Abstract
Spectroscopic investigation supported by molecular modeling methods has been used to describe the inclusion complex of β-cyclodextrin (β-CD) with N-[(4-sulfonamidophenyl) ethyl]-5-(1,2-dithiolan-3-yl) pentanamide in solution and in solid state. By using UV–Vis absorption, the stoichiometric ratio of the complex was found to be 1:1 and the stability constant was evaluated as 1.3415.104 (mol/L)−1. Solid state characterization by FT-IR spectroscopy provided remarkable evidences of the formation of inclusion system. Moreover, semi-empirical calculations using PM3 level of theory and hybrid method ONIOM2 clearly indicate that the formed complexes are energetically favored in vacuum and in solution. From NBO analysis, the mutual interactions between β-CD and SULPA were analyzed and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sulfonamides are a very useful structure, leading to a large family of compounds exhibiting a wide variety of pharmacological activities such as antibacterial, hypoglycemic, diuretic, anticonvulsant, anti-carbonic anhydrase, antithyroid, antitumor, HIV protease inhibitor activities and anti-inflammatory [1, 2]. Among this broad spectrum of activities showed by sulfonamides, their role as inhibitors of the zinc containing metalloenzyme carbonic anhydrase (CA) has been discovered and clinically exploited, However, most of the clinically used sulfonamides present unfavorable physicochemical properties such as low water solubility and bad therapeutic index, owing to several undesired side effects [3, 4].
Modern drug design not only focuses on the pharmacological activity of a drug such as a compound but also considers its ability to be absorbed and to reach its site of action. Low water solubility continues to influence the development of many potential drug candidates [5, 6]. This is the reason why different strategies using prodrug, solid dispersions, salt compounds and complexes have been developed to exceed the problem of poor solubility of drugs [7, 8]. Among all the solubility enhancement techniques, inclusion complex formation technique has been employed more precisely to improve the aqueous solubility, dissolution rate, and bioavailability of poorly water soluble drugs. Inclusion complexes are formed by the insertion of the hydrophobic molecule (known as guest) into the cavity of another molecule or group of molecules (known as host).The most commonly used host molecules are cyclodextrins [9].
Cyclodextrins (CDs) are seductive cyclic oligosaccharides composed of glucopyranose units. The most used CDs are α-, β-, and γ-CDs, which are built up from six, seven and eight glucopyranoses, respectively. Due to their unique structure, characterized by a chiral and hydrophobic cavity, CDs have been successfully used as chiral selectors and drug delivery systems, due to its exterior hydrophilic surface and interior hydrophobic cavity.
Complexation of a large variety of guest compounds with CDs or modified cyclodextrins can enhances the apparent water solubility of the guest, increase stability against the effects of light, heat, and oxidation, mask undesirable physiological effects, in addition to other important properties [10, 11].
Recently, there has been considerable interest in the synthesis and pharmacological property of human carbonic anhydrase (hCA) inhibitors [12–15].Interestingly, the inhibition of transmembrane, tumor-associated to isozyme hCA IX with a library of aromatic and heteroaromatic sulfonamides has been investigated. Unfortunately, most of these compounds have very limited water solubility and exhibit some serious side effects due particularly to their low selectivity by inhibiting the other CA isozymes of major physiological functions [16–18].
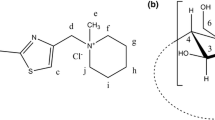
Previously, we have synthesized and studied a new class of carbonic anhydrase inhibitors, mainly the N-[(4-sulfonamidophenyl)ethyl]-5-(1,2-dithiolan-3-yl)pentanamide (Fig. 1), a specific CAIX inhibitor exhibiting an effective antitumor activity [19]
This poorly water soluble compound that we have denoted by SULPA bears a chiral carbon atom and therefore occurs as R- and S-enantiomers.
Furthermore, according to a Nano approach, which consisted in grafting this compound onto gold nanoparticles (AuNPs), we found that the activity and selectivity of this lipoic acid tailed sulfonamide were dramatically enhanced. However, knowing that the 1,2-dithiolane ring in SULPA compound is the source of the fascinating biological properties of lipoic acid, but also of its low solubility in water, instability to light, heat and alkaline conditions [20, 21]. For these reasons, we extend our investigation in this present paper to a Sugar approach in which we report an experimental and theoretical study of the inclusion complexation of β-CD with SULPA in the aim of improving its stability, aqueous solubility and inhibitor activity.
Experimental
Materials
The investigated compound namely: N-[(4-sulfonamidophenyl)ethyl]-5-(1,2-dithiolan-3-yl) pentanamide was synthesized, purified and characterized according to the procedure described in a previous work in the literature [19] and studied as a racemic mixture.
β-CD was purchased from Sigma. All used chemicals were of high purity (analytical grade). Double-distilled water was used throughout. β-CD hydrate (β-CD·12H2O) has a water content of 14 % which was taken into account when preparing its solutions.
