Abstract
Herbicides are used as management tools to improve habitat for native plants and animals, but their application may also have harmful effects on the native community. The federally threatened Oregon silverspot butterfly (Speyeria = Argynnis zerene hippolyta) resides in remnant native grasslands along the Pacific Northwest coast. However, like many grasslands, many of these areas have high incidences of invasive plants, such as false dandelion (Hypochaeris radicata) and velvet grass (Holcus lanatus). These and other invasive plants severely limit the abundance of the Oregon silverspot’s larval host plant, the early blue violet (Viola adunca). Selective herbicides, such as clopyralid and fluazifop-P-butyl, can reduce invasive plant abundance. However, non-target effects of these herbicides, and of adjuvants applied with these herbicides, on Oregon silverspots are unknown. In our study, we applied herbicides and adjuvants to host plants and Zerene silverspot (S. z. zerene) larvae, a subspecies closely related to Oregon silverspots. Responses in silverspot larvae measured in two experiments included survival, sex ratio, development time, mass, morphology, fecundity, and behavior. Our results suggest that negative effects of herbicides, clopyralid and fluazifop-P-butyl, and adjuvants, Agri-Dex® and Nu-Film®-IR, are limited. However, we detected weak effects from clopyralid and fluazifop-P-butyl with and without Agri-Dex® on larval and pupal development time and pupal mass.
Implications for insect conservation
Our study contributes to the growing literature on non-target effects of herbicides on butterflies, which suggests that butterfly responses are species- and chemical-specific. For Speyeria species, our results indicate that the risks posed by the herbicides we examined are low. In management settings where herbicides are used to combat invasive species posing a conservation threat to native communities, monitoring the direct and indirect effects of herbicides on Oregon silverspots or other Speyeria butterflies will shed additional light on the risk–benefit tradeoffs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive plants are a leading threat to at-risk butterfly populations (Keeler et al. 2006; Wagner and Van Driesche 2010; Florens et al. 2010; Hanula and Horn 2011; LaBar and Schultz 2012; Gallien et al. 2017; Augustine and Kingsolver 2017; Zhang and Miyashita 2018; Kral-O’Brien et al. 2018; Moroń et al. 2018; Bennion et al. 2020). In the United States, invasive plants are associated with the decline of 31 out of 33 butterflies currently listed as threatened or endangered under the U.S. Endangered Species Act, and 24 out of 26 recovery plans for these butterflies recommend invasive plant control (USFWS 2020a). Multiple management tools have been used to reduce invasive plant competitors in butterfly habitat, including mechanical methods (e.g., manual removal and mowing), chemical methods (e.g., herbicides), burning, and grazing by livestock (Huntzinger 2003; Vogel et al. 2007; Dennehy et al. 2011; Moranz et al. 2014). In cases where mechanical methods fail to combat invasive plants or when burning or grazing are not possible, selective herbicides targeting specific plants are a promising alternative (Dennehy et al. 2011; LaBar and Schultz 2012). However, herbicides, though formulated to kill plants, can still negatively impact butterflies and other insects (Eliyahu et al. 2003; Herbert et al. 2014; Saska et al. 2016; Pereira et al. 2018; Rainio et al. 2019).
Under laboratory and greenhouse conditions, several studies have documented direct effects of herbicides on butterflies (Kutlesa and Caveney 2001; Russell and Schultz 2010; Stark et al. 2012; Bohnenblust et al. 2013; Schultz et al. 2016). A number of studies have detected reduced larval survival (Kutlesa and Caveney 2001; Russell and Schultz 2010; Stark et al. 2012; Schultz et al. 2016) and, in one study, investigators observed reduced fecundity in females (Stark et al. 2012); either of these effects could be problematic for conserving populations of at-risk butterflies. Other life history variables, such as development time, mass, morphology, or behavior of individual butterflies, are also known to be impacted by herbicides (Russell and Schultz 2010; Stark et al. 2012; Bohnenblust et al. 2013; Schultz et al. 2016). For example, herbicides can accelerate larval and pupal development time (Russell and Schultz 2010), which can cause long-term impacts on butterfly populations, such as population asynchrony (Lingren et al. 1988; Jones and Aihara-Sasaki 2001). Although all these studies captured direct effects of herbicides on butterflies, which are best observed under controlled conditions, laboratory experiments are not able to document indirect costs or benefits of herbicides associated with changes to habitat conditions.
