation [13]:
where i counts the sample number and j the studied proton.
where [G] = ct = 1 mM and [H] varying between 0 and 14 mM.
If the studied proton belongs to the guest or host molecule, X = G or H respectively. Δδc(j) represents the chemical shift difference (for a given proton) between the free component and the pure inclusion complex. Equation (1) involves no approximations and correlates the total concentrations of the guest and host molecules with the observed difference in the chemical shift:
We used a software [14] based on an iteration procedure following specific algorithms in order to fit the experimental values of Δδ(i,j) to the appropriate equation. Each iteration sets up a quadratic programme to determine the direction of search and the loss function,
until the search converges.
The treatment of the whole set of protons studied yields one single K value characterizing the inclusion process and a set of calculated Δδc(i,j) values.
In our case, we applied Eq. (1) for a set of protons consisting in Ha, Ha′, Hb, Hb′ and Hc of 1MPTMPC. The association constant obtained using the above described procedure is K = 925 M−1, with E = 2.0246 × 10−4 and a correlation factor r = 0.9992.
XRPD results
XRD is a powerful technique for identification and characterization of the materials in solid state and is routinely used for the identification and sometime crystal structure determination of the CD complex. The crystalline state of 1MPTMPC, β-CD and the possible inclusion compound 1MPTMPC/β-CD where examined by XRD. Looking closely at the Fig. 9 we can observe the high degree of crystallinity for 1MPTMPC and β-CD, compared to the inclusion compound 1MPTMPC/β-CD. Although low crystallinity has been achieved, we can still identify the characteristic peaks of the compound 1MPTMPC/β-CD. As we can see in Fig. 9 the characteristic peaks corresponding to the pure substances (1MPTMPC and β-CD) are not present in the diffraction pattern of 1MPTMPC/β-CD. New peaks can be observed for example at 5.4°, 8.8°and 10.7° difractogram of the inclusion complex. Unfortunately, the low crystallinity of the diffraction pattern obtained for the 1MPTMPC/β-CD does not allow us a full structural characterization by crystal structure determination.
Differential scanning calorimetry (DSC)
DSC represents useful method that reveals information on solid-state interactions between a drug and cyclodextrin. The DSC thermograms of pure components and of 1MPTMPC:β-CD inclusion compound are presented in Fig. 10.
On the DSC curve of the compound 1MPTMPC one can observe two sharp endothermic peaks, the first, with Tonset = 63.1 °C, Tpeak = 66.6 °C and ΔH = − 152.8 J g−1, corresponds of the residual solvent elimination and the second, with Tonset = 213.4 °C, Tpeak = 219.1 °C [5] and ΔH = − 183.4 J g−1, to melting of the compound, followed by its decomposition. The DSC curve of β-CD revealed a broad endothermic signal in the 70–120 °C temperature range, with Tpeak = 95.8 °C and ΔH = − 204.1 J g−1, that corresponds to the loss by evaporation of the water molecules existing as residual humidity, as well as those included in the cavity [15, 16]. From 290 °C the melting, respectively the decomposition of compound occurs.
The thermogram of the 1MPTMPC:β-CD inclusion compound shows a single broad endothermic peak of weak intensity between 42 and 90 °C with Tpeak = 51.9 °C and ΔH = − 204.1 J g−1, corresponding to the loss of residual solvent molecules still present in this complex. This small peak might indicate that by integrating of the molecule of 1MPTMPC inside the nanocavity of β-CD the major removal of water molecules occurs. The 1MPTMPC’s melting peak disappears from the thermal profile of inclusion compound, which presents thermal stability up to around 250 °C.
Computational results
The molecular docking was achieved using the Lamarckian genetic algorithm. To insure good statistics and clustering, 1000 runs were performed, each starting with a different random generation seed. The conformation with the maximum binding energy presented in Fig. 11, had an occurrence of about 32%, while all the other most populated conformations, from the 19 clusters obtained, had an occurrence of less than 11%.
In this geometry, the (trifluoromethyl)phenyl-thiazol subunits of the 1MPTMPC molecule penetrate the β-CD cavity from the wider/secondary rim and occupies it entirely, the methyl-piperidinium subunit hanging outside the secondary rim. The average distance between the closest hydrogens in phenyl ring and the inner hydrogens in β-CD is about 2.4 Å, with a minimum of 1.61 Å and a maximum of 2.9 Å. This conformation was favored in both AutoDock 4.2 and AutoDock Vina runs.
Conclusions
Both ITC and 1H NMR are suitable techniques able to characterize, in solution, the molecular recognizing processes in host–guest systems. The association constant K obtained by 1H NMR and ITC are in good agreement and both methods sustain a 1:1 stoichiometry.
