Abstract
Assessment of interaction between β-cyclodextrin and 3-carboxy-1-[(2-phenyl-1,3-thiazol-4-yl) methyl]pyridin-1-ium iodide (3CPTMPI) in aqueous solution were investigated by isothermal titration calorimetry (ITC) and 1D and 2D 1H NMR spectroscopy at 298 K. Thermodynamic analysis using ITC revealed that the association constant of β-cyclodextrin and 3CPTMPI is 441.6 M−1 with favorable enthalpy and entropy changes. These thermodynamic parameters indicate that the binding is dominated by hydrophobic interactions, which is in agreement with inclusion complex formation. The details of β-CD/3CPTMPI molecular interaction was analyzed by 1H 2D NMR allowing the proposition of an inclusion model for 3CPTMPI into β-CD. Rotating frame NOE spectroscopy (ROESY) was used to certain the solution geometry host–guest complex. The results reveal that the 3CPTMPI molecule penetrates into β-CD cavity with both aromatic and thiazol rings. For this type of inclusion complex, the association constant K obtained by 1H NMR and ITC are in good agreement and both methods sustain a 1:1 stoichiometry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since their discovery, antibiotics have dramatically improved public health by enabling millions of people to live longer, more productive lives. The very success of these drugs has often resulted in an inappropriate and irrational use of antimicrobial drugs that has led to the development of antimicrobial resistance, a worldwide public health problem that continues to grow. Despite the large number of antimicrobial agents for medical use, increasing the number of pathogens resistant to different classes of antibiotics and infectious diseases led to the need to develop a new approach to antimicrobial therapy. The disadvantage of using antibiotics in therapy is their low solubility in water. For this reason it is necessary to develop technologies for improve the aqueous solubility. One of these is the encapsulation of the antibiotic in cyclodextrin.
Cyclodextrins (CDs) are cyclic oligosaccharides containing six (α-CD), seven (β-CD) or eight (γ-CD) α-1,4-linked glucopyranose units with a hydrophilic hydroxyl group on their outer surface and a hydrophobic cavity in the center. The hydrophilic exterior of the CD molecules can make them water soluble, but the hydrophobic cavity provides an environment for appropriate sized non-polar molecules. In aqueous solution CDs are capable of forming inclusion complex with many molecules by taking up a whole molecule or some part or it, into the cavity. These non-covalent complexes offer a variety of physicochemical advantages over uncomplexed molecules including increased water solubility and stability [1–4].
In this context, new quaternary ammonium compounds derivatives of 2-aryl-thiazole were synthesized. The thiazole ring is present in a variety of therapeutic agents. These agents can exhibit significant anti-cancerous, antimicrobial, anti-diabetic, anti-inflammatory, antiviral or analgesic activity [5–7]. On the other hand, the quaternary ammonium group is present in antiseptic, antispasmodic or spasmodic compounds [8–13].
Depending on the nucleophilic component, the quaternary ammonium salt is illustrated in the following scheme:
After the investigation of the antispasmodic potential, the quaternary ammonium derivatives have favorably influenced the contractile effect [14]. The inclusion of the 3-carboxy-1-[(2-phenyl-1,3-thiazol-4-yl) methyl]pyridin-1-ium iodide (3CPTMPI) into cyclodextrin could offer the possibility of controlling drug delivery in biological systems, extending the antispasmodic effect and reducing its toxicity.
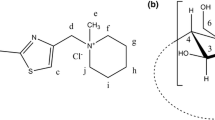
For these reasons, our contribution focuses on the characterization of the complexation thermodynamics of β-CD with 3CPTMPI (Fig. 1) using isothermal titration calorimetry (ITC) and to relate the thermodynamic parameters with the complex structure, by means of 1H NMR.
Experimental
Materials
β-cyclodextrin (water content 8 mol/mol) was purchased from Sigma Chimie GmbH. Germany and 3CPTMPI compound was synthesized by the Iuliu Haţieganu University, Pharmacy Faculty Cluj-Napoca. Both chemicals were used without any further purification. Deuterium oxide (99.7 % D) was obtained from Heavy Water Plant Romag—Prod, Romania.