Under field conditions, few studies have documented direct and indirect effects of herbicides on insects (Agnello et al. 1986; Martínez et al. 2001; Watts et al. 2015; Pereira et al. 2018), with most having been focused on butterflies (Blake et al. 2011; LaBar and Schultz 2012; Glaeser and Schultz 2014; Schultz and Ferguson 2020). No studies have systematically detected a direct effect, positive or negative, on butterfly egg or larval survival in areas treated with herbicides (LaBar and Schultz 2012; Glaeser and Schultz 2014; Schultz and Ferguson 2020). However, one study documented an increase in butterfly abundance and diversity in herbicide-treated sites (Blake et al. 2011). Two studies examined butterfly residence time; one study did not detect an effect in sites treated with herbicides (Glaeser and Schultz 2014), while investigators in the other study observed reduced time spent in herbicide-treated areas (LaBar and Schultz 2012). In addition, all studies cited here documented the potential for indirect effects of herbicides on butterflies through their habitat. For example, a number of studies detected a decrease in invasive plant abundance (Blake et al. 2011; Glaeser and Schultz 2014; Schultz and Ferguson 2020), while investigators in one study observed an increase in native plant abundance and diversity (Blake et al. 2011), suggesting effective management of butterfly habitat. Despite their potential negative effects on butterflies, herbicides are one of the most promising management tools for maintaining suitable butterfly habitat (Dennehy et al. 2011; LaBar and Schultz 2012).
To evaluate the use of herbicides for butterfly conservation, we undertook a controlled experiment in a greenhouse setting to assess their direct non-target effects. We used the Oregon silverspot (Speyeria zerene hippolyta) system to evaluate effects of herbicides on butterflies. A recent genomic study suggests that Speyeria be regarded as a subgenus of Argynnis rather than a separate genus (Zhang et al. 2020). For the sake of consistency with other ecological and conservation-related studies as well as usage by federal agencies, such as the U.S. Fish and Wildlife Service (USFWS), and for ease of adoption by practitioners in insect conservation, we used the name Speyeria in our study. The Oregon silverspot is a federally threatened butterfly whose survival depends on habitat restoration (McCorkle and Hammond 1988; Bierzychudek and Warner 2015). A reliance on habitat restoration is common for many declining butterflies (Schultz et al. 2008; Wagner and Van Driesche 2010), including two other U.S. federally listed S. zerene subspecies: the Behren’s silverspot (S. z. behrensii; endangered) and Myrtle’s silverspot (S. z. myrtleae; endangered; Hammond and McCorkle 1983; Sims 2017). Oregon silverspots and other federally listed S. zerene subspecies mostly reside in fragmented coastal grasslands from which it is particularly difficult to successfully remove invasive plants using non-chemical methods (USFWS 2009, 2016; Silvernail 2017). We conducted a greenhouse study to assess direct non-target effects of a forb-specific herbicide and a grass-specific herbicide. We viewed this greenhouse study as an important precursor to field studies that will be able to evaluate both direct and indirect effects of herbicides on the butterfly population. Most previous studies evaluating the effects of herbicides on butterflies documented the effects of grass-specific herbicides; however, because managers would like to control for both invasive grasses and forbs, we documented the effects of each type of herbicide. In addition, to our knowledge, no prior studies have systematically examined effects of any herbicide on members of the genus Speyeria. As such, our results are applicable to other at-risk Speyeria butterflies, including the callippe silverspot (S. callippe callippe; federally endangered), Great Basin silverspot (S. nokomis nokomis; under federal review), and regal fritillary (S. idalia; under federal review).
We evaluated the effects of clopyralid and fluazifop-P-butyl on a surrogate subspecies, the Zerene silverspot (S. z. zerene), which is in the same species complex as the Oregon silverspot (McHugh et al. 2013; De Moya et al. 2017; Warren et al. 2017; Pelham 2021). Prior to this study, USFWS was aware of the potential value of using clopyralid and fluazifop-P-butyl in combination with either the Agri-Dex® or Nu-Film®-IR adjuvants, particularly in spring and fall, as management tools for habitat occupied by the Oregon silverspot, but lacked sufficient information regarding potential impacts for the butterfly (R. G. Chuck pers. comm., USFWS). In two experiments, we measured survival, sex ratio, development time, mass, morphology, fecundity, and behavior of the Zerene silverspot, a suitable surrogate subspecies, in response to herbicide applications.