Fresh solutions were prepared just before taking measurements. The concentration of β-CD was varied from 10−4 to 10−5 M. The concentration of the SULPA (C16H24O3N2S3) solutions was equal to 10−5 M. The experiments were carried out at room temperature.
Preparation of inclusion complex in solution
The concentration of stock solution of the SULPA was 10−2 M. The stock solution (0.1 ml) was transferred into 100 ml volumetric flasks.The final concentration of SULPA in all the flasks was 10−5 M. The experiments were carried out at room temperature (25 °C). Varying concentrations of β-CD solution (ranging from 2.0.10−5 to 1.0.10−4 M) was added. The mixed solution was shaken thoroughly and analyzed.
Preparation of inclusion complex in solid state
The inclusion complexes were prepared by using co-precipitation method as follows: To a stirred saturated solution of β-CD in water (2 %) was added slowly a solution of SULPA (1 equiv.) in methanol: water system (10:90, v/v). The mixture was stirred vigorously for 24 h at room temperature. The solution became turbid and the precipitated complex was recovered by filtration and washed with small amount of methanol and water to remove uncomplexed compound and β-CD, respectively. The precipitate was then dried in vacuum at room temperature for two days and stored in an airtight bottle.
Instruments
The electronic absorption spectral measurements were obtained by means of a Shimadzu spectrophotometer (Model U-1800 PC UV–Vis spectrophotometer) equipped with a xenon arc lamp. The wavelengths (λ) are recorded in the range of 400–220 nm and the measurements were carried out in acetonitrile.
FT-IR spectra of the samples (β-CD, SULPA and their inclusion complex powder) were obtained on a Spectrum one Perkin Elmer FT-IR. The samples were first ground and mixed thoroughly with KBr: an infrared transparent matrix. The KBr disks were prepared by compressing the powder. The scans were obtained from 500 to 4000 cm−1 at resolution of 1 cm−1.
Molecular modeling
Molecular modeling of CD complexes is among computational techniques and theoretical methods that are used to predict their molecular behaviors, specifically, information about their geometry and the nature of interactions between the host and the guest molecules.
In our study, the initial geometry of SULPA structure was constructed with the use of Hyperchem software 7.5 molecular modeling package [22]. The starting structure of β-CD was constructed with CS Chem3D Ultra (Version 10, Cambridge software) from the crystal structure. Molecular modeling of the inclusion complex for 1:1 stoichiometry and all calculations were performed using Gaussian 09 program [23].
The inclusion process was emulated manually by translating the guest molecule into the β-CD cavity along the Z axis from −10 Å to +10 Å with 1 Å step By using semi-empirical quantum mechanical calculations at PM3 level of theory, the geometry optimization and the energy minimization were carried out as described in the literature [24] (details were also provided in Supporting Information).
It is generally accepted that the approach of the guest molecules to the CD cavity is more favorable towards the wider rim side of the cavity, therefore, as showed below in Fig. 2, we optimized both the two possible orientations labeled A and B:
-
(i)
Dithiolane ring of SULPA inserted into the wide rim of β-CD, namely A model.
-
(ii)
Arylsulfonamide ring of the guest inserted into the wide rim of β-CD, namely B model.
The coordinate systems and inclusion process of SULPA into β-CD according to both orientations A and B are shown in Fig. 2.
The solvent effects on the conformational equilibrium have been investigated using the PCM model for water (H2O) (e = 78.39), dimethylsulfoxide (DMSO) (e = 46.7) and acetonitrile (CH3CN) (e = 36.2) as solvents with PM3 method.
The complexation energy (Ecomplex) upon complexation between β-CD and SULPA is calculated for the minimum energy structures according to the following equation [25, 26].
Where E(complex), E(β-CD) and E(SULPA) are the total energy of the optimized most stable complex, the free optimized β-CD and the free optimized guest SULPA molecule, respectively.
The density functional theory (DFT) single- point calculation is applied to calculate also the deformation energy (EDEF) of each component (host or guest), based on the following equation:
Where \(E\left[ {\text{component}} \right]_{sp}^{opt}\) stands for the single point energy of the component on the configuration taken from the optimized complex geometry and \(E\left[ {\text{component}} \right]_{opt}\) its energy when it is optimized in free form. The energy values were obtained from Eq. (2) by decomposing the optimized complex and performing a single point energy calculation on each resulting species.
For a deeper understanding of the encapsulation process and to improve the precision of the theoretical results, an ONIOM method of calculation was used. This method allows different levels of theory to be applied to different parts of a molecular system [27]. In the two-layered ONIOM method (ONIOM2), the molecular system being studied is split into two layers: an inner and an outer layer. The inner layer consists of the most critical elements of the system, and the remainder of the system includes outer layer. In the terminology used by Morokuma et al. [28], the ONIOM energy is described as:
where E (high, model), E (low, real) and E (low, model) represent the energy of the inner layer (SULPA) treated with the high level of theory, the energy of the entire system (the complex (β-CD/SULPA)) treated with the low level of theory and the energy of the model system(β-CD) also treated with the low level of theory, respectively.