Apparatus
The isothermal titration calorimetric experiments were carried out on a Nano ITC 2G calorimeter (TA Instruments, New Castle, Delaware, USA) at 298 K next to the electrically and chemically calibration. The volume of the titration and reference cells was 1 mL. Small aliquots of a solution are injected under computer control into the titration cell at pre-defined time intervals [15, 16]. The recorded quantity is the rate of heating as a function of time. The individual pulses are integrated to obtain plots recording the ratio of heat to the amount of injected molecules.
The 1H NMR experiments were performed on a Bruker Avance III, spectrometer operating at 500.13 MHz and equipped with a broadband observe probe. The NMR spectra were recorded in D2O solution at 298 K and all chemical shifts were measured relative to TMS.
Isothermal titration calorimetry (ITC)
Calorimetric titration experiments were carried out at constant temperature by injecting 15 mM β-CD solution into the sample cell containing 0.75 mM 3CPTMPI, keeping the stirring speed fixed at 350 rpm. From a computer controlled 250 µL Hamilton syringe, aliquots of 10 µL have been injected with a 400 s interval which was sufficiently long for the signal to return to the baseline and to ensure the equilibrium for the system. The heat associated with each injection was calculated by integration of the peaks in the heat flow curve using the calorimeter software. The first injection was generally imprecise and was ignored. Dilution heat of the host was measured under identical experimental conditions by injecting the β-CD into water. In general, heats of dilution were very small compared to the heats of complexation. The blank effects were subtracted in order to correct for dilution, mixing and injection effects.
1H NMR measurements
In order to study the stoichiometry between 3CPTMPI and β-CD in solution by NMR spectroscopy, two stack solutions in D2O, both having 1 mM were prepared. Based on these two equimolar solutions, a series of eleven samples (i = 1–11) containing both the 3CPTMPI and β-CD molecules were prepared. This was accomplished by mixing the two solutions to constant volume at varying proportions, so that a complete range (0 < r < 1) of the ratio r = [X]/([H] + [G]) was sampled. X = H or G and [H] and [G] are the total concentrations of the host (β-CD) and guest (3CPTMPI), respectively. Thus the total concentration [H]t + [G]t = 1 mM was kept constant for each solution. In order to determined the association constant we used a second set of samples with [G] = ct = 1 mM and [H] varying between 0 and 15 mM.
For each 1H NMR experiment, between 32 and 128 transients were collected into 65 K data points over a 5000 Hz spectral window, using a 2 s relaxation delay. The 2D ROESY spectra were acquired in the phase sensitive mode and residual water suppression, using Bruker standard parameters (pulse program roesyphpr). Each spectrum consisted of a matrix of 8/1 K data points covering a spectral width of 5000 Hz. The spectra were obtained with a spin-lock mixing time of 500 ms, relaxation delay 4.3 s and 32 scans were recorded. The 2D 1H-1H COSY NMR spectra were acquired using 8 scans and a Bruker standard program (pulse program cosygf45). Each spectrum consisted of a matrix of 4096/2048 data points covering a spectral width of 2250 Hz, relaxation delay 2 s and 8 scans were recorded.
Results and discussions
Determination of the association constant by ITC
ITC is frequently used to provide a complete profile of the thermodynamic parameters which characterize the molecular complexes and seems to be the most sensitive method for measuring the total heat changes during the complexation process [17–19].
In a ITC experiment, aliquots of a concentrated solution of cyclodextrin are added in a time-controlled manner to a cell containing the interacting 3CPTMPI molecule, which is maintained at constant temperature. During cyclodextrin addition, the molecules interact and the observed released heat is directly proportional to the amount of binding compound added with the solution aliquot. As the population of interacting molecules in the cell becomes more saturated with cyclodextrin, the heat signal diminishes until only the background heat of dilution is observed. After integrating the heat as a function of molar ratio between the two reactants, it is possible to fit the integration curve for to determine the association constant, the stoichiometry and a thermodynamic profile of the interaction.