Methods
Study species and system
Oregon silverspots reside in remnant native grasslands along the coast of Oregon and Northern California (USFWS 2020b). These butterflies are univoltine and can be seen flying from July through September (McCorkle and Hammond 1988; James and Nunnallee 2011; Pyle and LaBar 2018). During that time, mating occurs shortly after female eclosion, and females oviposit within or adjacent to areas containing early blue violets. After approximately two weeks, eggs hatch and newly hatched larvae eat their eggshells and enter diapause, overwintering in leaf litter as first instar larvae. Larvae end diapause in April or May when their host plants begin emerging from the ground. Larvae feed on host plants until pupating in June. Adults emerge in July through September.
We used the Zerene silverspot as a surrogate subspecies for the Oregon silverspot to test for the potential consequences of using two herbicides, clopyralid and fluazifop-P-butyl, each applied with one of two adjuvants, Agri-Dex® and Nu-Film®-IR, in habitat occupied by Oregon silverspots. The Zerene silverspot is a common subspecies in the same species complex as the Oregon silverspot (McHugh et al. 2013; De Moya et al. 2017; Warren et al. 2017; Pelham 2021). Unlike Oregon silverspots, Zerene silverspots reside in montane grasslands in the southern Cascade Range (James and Nunnallee 2011; Pyle and LaBar 2018). Despite having a different distribution, the primary life history difference between Zerene and Oregon silverspots is the presence of a reproductive diapause. Reproductive diapause, a period after mating and before active oviposition, is common in Speyeria (Kopper et al. 2001; James 2008; James and Nunnallee 2011; James and Pelham 2011; Sims and Shapiro 2014). Zerene silverspots and other S. zerene subspecies from low to mid-elevations typically have a reproductive diapause, while subspecies from higher elevations or coastal areas, like Oregon silverspots, appear to either lack, or have a reduced, reproductive diapause (Sims 1984, 2017; McCorkle and Hammond 1988; Sims and Shapiro 2016). Another difference between the two S. zerene subspecies is that the Oregon silverspot experiences an extended larval development period, which seems to be asynchronous (McCorkle and Hammond 1988). Otherwise, Zerene and Oregon silverspots share similar life histories, are univoltine, overwinter as first instar larvae, and use early blue violets as a larval food source.
Chemicals
We tested four chemicals on the Zerene silverspot: two herbicides, clopyralid and fluazifop-P-butyl, and two adjuvants, Agri-Dex® and Nu-Film®-IR. Clopyralid is used to reduce annual and perennial broadleaf weeds, such as false dandelion (Hypochaeris radicata), yellow starthistle (Centaurea solstitialis), spotted knapweed (C. stoebe), and honey mesquite (Prosopis glandulosa; Morghan et al. 2003; Ansley and Castellano 2006; Silvernail 2017; MacDonald et al. 2019). This broadleaf-specific herbicide mimics the plant growth hormone auxin, causing uncontrolled and disorganized growth leading to death (Sterling and Hall 1997; Tu et al. 2001). Fluazifop-P-butyl is used to reduce tall nonnative grasses, such as velvet grass (Holcus lanatus), tall oatgrass (Arrhenatherum elatius), and tall fescue (Festuca arundinacea), while having a limited impact on native bunchgrasses such as Roemer’s fescue (F. roemeri) and red fescue (F. rubra; Silvernail 2017; Bennion et al. 2020), which are bunchgrasses found in Oregon silverspot habitat. This grass-specific herbicide inhibits acetyl-coenzyme A carboxylase, thus disrupting lipid synthesis, cell membrane formation, and plant growth in grasses with intercalary meristem growth (Walker et al. 1988; Luo et al. 2004). Both clopyralid and fluazifop-P-butyl have limited effects on the visual appearance of early blue violets. Clopyralid can increase stem height and curl leaves, while fluazifop-P-butyl does not have obvious effects on the appearance of host plants (Silvernail 2017).