Results and discussion
Absorption spectral characteristics of SULPA in β-CD
The inclusion phenomena in acetonitrile solution of the SULPA within the hydrophobic cavity of the β-CD was investigated through UV/Vis spectroscopy (Fig. 3). The absorption spectrum of the SULPA (1.00.10−5 M) itself without β-CD exhibits a single absorption maximum at 224 nm, in addition, there was no noticed spectral shift in the presence of β-CD (1.00.10−5 M) except an increase in the intensity.
Figure 4 shows absorption spectra of SULPA solution (1.00.10−5 M) containing various concentrations of β-CD (between 2.00.10−5 and 1.00.10−4 M). Upon the stepwise addition of β-CD, the absorption peak at 224 nm gradually increases in intensity, but don’t shows any shift in the wave peak (bathochromic or hypsochromic shift) this behavior might suggest strong interactions between β-CD and SULPA and formation of inclusion complex. Further, the increase of absorbance when adding β-CD solutions has been attributed to the enhanced dissolution of SULPA through the hydrophobic interaction of β-CD cavity [29, 30].
Determination of the stoichiometry of the complex
The stoichiometry of the complex β-CD/SULPA was studied using the Job method, also known as the continuous variation method [31, 32]. Briefly, the experiments use stock solutions with different volumes of equal molar concentrations of Host and Guest components which were mixed in such a way that their total volume remains constant and absorbance was plotted versus molar ratio (the molar of β-CD divided by the total molar of β-CD and SULPA).
Where Vβ-CD and VSULPA are the volumes of β-CD and SULPA solutions, respectively.
The Job’s plot is shown in Fig. 5a where we noticed that the UV absorbance changes between free and complexed SULPA. The Job’s plot maximum peak was obtained at molar ratio = 0.5, which indicates that a complex with a 1:1 stoichiometry between β-CD and SULPA is predominant in the solution. Besides that, the presence of isosbestic point in the absorption spectra Fig. 5b is a strong indicative of the 1:1 inclusion complex formation [33].
Determination of the stability constant of the complex
The stability constant value of host–guest complexes is a useful index of their binding strength. In our study, the stability constant (K) of β-CD/SULPA complex has been determined in acetonitrile, with Benesi–Hildebrand method [34–36], which comprises varying the concentration of β-CD while fixing that of SULPA one. In this approach, we expressed the ratio of the concentration of SULPA and maximum absorbance versus the inverse of the concentration of β-CD.
Assuming that the β-CD forms an inclusion complex of 1:1 stoichiometry with the SULPA.
In such case, the inclusion complex is present in solution in dynamic equilibrium with its constituents as shown below in Eq. (5), on which the Benesi–Hildebrand Eq. (6) can be applied.
Thus, the plot of [SULPA]/Abs versus (1/[β-CD]), displayed in Fig. 6, shows a good linearity with a correlation factor of R2 = 0.998. This confirms the formation of the inclusion complex between β-CD and SULPA with a stoichiometry of 1:1.
From the intercept (1/ε) and the slope (1/Kε) values of this plot shown in (Fig. 6), the constant stability (k) for the host–guest inclusion complex was calculated and proved to be equal to 1.34.104 (mol/L)−1 at 25 °C in acetonitrile solution. The higher value of the formation constant of β-CD/SULPA inclusion complex suggests that β-CD forms stable inclusion complex with SULPA molecule and reveals the presence of strong interactions between the host and the guest [37].
Now, suppose the case where a 2:1 stoichiometry is predominant, since then, the applicable equation becomes:
According to this equation, when the trace of [SULPA]/Abs versus 1/[β-CD]2 is performed with the same data above, we do not get a straight line. This observation suggests that the composition of the complex is not 2:1 and therefore the possibility of forming an inclusion complex with stoichiometry 2:1 between β-CD and SULPA is discarded [38].
Fourier transform infrared spectroscopy (FT-IR) study
FT-IR is a very useful tool to confirm the formation of the inclusion complexes. Indeed, the bands resulting from the included part of the guest molecule are generally shifted or their shapes and intensities altered [39].
The FT-IR spectra of β-CD, SULPA, and their inclusion complex are presented in (Fig. 7). The β-CD (Fig. 7b) showed its signature peaks observed at frequencies: 3413, 2926 and 1027 cm−1 which are corresponding respectively to the symmetric and asymmetric stretching of OH, CH2 stretching, and bending vibration of C–O–H groups in β-CD structure. The infrared spectra of the complex SULPA/β-CD (Fig. 7c) taken in the region of 4000–500 cm−1 was compared with that of the free guest SULPA (Fig. 7a).