Taking into account, the equilibrium in guest–host reaction is described by the following hypothetical scheme:
where [H], [G], and [C] stand for the concentrations of 3CPTMPI, β-CD and of the complex respectively.
In an ITC experiment the reaction heat per mole of injected molecules after the ith injection is given by:
where \(r_{0} = \frac{{G_{0} }}{{H_{0} }};\quad r_{i} = \frac{{G_{i} }}{{H_{i} }} = r_{0} \frac{iv}{V}\;\), H0 and G0 are the initial concentrations of 3CPTMPI and of β-CD respectively, ΔH is the reaction enthalpy, V is the volume of reaction cell and v is the small volume injected at each titration step [15].
The software supplied with the calorimeter was used to fit thermodynamic parameters K and ΔH to the heat profiles.
ITC thermogram showing the raw signals obtained from the titration of 0.75 mM 3CPTMPI with 15 mM β-CD at 298 K (Fig. 2) and the incremental heat/mol of added ligand as a function of molar [β-CD]/[3CPTMPI] ratio (Fig. 3). All the measured heats were exothermic.
The reaction enthalpy (ΔH) of the host–guest inclusion compounds is −2.53 kcal/mol. This value is negative, which indicates that the formation of host–guest inclusion complexes is a weak exothermic process. The entropy effect (ΔS) of the host–guest complexes in 1:1 stoichiometry is ΔS = 3.58 cal/mol K, which is positive and make evidently larger contribution to the negative charge of Gibbs energy (ΔG = −3.61 kcal/mol) than the heat effect do. The positive entropy effect may be due to the total result of the host–guest combination, which has negative contribution to entropy and the releasing water molecules from the cavity, which has positive contribution to entropy. The analysis of the thermodynamic data reveals that the driving force of the binding process is a hydrophobic interaction with the released water molecule from the β-CD cavity, which has positive contribution to entropy [20–22].
Calorimetric measurements of the interaction of β-CD with 3CPTMPI have shown that the stoichiometry of the host–guest complex is 1:1 and the association constant was K = 441.6 M−1.
Whatever the stoichiometry obtained, the most described mechanism for the cyclodextrins–guest interaction is that, in an aqueous solution, the slightly apolar cyclodextrin cavity is occupied by water molecules which are energetically unfavored (polar–apolar interaction), and therefore can be readily used by appropriate ‘guest molecules’ which are less polar than water resulting in a more stable lower energy state. From a thermodynamic point of view, a stronger interaction of a molecule with the cyclodextrins is evidently expressed not only by a higher value of the binding constant, but also by a more negative value of ΔH and ΔG indicating that the interaction is stronger and more spontaneous, respectively [17].
Determination of the stoichiometry by 1H NMR
NMR is a technique which provides the most evidence for the inclusion of a guest molecule into the hydrophobic CD cavity in solution. Inclusion of 3CPTMPI in β-CD cavity is evidenced by the change in chemical shifts of some of the guest and host protons, in comparison with the chemical shifts of the same protons in the free components. Figure 4 represents the 1H NMR spectrum of 3CPTMPI with assignment of the signals (in the presence of β-CD), considering the numerical order from Fig. 1a. For a good assignment of 1H NMR spectrum, we take into account the two-dimensional 1H-1H COSY NMR spectrum (COrrelation SpectroscopY) of 3CPTMPI in the presence of β-CD, which describes the interactions between neighbor protons (Fig. 5) [23]. We used this experiment to put in evidence the existing proton homonuclear spin–spin coupling in 3CPTMPI molecule. Partial 1H NMR spectra of pure components and 3CPTMPI: β-CD mixture in a 1:1 molar ratio is presented in Figs. 6 and 7 and the chemical shifts for all protons will be given in Table 1.
When comparing spectra from Fig. 6, it is possible to observe a subtle signal broadening and chemical shifts variation for some aromatic protons of 3CPTMPI, due to the presence of β-CD. As expected, the H3 and H5 protons located inside the cavity are appreciably shifted (see Fig. 7), evidencing the existence of an interaction between the 3CPTMPI molecule and the interior of the β-CD cavity. The observed broadening may be due to the restricted motion of a 3CPTMPI molecule inside a β-CD cavity and by the complexation association–dissociation process.