The microcalorimetry allowed us to study inclusion reaction between β-cyclodextrin and 1MPTMPC and to determine all the thermodynamic parameters of the cyclodextrin complexation. The complexation-induced chemical shift of H3 and H5 protons of β-CD are those expected as a result of interaction with those protons of 1MPTMPC, with the aromatic ring included in the β-CD cavity and the thiazol ring partially outside the secondary rim. The ROESY experiment indicates that the 1MPTMPC is included with the aromatic ring into the cyclodextrin cavity, having 1:1 stoichiometry.
The 1MPTMPC:β-CD supramolecular complex, in a 1:1 molar ratio was synthesized through evaporation and drying method. The formation of the complex was confirmed by X-Ray diffraction on powder and its thermal behavior was investigated by DSC. The differences in the thermal profile, compared to the active ingredient and β-CD also indicate the formation of the inclusion complex.
Numerical simulation of the inclusion process of 1MPTMPC in β-CD cavity was performed using a genetic algorithm to scan among possible relative orientations. The obtained maximum binding energy geometry of the inclusion complex corroborates well to ROESY experiment.
References
Lemke, T.L., Williams, D.A.: Foye’s Principles of Medicinal Chemistry, 7th edn. Lippincot Williams & Wilkinins, Philadelphia (2013)
Brunton, L.: The Pharmacological Basis of Therapeutics-II Neuropharmacology: Muscarinic Receptor Agonist and Antagonist, 12th edn. The McGraw-Hill Companies, San Diego, California (2011)
Palage, M., Nechifor, M., Mureşan, A.: The effect of the thiazole ammonium quaternary salts on the blood pressure to rats. Cluj. Med. LXXIII(4), 614–617 (2000)
Palage, M., Oniga, S., Parnau, A., Zaharia, V., Belegeanu, C., Vlase, L., Muresan, A.: Synthesis and physico-chemical characterisation of some quaternary ammonium salts 2-aryl thiazole derivatives. Farmacia LVII(5), 598–608 (2009)
Ielciu, I., Voştinaru, O., Oniga, S., Mogoşan, C., Vlase, L., Pârnău, A., Araniciu, C., Palage, M.: Synthesis and effects of some new 2-Aryl-thiazole ammonium salts on isolated ileum motility. Dig. J. Nanomater. Biostruct. 8(3), 1089–1099 (2013)
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S., et al.: AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30(16), 2785–2791 (2009)
Trott, O., Olson, A.J.: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31(2), 455–461 (2010)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A. Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., CammiR, PomelliC., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople JA: Gaussian 09, Revision C.01. Gaussian Inc, Wallingford (2004)
Caira, M.R., Miller, J.L., Nassimbeni, L.R.: β-Cyclodextrin inclusion complexes of Mg2+ and Ca2+ salts of meclofenamic acid: preparation and structural characterization. Supramol. Chem. 18(7), 553–559 (2006)
Todorova, N.A., Schwarz, F.P.: The role of water in the thermodynamics of drug binding to cyclodextrin. J. Chem. Thermodyn. 39, 1038–1048 (2007)
Floare, C.G., Pirnau, A., Bogdan, M.: 1H NMR spectroscopic characterization of inclusion complexes of tolfenamic and flufenamic acids with β-cyclodextrin. J. Mol. Struct. 1044, 72–78 (2013)
Pirnau, A., Floare, C.G., Bogdan, M.: The complexation of flurbiprofen with cyclodextrin: a NMR study in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 78, 113–120 (2014)
Bogdan, M., Caira, M.R., Farcas, S.I.: Inclusion of the niflumic acid anion in beta cyclodextrin: a solution NMR and X-ray structural investigation. Supramol. Chem. 14, 427–435 (2002)
Floare, C., Balibanu, F., Bogdan, M.: CONSTEQ: a program for the calculation of the equilibrium constants using spectroscopic data. Stud. Univ. Babes-Bolyai Phys. L4a, 451 (2005)
Castro-Hermida, J.A., Gomez-Couso, H., Ares-Mazas, M.E., Gonzales-Bedia, M.M., Castaneda-Cancio, N., Otero-Espinar, F., Blanco-mendez, J.: Anticryptosporidial activity of furan derivative G1 and its inclusion complex with beta-cyclodextrin. J. Pharm. Sci. 93, 1197–1206 (2004)
Rotich, M.K., Brown, M.E., Glass, B.D.: Thermal studies on mixtures of benzoic and salicylic acids with cyclodextrins. J. Therm. Anal. 73, 671–686 (2003)
Acknowledgements
Financial support from the Ministry of Research and Innovation—MCI, Core Programme, Project PN 18 03 02 01 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mic, M., Pîrnău, A., Floare, C.G. et al. Interaction of 1-methyl-1-({2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-4-yl}methyl) piperidinium chloride with β-CD: spectroscopic, calorimetric and molecular modeling approaches. J Incl Phenom Macrocycl Chem 92, 195–204 (2018). https://doi.org/10.1007/s10847-018-0830-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0830-0