The spectral region from 4000 to 3000 cm−1 is difficult to analyze for β-CD and its complexes due to the co-presence of primary and secondary OH groups of β-CD and water molecules of crystallization in this region. In the SULPA spectrum, the peaks between 3500 and 3400 cm−1 are due to asymmetric and symmetric vibrations of the amino group (NH2).The band for (N–H) of the amidic group is observed around 3345 cm−1. The peaks appearing at 3078, 1619 and 1545 cm−1 respectively, are attributed to C–H stretching of aromatic ring, to the amidic (C=O) stretching and to NH deformation. The strong absorptions observed at 1324 and 1152 cm−1 are assigned successively to SO2 asymmetric and symmetric stretching. The bands positioned at 1097, 920, 810, and 576 cm−1 are assigned to C–H deformation in phenyl ring, S–N stretching, S–O stretching and S–S stretching in 1,2-dithiolan ring, respectively.
In the complex, the less resolved band at 3338 cm−1 corresponding to the (N–H) stretching of amidic group was shifted by 7 cm−1 and its intensity was slightly diminished. In contrast, the bands observed at 3078 and 920 cm−1 which are attributed successively to C–H stretching of aromatic ring and S–N stretching in sulfonamide group remained unchanged. The band assigned to (C=O) of the amidic group positioned at 1622 cm−1 became shorter and shifted by 3 cm−1 to higher frequencies. An appreciable shift of 43 cm−1 to a lower frequency (533 cm−1) and reduction in intensity of the characteristic disulfide bridge S–S stretching band was observed.
According to the above FT-IR analysis, we might suggest that these changes in the characteristic bands of free SULPA can be due to the formation of β-CD/SULPA inclusion complexes in solid state. Thus, only one ring of the guest molecule namely the dithiolan ring seems to be predominantly embedded into the hydrophobic cavity of β-CD. By contrast, these results indicate that the Arylsulfonamide moiety remains outside of the CD cavity.
The dithiolan ring is the most fragile part of SULPA, as it is common for this group to be reduced enzymatically or chemically to thiols or be cleaved when SULPA is under degradation conditions [40]. Therefore, the inclusion of the dithiolan ring into CD cavity and the consequent protective effects on this moiety is the determinative factor for SULPA stability.
Molecular modeling studies
Molecular modeling investigations using semi-empirical PM3 method
Host–guest systems using the complexation of biomolecules with cyclodextrins (CDs) is an ideal model for mimicking enzyme-substrate interactions [41]. Currently, computational methods used in molecular modeling have became an important tool to study such supramolecular systems. Among the most widely used of these methods, we can mention: molecular mechanics (MM), molecular dynamics (MD), semiempirical methods such as Austin Model 1 (AM1), Parametrization Model 3 (PM3), Parametrization Model 6 (PM6), Own N-layered Integrated molecular Orbital and molecular Mechanics (ONIOM) method, Natural Bond Orbital (NBO) analysis, Hartree–Fock (HF) and Density Functional Theory (DFT) [42].
To validate our experimental results described above, PM3 method was particularly adopted to investigate the encapsulation process of SULPA in β-CD cavity, since this method has been shown to be a powerful tool in the conformational study involving CD inclusion complexes [43].The molecular modeling analysis was performed on the optimized geometry of the inclusion complexes of the 1:1 stoichiometry with the lowest energy which were obtained during the simulation of the inclusion process in each one of the orientations considered, as detailed in “Molecular modeling” section.
In vacuum study
In the first approach of this study, calculations were carried out in vacuum.
Figure 8 depicts the graphical representation of the complexing energy (kcal/mol) changes involved during the inclusion passing and circling processes of β-CD with SULPA versus the displacement along the Z axis and the rotational angle, respectively for the two possible models A and B.
Passing process
Interestingly, in the passing process, complexation energy is the lowest −24.02 kcal/mol), when SULPA is precisely located at the optimum coordinate Z equal to 3 Å for the A complex. However, for the B complex the optimum coordinate Z was located at −3 Å with complexation energy of −22.61 kcal/mol (Fig. 8a). Consequently, we conclude that the A model, in which the SUPRA molecules penetrate into the CD cavity with dithiolane ring, is the most favorable.
Circling process
In the circling process the complexing energy is the lowest (−23.36 kcal/mol) when θ is equal to 80° for the A complex, but for the B complex, the optimum angle was found to be equal to 340° with complexing energy of −24.95 kcal/mol (Fig. 8-b).
One can notice that in both passing and circling processes, the curves show several local minima, where all complexing energies for the two orientations are negative, indicating that the inclusion process of the SULPA in the β-CD is thermodynamically favorable.
Effect of solvents
In the second approach, we undertook PM3 calculations in solutions in order to examine the influence of the solvation effect on the complexation energy, deformation energy and dipole moments of the inclusion complexes. Since in solution, results describe really the binding behavior of β-CD and SULPA than in vacuum; the key features in the complexation of β-CD with SULPA in presence and in absence of solvents are summarized in Table 1.
Like the results obtained in the vacuum, the values of complexation energy in solution listed in Table 1 indicate that the A model is the most favorable for complexation. In all cases, we notice that the energy difference between the two models using H2O, CH3CN and DMSO as solvents is almost the same (around 1.9 kcal/mol) in favor of A model.