Determination of the stoichiometry of the 3CPTMPI–βCD complex, by the continuous variation method was based on 1H NMR spectra obtained by mixing the two solutions to constant volume at varying proportions, so that a complete range (0 < r < 1) of the ratio r = [X]/([A] + [B]) was sampled. X = A or B and [A] and [B] are the total concentrations of the host (β-CD) and guest (3CPTMPI), respectively. Thus the total concentration [A] + [B] = [C] = 1 mM was kept constant for each solution [24]. The resulting continuous variation plots demonstrate that because r has a maximum value of 0.5 and highly symmetrical shapes (Fig. 8), the complex has 1:1 stoichiometry.
ROESY experiments
While the 1D NMR provides unambiguous evidence on the formation of a complex, ROESY experiments provide information on the dynamics and the averaged relative inter- and intramolecular proton distances. In the present study, to gain further information on the inclusion complexation mode and additional insights into the dynamic structure, a 2D ROESY 1H NMR spectrum was acquired. Due to the rapid dynamics of the complexation process, the ROESY effects were only qualitatively used and no conclusions on intermolecular distances were extracted. An expansion of the ROESY spectrum of the 3CPTMPI : β-CD complex is reported in Fig. 9.
The 2D NMR spectrum shows several intermolecular cross-peaks between H3 and H5 protons of β-CD and protons of both aromatic and thiazol rings and methylene group of 3CPTMPI, demonstrating the inclusion of these groups in the β-CD cavity. Based on these findings, the geometrical structure of the 3CPTMPI:β-CD inclusion complex, having 1:1 stoichiometry, can be schematically presented as shown in Fig. 10.
Evaluation of the association constant
In order to determine the extent of the intermolecular binding between 3CPTMPI and β-CD, the association constant has been evaluated. The association constant, K for a 1:1 complex can be determined according to the following equation [25]:
where i counts the sample number and j the studied proton.
where [G] = ct = 1 mM and [H] varying between 0 and 15 mM and the studied proton belongs to the guest or host molecule. \(\Delta {{\delta }}_{\text{c}}^{{\left( {\text{i}} \right)}}\) represents the chemical shift difference (for a given proton) between the free component and the pure inclusion complex. Equation (3) involves no approximations and correlates the total concentrations of the guest and host molecules with the observed difference in the chemical shift:
We used a software [26] based on an iteration procedure following specific algorithms in order to fit the experimental values of Δδ(i,j) to the appropriate equation. Each iteration sets up a quadratic programme to determine the direction of search and the loss function,
until the search converges.
The treatment of the whole set of protons studied yields one single K value characterizing the inclusion process and a set of calculated \(\Delta {{\delta }}_{\text{c}}^{{\left( {\text{i,j}} \right)}}\) values. The programme is quite flexible since it allows observing the chemical shift variation of the host, guest or both molecules as a function of variable guest or host concentrations.
In our case, we applied Eq. (3) for a set of protons consisting in Hi, Hh, Hf and He of 3CPTMPI. The association constant obtained using the above described procedure is K = 498 M−1, with E = 3.46 × 10−4 and a correlation factor r = 0.9979.
Conclusions
Both ITC and 1H NMR are suitable techniques able to characterize the molecular recognizing processes in host–guest systems. The microcalorimetry allowed us to study inclusion reaction between β-cyclodextrin and 3CPTMP and to determine all the thermodynamic parameters of the cyclodextrin complexation.
The complexation—induced chemical shift of H3 and H5 protons of β-CD are those expected as a result of interaction with those protons of 3CPTMPI which are oriented with aromatic and thiazol rings towards the β-cyclodextrin cavity, thus confirming a 3CPTMPI/β- CD interaction.
The ROESY experiment indicates that the 3CPTMPI is included with the aromatic ring into the cyclodextrin cavity, having 1:1 stoichiometry.
For this type of inclusion complex, the association constant K obtained by 1H NMR and ITC are in good agreement and both methods sustain a 1:1 stoic7hiometry.