We used the following herbicide formulations: clopyralid as Stinger® (40.9% clopyralid, Dow AgroSciences LLC, Indianapolis, Indiana) and fluazifop-P-butyl as Fusilade® DX (24.5% fluazifop-P-butyl, Syngenta Crop Protection, LLC, Greensboro, North Carolina). Herbicides are applied with an adjuvant or “spreader-sticker” that is designed to increase the efficacy of the herbicide (Gauvrit and Cabanne 1993). We used the following adjuvant formulations: Agri-Dex® (99% heavy range paraffinic oil, polyol fatty acid esters, and polyethoxylated derivatives thereof, Helena Chemical Company, Collierville, Tennessee) and Nu-Film®-IR (96% poly-1-p-menthene, Miller Chemical and Fertilizer Corporation, LLC, Hannover, Pennsylvania). Agri-Dex® (hereafter Agri-Dex) is used in coastal grassland restoration in Oregon (Silvernail 2017), while Nu-Film®-IR (hereafter Nu-Film) is used broadly in grassland restoration in the Pacific Northwest (Dennehy et al. 2011; Bennion et al. 2020).
Herbicide exposure experiment
To test for effects of herbicides and adjuvants on the Zerene silverspot, we conducted an experiment to measure responses of post-diapause larvae treated with chemicals and reared on early blue violets treated with chemicals. Treatments included distilled water for untreated (U), Agri-Dex (A), Nu-Film (N), clopyralid (C), clopyralid with Agri-Dex (CA), clopyralid with Nu-Film (CN), fluazifop-P-butyl (F), fluazifop-P-butyl with Agri-Dex (FA), and fluazifop-P-butyl with Nu-Film (FN). Treatments with one chemical (A, N, C, and F) allowed us to test for effects between herbicides and adjuvants; however, in management, chemicals are applied as a mixture of herbicide, adjuvant, and water. To isolate treatment effects from other factors affecting responses, we conducted this experiment in a single bay in the greenhouse at Washington State University (WSU) Vancouver.
We administered treatments to post-diapause larvae and adjacent host plants, which followed methods from Russell and Schultz (2010) and Schultz et al. (2016). Host plants were supplied by two Oregon nurseries and larvae came from females belonging to a single population in an Oregon montane grassland (Supplemental Material S1). We applied treatments to 360 larvae (40 larvae per each of the nine treatments) on three dates based on when larvae reached second instar. We treated 180 second instar larvae on May 31, 2018 (20 larvae per treatment), 90 on June 2, 2018 (10 larvae per treatment), and 72 on June 6, 2018 (8 larvae per treatment). In addition, we treated 18 first instar larvae on June 6, 2018 (2 larvae per treatment). Larvae were randomly assigned to treatments. We applied treatments using an R&D Precision CO2 powered (276 kPa) backpack sprayer (R&D Sprayers, Opelousas, Louisiana) with a handheld wand and flat fan 8002VS nozzle. We used the manufacturer’s recommended field rates of 4.7 mL Stinger®, 12.4 mL Fusilade® DX, 25.0 mL Agri-Dex®, and 2.99 mL Nu-Film®-IR per 1000 mL for each mixture. We applied distilled water to larvae and plants assigned to the U treatment prior to using the equipment to administer other treatments. To administer the spray, we held the nozzle 1 m above the larvae and host plants, making a single overspray pass with the appointed treatment at 1.6 s/m2. To prevent cross-contamination, we bottom-watered host plants for the duration of the experiment.
Larvae were reared on host plants subjected to the same treatment as the larvae. We transferred larvae to other treated host plants when current host plant material was low or depleted. When adults eclosed, we marked them with unique color codes on their ventral hindwings and placed them in netted enclosures in the greenhouse. Adult enclosures contained 1–6 individuals belonging to the same sex and treatment with Gatorade® provided as an artificial nectar source (Russell and Schultz 2010; Schultz et al. 2016). We mated adults from the same treatment by introducing males to females in netted enclosures. After mating, we transferred each female to a paper bag with two leaves from host plants in the same treatment and strips of paper as a medium for oviposition (Anderson et al. 2010).