Dipole changes
As depicted in Table 1, we noticed that both A complex and B complex showed dipole moments in acetonitrile, water and DMSO higher than the corresponding isolated SULPA molecule in the same medium which is an indication of the augmentation of the polarity and the intermolecular interactions in the solution [44].
In addition, the dipole moment of B complex is 1.97 D higher than that of A complex in vacuum. And it is the A complex which possesses the higher dipole moments values in solution and its polarity was dramatically increased when passing from vacuum(4.70 D) to solution as shown in Table 1. From these results, it can be concluded that the polarity of these inclusion complexes have a closed relation with the way in which the SULPA molecule is included in the β-CD molecule and with the medium in which the complexation was carried out.
Geometrical parameters and deformation energy during inclusion process
According to Pathigoolla et al. [45], the flexibility of guests and β-CD can favor interactions because they can modify their conformations to ensure a better inclusion and enhance the complexation entropy. The deformation energy for each component, the host or the guest molecules, throughout the formation of the complex, was defined as the difference between the single point energy of the component in the optimized complex and its energy when it is optimized in free form.
Calculations on the deformation energy in vacuum reported in Table 1 demonstrate that both β-CD and the SULPA molecule in the A model require more energy than that of the B model in order to adapt their structures for the formation of their complexes; the corresponding values are, respectively, 1.02 and −3.83 kcal/mol for β-CD and 1.15 and 0.91 kcal/mol for SULPA molecule respectively. These results are also confirmed with calculation carried out in solution with CH3CN, H2O and DMSO solvents. The Conformational flexibility of the guest and host structures are one of the important structural requirements for complexation process. This can be more supported by the geometric parameters (bond distances, bond angles and dihedral angles) of SULPA before and after inclusion in β-CD (Results are provided in the supplementary data).
It is clear that inside β-CD (in both models A and B), the SULPA molecule has completely changed its initial topology. The alteration was more significant in dihedral angles, particularly, in aliphatic chain of lipoyl moiety and in the dithiolane ring, which indicate that SULPA adapt a better conformation inside the host cavity to form a more stable inclusion complex.
Hydrogen bounding in the complex
From the above discussions on the PM3 optimized structures, we can confirm that in both models, the SULPA molecule is inserted in the hydrophobic cavity of β-CD. Further insight, reveals that there are intermolecular H-bonds in these structures (shown as black dashed line in Fig. 9); which have a crucial role in stabilization of complexes, in particular those having bond lengths ranging from 2.4 to 3.1 Å, just falling in the reported data [46], such as H bonds established between the oxygen atoms O(162) of amide groups H–N–C=O in SULPA molecule and hydrogen atoms H(141) and H(136) of secondary and primary hydroxyl groups of β-CD in A and B complexes respectively and hydrogen atoms H(185) of amide groups H–N–C=O in SULPA molecule with the oxygen atom O(78) of primaryry hydroxyl groups of β-CD. The insertion of SULPA into the hydrophobic cavity of β-CD changes the preference of hydrogen bridges and leads to the decrease of flexibility of the guest and increase of its stability.
DFT single point and Hartree–Fock calculations
The evidence for the formation of inclusion complex preferably in A model was proved by using a more accurate method. Thus, by proceeding with DFT single point calculations at the B3LYP/6-31 G(d) levels and Hartree–Fock HF/6-31G(d) to the PM3 optimized geometries, we can observe from calculation results summarized in Table 1, that in vacuum as in acetonitrile solution, the single point energy calculated by DFT and HF is lower in the A complex than that in the B complex. This provides important support to our above result and thus, predicts that the dithiolane ring has a preference to be embedded in cavity of the β-CD rather than the Arylsulfonamide moiety. Finally it is important to notice that in all cases, the results of both methods DFT and HF are converging and are in agreement with those obtained by the PM3 semi-empirical method in solutions.
ONIOM2 calculations
In order to further understand molecular recognition between β-CD and SULPA, we rationalized our study by adopting the ONIOM2 method. Since this hybrid method allows a high level calculation on just a small part of the critical molecular system and incorporate the effects of the remaining items at a lower level of theory, to give an expression of energy compatible with similar accuracy to a high calculation level on the complete system [47]. Within this approach, we submitted the host molecule β-CD to the low level of quantum calculations PM3 method, while the guest molecule SULPA was treated by the [(B3LYP/6-31G(d):PM3) high level of DFT theory and (RHF/6-31G(d):PM3). As can be seen in Table 2, we reported and compared the energetic values (EONIOM2) in vacuum and in acetonitrile for the two models A and B computed with ONIOM2 method, to those obtained from PM3 calculations. We found that ONIOM2 calculations confirm PM3 results. Indeed, both ONIOM2 (B3LYP/6-31G (d):PM3) and ONIOM2 (RHF/6-31G (d):PM3) allowed A model to be more favorable than B model by respectively 0.13, 0.26 kcal/mol in vacuum and 1.42, 1.75 kcal/mol in acetonitrile. We notice that the relative energy difference of the optimized complexes computed by the ONIOM2 method has almost the same order of magnitude than that obtained by PM3 method. Consequently, this is another proof of the tailoring and the effectiveness of the PM3 semi-empirical calculations in inclusion complexes.