References
di Cagno, M., Stein, P.C., Skalko-Basnet, N., Brandl, M., Bauer-Brandl, A.: Solubilization of ibuprofen with β-cyclodextrin derivatives: Energetic and structural studies. Journal of Pharmaceutical and Biomedical Analysis 55(3), 446–451 (2011)
Sun, D.Z., Li, L., Qiu, X.M., Liu, F., Yin, B.L.: Isothermal titration calorimetry and 1H NMR studies on host-guest interaction of paeonol and two of its isomers with beta-cyclodextrin. Int. J. Pharm. 316, 7–13 (2006)
Mazzaferro, S., Bouchemal, K., Gallard, J.F., Iorga, B.I., Cheron, M., Gueutin, C., Steinmesse, C., Ponchel, G.: Bivalent sequential binding of docetaxel to methyl-β-cyclodextrin. Int. J. Pharm. 416, 171–180 (2011)
Chadha, R., Gupta, S., Shukla, G., Jain, D.V.S., Singh, S.: Characterization and in vivo efficacy of inclusion complexes of sulphadoxine with β-cyclodextrin: calorimetric and spectroscopic studies. J. Incl. Phenom. Macrocycl. Chem. 71(1–2), 149–159 (2011)
Mislin, G.L., Burger, A., Abdallah, M.A.: Synthesis of new thiazole analogues of pyochelin, a siderophore of Pseudomonas aeruginosa and Burkholderia cepacia. A new conversion of thiazolines into thiazoles. Tetrahedron 60, 12139–12145 (2004)
Wilson, K.J., Illig, C.R., Subasinghe, N., Hoffman, J.B., Rudolph, M.J., Soll, R., Molloy, C., Bone, R., Green, D., Randall, T., Zhang, M., Lewandowski, F.A., Zhou, Z., Sharp, C., Maguire, D., Grasberger, B., Desjarlais, R.L., Spurlino, O.: Synthesis of thiophene-2-carboxamidines containing 2-aminothiazoles and their biological evaluation as urokinase inhibitors. J. Bioorg. Med. Chem. Lett. 11, 915–918 (2001)
Beale, J.M., Block, J.H.: Wilson’s and Gisvold’s textbook of organic medicinal and pharmaceutical Chemistry, vol. 349, 12th edn, pp. 281–324. Lippincott, Williams and Wilkins, Philadelphia (2011)
Stenlake, J.B., Dhar, N.C., Haddow, J., McDonald, I.M., Maehr, R.B., Wastila, W.B.: Neuromuscular blocking agents. Some approaches to short acting compounds. Eur. J. Med. Chem. 27, 463–477 (1992)
Stenlake, J.B., Dhar, N.C., Henderson, C.F., Maehr, R.B., Scharver, J., Wastila, W.B., Midgley, J.M.: Neuromuscular blocking agents. Approaches to short-acting compounds 2. Bis-thiazolium salts. Eur. J. Med. Chem. 28, 415–418 (1993)
Komloova, M., Musilek, K., Horova, A., Holas, O., Dohnal, V., Gunn-Moore, F., Kuca, K.: Preparation, in vitro screening and molecular modelling of symmetrical bis-quinolinium cholinesterase inhibitors—implications for early Myasthenia gravis treatment. Bioorg. Med. Chem. Lett. 21, 2505–2509 (2011)
Musilek, K., Komloova, M., Zavadova, V., Holas, O., Hrabinova, M., Pohanka, M., Dohnal, V., Nachon, F., Dolezal, M., Kuca, K., Jung, Y.S.: Preparation and in vitro screening of symmetrical bispyridinium cholinesterase inhibitors bearing different connecting linkage—initial study for Myasthenia gravis implications. Bioorg. Med. Chem. Lett. 20, 1763–1766 (2010)
Yu, Q.S., Holloway, H.W., Luo, W., Lahiri, D.K., Brossi, A., Greig, N.H.: Long-acting anticholinesterases for myasthenia gravis: synthesis and activities of quaternary phenylcarbamates of neostigmine, pyridostigmine and physostigmine. Bioorg. Med. Chem. 18, 4687–4693 (2010)