Thermodynamic parameters of inclusion complexes
The statistical thermodynamic calculations carried out at 1 atm and 298.15 K by PM3, were also performed to confirm the completeness of our optimization. The thermodynamic parameters (enthalpy, entropy, free energy) of the guest, host and inclusion complexes are summarized also in Table 2. The obtained results demonstrate that 1:1 β-CD/SULPA complex is favored by a negative enthalpy change, suggesting that the complexation is exothermic and is an enthalpy-driven process in vacuum. Furthermore, the enthalpy change of complexes is more negative for A complex indicating a stronger interaction between β-CD and SULPA and showing that the A model is the most thermodynamically favorable. The thermal Gibbs free energy change ∆G is also more negative for A complex which implies that the inclusion process proceeded spontaneously at 298.15 K and the A complex is the most thermodynamically stable. Both enthalpy change ∆H and entropy change ∆S are negative, which indicate that the inclusion process is an exothermic and enthalpy controlled process [48]. The corresponding negative entropy change is assigned to a diminution of the degrees of freedom of the guest while the negative enthalpy change (∆H) arose from the Van-der-Waal’s interactions and H-bonding between the host and the guest which play an important role in the inclusion complexes.
Frontier molecular orbital (FMO) study
The charge transfer interaction occurs in CD molecular recognition phenomenon plays a crucial part in the chemical stability of the supramolecular self assembling system. The most important terms in this kind of interaction are contributed from the charge transfer between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), called frontier molecular orbitals (FMO), which are the most important orbitals in a molecule.
In simple molecule orbital theory approaches, the HOMO energy (EHOMO) is in relationship with the ionization potential (I) and LUMO energy (ELUMO) has been used to estimate the electron affinity (A) [49]. The energy gap (EHOMO–ELUMO) between HOMO and LUMO reflects the eventual charge transfer interaction within the molecule, which explains the chemical reactivity and stability of this molecule and also influence its biological activity.
So, a molecule with a large frontier orbital gap is less polarizable and is generally associated with a low chemical reactivity and high stability [50].
In our study, the HOMO–LUMO analysis (Fig. 10) has been carried out to elucidate information regarding the inclusion complexation between β-CD and SULPA. The calculation was conducted in vacuum and in acetonitrile using the PM3 method. According to the results reported in Table 2, the HOMO–LUMO energy gaps Δ(HOMO–LUMO) of the two complexes (A model and B model) are expected to be −6.61 and −6.66 eV in vacuum and −6.86 and −6.81 eV in acetonitrile, respectively. Consequently, we can conclude that the two complexes are highly stable [51] and this result is in good agreement with our other theoretical calculations.
Conclusions
In this study, the inclusion complex of β-CD with N-[(4-sulfonamidophenyl)ethyl]-5-(1,2-dithiolan-3-yl)pentanamide was successfully prepared by co-precipitation method and characterized in both the liquid phase and the solid state using (UV–Vis) and (FT-IR) spectroscopy. By Job method, and Benesi–Hildebrand equation, respectively, the complex was found to present a 1:1 stoichiometry and its formation constant (K) in solution was evaluated. Additionally, a molecular modeling investigation of the interaction between β-CD and SULPA was carried out using PM3, HF, DFT, ONIOM methods and NBO analysis, which allow us to define and analyze two possible orientations of the guest molecule inside the host cavity. The statistical thermodynamic calculations (ΔG, ΔH, ΔS) and HOMO, LUMO orbital investigations suggest that the inclusion complex β-CD/SULPA is enough stable and the simulation results indicate that the A complex is more favored than B complex in presence and in absence of solvent. In particular, it is important to note that in both vacuum and solution, the 1, 2-dithiolane ring in the molecule that is most sensitive to chemical or physical attacks was entrapped into the β-CD cavity and therefore was sufficiently well protected.
References
Maren, T.H.: Relations between structure and biological activity of sulfonamides. Ann. Rev. Pharmacol. Toxicol. 16, 309–327 (1976)
Weidle, U.H., Tiefenthaler, G., Georges, G.: Proteases as activators for cytotoxic prodrugs in antitumor therapy. Cancer Genomics Proteomics 11, 67–79 (2014)
Supuran, C.T., Scozzafava, A.: Carbonic anhydrase inhibitors. Curr. Med. Chem. Immunol. Endocr. Metab. Agents 1(37), 61–97 (2011)
Frost, S.C., McKenna, R. (eds.): Carbonic Anhydrase, Mechanism, Regulation, Links to Disease, and Industrial Applications. Springer, Dordrecht (2014)
Lipinski, C.A., Lombardo, F., Dominy, B.W., Feeney, P.J.: Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001)
Dahan, A., Hoffman, A.: Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J. Control. Release. 129, 1–10 (2008)
Savla, S., Surjusee, A., Rokade, V., Sawant, S., Kadu, P.: Approaches to improve solubility of poorly water soluble drugs. W J PP S 4, 610–626 (2015)
Torchilin, V.P.: Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release. 73, 137–172 (2001)
Saraf, S.A., Tripathi, G.K., Pandey, M., Yadav, P., Saraf, S.K.: Development of meloxicam formulation utilizing ternary complexation for solubility enhancement. Pak. J. Pharm. Sci. 24(4), 533–538 (2011)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cycloextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85, 1017–1025 (1996)
Pastorekova, S., Parkkila, S., Pastorek, J., Supuran, C.T.: Carbonic anhydrases, current state of the art, therapeutic applications and future prospects. J. Enzym. Inhib. Med. Chem. 19, 199–229 (2004)
Supuran, C.T.: Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 27, 759–772 (2012)
Aggarwal, M., Boone, C.D., Kondeti, B., McKenna, R.: Structural annotation of human carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 28, 267–277 (2013)
Hassan, I., Shajee, B., Waheed, A., Ahmad, F., Sly, W.S.: Structure, function and applications of carbonic anhydrase isozymes. Bioorg. Med. Chem. 21, 1570–1582 (2013)
Lock, F.E., McDonald, P.C., Lou, Y., Serrano, I., Chafe, S.C., Ostlund, C., Aparicio, S., Winum, J.Y., Supuran, C.T., Dedhar, S.: Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 32, 5210–5219 (2013)
Durgun, M., Turkmen, H., Ceruso, M., Supuran, C.T.: Synthesis of Schiff base derivatives of 4-(2-aminoethyl)-benzenesulfonamide with inhibitory activity against carbonic anhydrase isoforms I, II, IX and XII. Bioorg. Med. Chem. Lett. 25, 2377–2381 (2015)
Krall, N., Pretto, F., Neri, D.: A bivalent small molecule-drug conjugate directed against carbonic anhydrase IX can elicit complete tumour regression in mice. Chem. Sci. 5, 3640–3644 (2014)
Stiti, M., Cecchi, A., Rami, M., Abdaoui, M., Barragan-Montero, V., Scozzafava, A., Guari, Y., Winum, J.Y., Supuran, C.T.: Carbonic anhydrase inhibitor coated gold nanoparticles selectively inhibit the tumor-associated isoform IX over the cytosolic isozymes I and II. J. Am. Chem. Soc. 130, 16130 (2008)
Maeda, H., Onodera, T., Nakayama, H.: Inclusion complex of α lipoic acid and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 689(1–2), 201–206 (2010)
Zhang, S.J., Ge, Q.F., Guo, D.W., Hu, W.X., Liu, H.Z.: Synthesis and anticancer evaluation of α-lipoic acid derivatives. Bioorg. Med. Chem. Lett. 20, 3078–3083 (2010)
Hyperchem. Release 7.51 for windows. Hypercube Inc. (2002)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Jr. Montgomery, J.A., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J. C.T. Wallingford, Gaussian, Inc, (2009)
Yan, C.L., Li, X.H., Xiu, Z.L., Hao, C.: A quantum-mechanical study on the complexation of β-cyclodextrin with quercetin. J. Mol. Struct. (Theochem) 764, 95–100 (2006)
Tafazzoli, M.: M. hiasi, Structure and conformation of α, β and γ-cyclodextrin in solution, Theoretical approaches and experimental validation. Carbohydr. Polym. 78, 10–15 (2009)
Yan, C.L., Xiu, Z.L., Li, X.H., Hao, C.: Molecular modeling study of β-cyclodextrin complexes with (+)-catechin and (−)-epicatechin. J. Mol. Graph. Model. 26, 420–428 (2007)
Dapprich, S.: I. Koma´romi, K.S. Byun, K. Morokuma, M.J. Frisch, A new ONIOM implementation in Gaussian 98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM 1, 461–462 (1999)
Kuno, M., Hannongbua, S., Morokuma, K.: Theoretical investigation on nevirapine and HIV-1 reverse transcriptase binding site interaction, based on ONIOM method. Chem. Phys. Lett. 380, 456–463 (2003)
Siva, S., Thulasidhasan, J., Rajendiran, N.: Host-guest inclusion complex of propafenone hydrochloride with α- and β-cyclodextrins: spectral and molecular modeling studies. Spectrochim. Acta. A 115, 559–567 (2013)