Musilek, K., Komloova, M., Zavadova, V., Holas, O., Hrabinova, M., Pohanka, M., Dohnal, V., Nachon, F., Dolezal, M., Kuca, K.: Preparation and in vitro screening of symmetrical bis-isoquinolinium cholinesterase inhibitors bearing various connecting linkage—implications for early Myasthenia gravis treatment. Eur. J. Med. Chem. 46, 811–818 (2011)
Ielciu, I., Voştinaru, O., Oniga, S., Mogoşan, C., Vlase, L., Pârnău, A., Araniciu, C., Palage, M.: Synthesis and effects of some new 2-aryl-thiazole ammonium salts on isolated ileum motility. Dig. J. Nanomater. Biostruct. 8(3), 1089–1099 (2013)
Fraceto, L.F., Pinto, L.M.A., Franzoni, L., Braga, A.A.C., Spisni, A., Schreier, S., de Paula, S., de Paula, E.: Spectroscopic evidence for a preferential location of lidocaine inside phospholipid bilayers. Biophys. Chem. 99, 229–243 (2002)
Pinto, L.M.A., Fraceto, L.F., Santana, M.H.A., Pertinhez, T.A., Oyama Jr, S., de Paula, E.: Physico-chemical characterization of benzocaine-b-cyclodextrin inclusion complexes. J. Pharm. Biomed. Anal. 39, 956–963 (2005)
Bouchemal, K., Mazzaferro, S.: How to conduct and interpret ITC experiments accurately for cyclodextrin–guest interactions. Drug Discov Today 17(11–12), 623–629 (2012)
Müller, B.-K., Ritter, H.: Scrutinizing ITC-study on the formation of inclusion complexes of nonionic surfactant Triton X-100 and cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 72(1–2), 157–164 (2012)
Waters, L.J., Bedford, S., Parkes, G.M.B., Mitchell, J.C.: Influence of lipophilicity on drug–cyclodextrin interactions: a calorimetric study. Thermochim. Acta 511, 102–106 (2010)
Chaires, J.B.: Calorimetry and thermodynamics in drug design. Annu. Rev. Biophys. 37, 135–151 (2008)
Chodera, J.D., Mobley, D.L.: Entropy-enthalpy compensation: role and ramifications in biomolecular ligand recognition and design. Annu. Rev. Biophys. 42, 121–142 (2013)
Wszelaka-Rylik, M., Gierycz, P.: Isothermal titration calorimetry (ITC) study of natural cyclodextrins inclusion complexes with drugs. J. Therm. Anal. Calorim. 111, 2029–2035 (2013)
Friebolin, H.: Basic One and Two Dimensional NMR Spectroscopy. Wiley-VCH, Weinheim (2005)
Fini, P., Castagnolo, M.: Determination of enthalpic interaction coefficients by ITC measurements. 2-Hydroxypropyl-β-cyclodextrin in aqueous solution of NaCl. J. Therm. Anal. Calorim. 66, 91–102 (2001)
Bogdan, M., Caira, M.R., Farcas, S.I.: Inclusion of the niflumic acid anion in beta-cyclodextrin: a solution NMR and X-ray structural investigation. Supramol. Chem. 14, 427–435 (2002)
Floare, C., Balibanu, F., Bogdan, M.: CONSTEQ —a program for the calculation of the equilibrium constants using spectroscopic data. Stud. Univ. Babes-Bolyai Phys. L4a, 451 (2005)
Acknowledgments
This work was financially supported by PCE-2011-3-0032 and PN 09-44 02 18 Projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mic, M., Pîrnău, A., Neamţu, S. et al. Molecular interaction of β-CD with 3-carboxy-1-[(2-phenyl-1,3-thiazol-4-yl) methyl]pyridin-1-ium iodide analyzed by isothermal titration calorimetry and NMR spectroscopy. J Incl Phenom Macrocycl Chem 83, 257–265 (2015). https://doi.org/10.1007/s10847-015-0561-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0561-4