Fisli, H., Bensouilah, N., Dhaoui, N., Abdaoui, M.: Effects of solvent, pH and β-cyclodextrin on the fluorescent behaviour of lomustine. J. Incl. Phenom. Macrocycl. Chem. 73, 369–376 (2012)
Mura, P.: Analytical techniques for characterization of cyclodextrin complexes in aqueous solution, a review. J. Pharm. Biomed. Anal. 101, 238–250 (2014)
Kadri, M., Dhaoui, N., Abdaoui, M., Winum, J.Y., Montero, J.L.: Inclusion complexes of 2-chloroethylnitrososulfamides (CENS) with β-cyclodextrin. Eur. J. Med. Chem. 39, 79–84 (2004)
Connors, K.A.: Binding Constants: The Measurement of Molecular Complex Stability, p. 143. Wiley, New York (1987)
Benesi, H.A., Hildebrand, J.H.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71, 2703–2707 (1949)
Szejtli, J.: Cyclodextrin Technology. Kluwer Academic Publishers, Dordrecht (1988)
Dhaoui, N., Fatfat, M., Abdaoui, M., Barragan-Montero, V.: Inclusion complexes of 2-chloroethyl nitrososulfamides CENS) in natural and modified β-cyclodextrins. Lett. Org. Chem. 6, 37–40 (2009)
Fisli, H., Bensouilah, N., Abdaoui, M.: Spectrofluorimetric determination of the antineoplastic agent lomustine based on the sensitizing effect of β-cyclodextrin. Luminescence 31, 871–880 (2016)
Bensouilah, N., Fisli, H., Dhaoui, N., Benali-Cherif, N., Abdaoui, M.: Solvent effects of N-nitroso, N-(2-chloroethyl)N′, N′-dibenzylsulfamid and its copper(II) and cobalt(II) complexes: fluorescence studies. Luminescence 28, 30–37 (2013)
Silverstein, R.M., Webster, F.X., Kiemle, D.: Spectrometric Identification of Organic Compounds, 7th edn. Wiley, New York (2006)
Carofiglio, T., Fornasier, R., Jicsinszky, L., Saielli, G., Tonellato, U., Vetta, R.: Capillary electrophoresis, ROESY NMR and molecular modelling study of the inclusion complex β-cyclodextrin/lipoic acid. Eur. J. Org. Chem. 2002, 1191–1196 (2002)
Breslow, R., Dong, S.D.: Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 98, 1997–2011 (1998)
Seridi, L., Boufelfel, A.: Molecular modeling study of Lamotrigine/β-cyclodextrin inclusion complex. J. Mol. Liq. 158, 151–158 (2011)
Rajendiran, N., Mohandoss, T., Venkatesh, G.: Investigation of inclusion complexes of sulfamerazine with α- and β-cyclodextrins, An experimental and theoretical study. Spectrochim. Acta A 124, 441–450 (2014)
Castro, R., Berardi, M.J., Cordova, E., de Olza, M.O., Kaifer, A.E., Evanseck, J.D.: Unexpected roles of guest polarizability and maximum hardness, and of host solvation in supramolecular inclusion complexes: a dual theoretical and experimental study. J. Am. Chem. Soc. 118, 10257–10268 (1996)
Pathigoolla, A., Sureshan, K.M.: Reverse-CD mimics with flexible linkages offer adaptable cavity sizes for guest encapsulation. Chem. Commun. 50, 317–319 (2014)
Rajendiran, N., Venkatesh, G., Mohandass, T.: Fabrication of 2D nanosheet through self assembly behavior of sulfamethoxypyridazine inclusion complexes with α- and β-cyclodextrins. Spectrochim. Acta A 123, 158–166 (2014)
Tschumper, G.S., Morokuma, K.: Gauging the applicability of ONIOM (MO/MO) methods to weak chemical interactions in large systems: hydrogen bonding in alcohol dimmers. J. Mol. Struct. TheoChem. 592, 137–147 (2002)
Bensouilah, N., Abdaoui, M.: Inclusion complex of N-nitroso, N-(2-chloroethyl) N′, N′-dibenzyl sulfamid with β-cyclodextrin, fluorescence and molecular modeling. C. R. Chim. 15, 1022–1036 (2012)
Pal, K., Chandra, F., Mallick, S., Koner, A.L.: Effect of solvents and cyclodextrin complexation on acid–base and photophysical properties of dapoxyldye. J. Photochem. Photobiol. A 306, 47–54 (2015)
Bani-Yaseen, A.D., Mo’ala, A.: Spectral, thermal, and molecular modeling studies on the encapsulation of selected sulfonamide drugs in β-cyclodextrin nano-cavity. Spectrochim. Acta A 131, 424–431 (2014)
Sayede, A.D., Ponchel, A., Filardo, G., Galia, A., Monflier, E.: J. Mol. Struct. THEOCHEM 777, 99–106 (2006)
Acknowledgments
This paper was supported in part by PNR (08/U24/2011). We acknowledge the Algerian Ministry of Higher Education and Scientific Research and General Direction of Scientific and Technologic research for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bouzit, H., Stiti, M. & Abdaoui, M. Spectroscopic and molecular modelling investigations of supramolecular complex of β-cyclodextrin with N-[(4-sulfonamidophenyl)ethyl]-5-(1,2-dithiolan-3-yl)pentanamide. J Incl Phenom Macrocycl Chem 86, 121–134 (2016). https://doi.org/10.1007/s10847-016-0647-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-016-0647-7