We recorded larval and pupal survival, larval and pupal development time, pupal and adult mass, adult morphology, sex, and lifetime fecundity of individual butterflies who were treated with chemicals as larvae (Doll et al. 2021). Individuals were monitored daily to record survival. Development time was recorded from the larval to pupal stages and from the pupal to adult stages. We weighed pupae 24 h after pupation. Adults were weighed and photographed for morphometric analysis. We measured abdomen length and width, left forewing length, and left hindwing area using ImageJ (Rasband 2018). Finally, we collected eggs from females every other day to record lifetime fecundity and recorded offspring survival and development time from eggs to pre-diapause larvae daily (Doll et al. 2021).
Oviposition behavior experiment
To test for an effect of herbicides and adjuvants on Zerene silverspot oviposition behavior, we conducted an experiment to measure egg-laying behavior on early blue violets treated with chemicals. Because clopyralid’s half-life in soils averages 1–2 months, an application in spring will potentially leave residual clopyralid on host plants when females oviposit in summer (Tu et al. 2001; Dow 2003). However, because fluazifop-P-butyl’s half-life in soils averages 1–2 weeks, an application in spring will likely not leave residual fluazifop-P-butyl in summer (Tu et al. 2001; Durkin 2014). Therefore, in this experiment, treatments included untreated (U), clopyralid with Agri-Dex (CA), and clopyralid with Nu-Film (CN). Treatments did not include clopyralid without an adjuvant because field protocols always include an adjuvant, and the goal of this experiment was to mimic field-based protocols which might be experienced by ovipositing butterflies in the wild. For this experiment, we propagated host plants and collected females from a single population in an Oregon montane grassland (Supplemental Material S2). To isolate treatment effects from other factors affecting oviposition behavior, we conducted this experiment in a single bay in the greenhouse at WSU Vancouver.
We followed methods from Glaeser and Schultz (2014) when observing oviposition behavior, which is based on methods from Singer (1982) and Singer et al. (1992). We measured post-alighting oviposition preference in staged encounters where females “accepted” an early blue violet by pressing their extruded ovipositor against the host plant and were removed before laying an egg (Singer 1982; Singer et al. 1992; Glaeser and Schultz 2014; Buckingham et al. 2016). We conducted observations between 10:30 AM and 3:30 PM. To conduct observations, we placed 25 females on five randomly selected host plants from each treatment for 5 min or until they displayed oviposition behavior (Doll et al. 2021). If females displayed oviposition behavior, we removed them for a minimum of 5 min before placing them on another host plant. In the absence of direct sunlight, we used overhead grow lights to simulate sunlight.
Statistical analyses
We conducted all analyses using R 4.0.2 (R Core Team 2020) using R packages “MASS” (Venables and Ripley 2002) for principal component analyses (PCA) and “lme4” (Bates et al. 2015) for all other analyses. To evaluate treatment effects on survival, development time, mass, fecundity, and oviposition behavior, we used statistical models and included treatments—with U as the baseline—and other experimental factors where appropriate. After checking to make sure all model assumptions were met, we made inference about treatment effects based on the size of treatment coefficients and whether the 95% confidence intervals on coefficients excluded zero. Finally, we calculated a binomial proportion and confidence interval around the estimated proportion to determine whether there was an even sex ratio for each treatment, and we used a PCA to explore adult morphology.
Herbicide exposure experiment
We analyzed treatment effects on survival from (1) post-diapause larvae to pupae (n = 358) and from (2) pupae to adults (n = 301) using two binomial generalized linear models (GLMs), with logit link functions. Prior to these analyses, we excluded two individuals due to death from mishandling larvae. For the analysis of larval survival, explanatory variables included treatment (U, A, N, C, CA, CN, F, FA, and FN) as well as other factors that might influence the outcome—the categorical variable of treatment date (May 31, June 2, and June 6, 2018), larval mass (g), and the interaction between treatment date and larval mass. However, for the analysis of pupal survival, only treatment was used as an explanatory variable because there were few observations of mortality in the pupal stage. Finally, we calculated binomial proportions along with confidence intervals to determine whether the sex ratio of adults was different from 1:1 for each treatment.
We assessed treatment effects on development time (days) from (1) post-diapause larvae to pupae and from (2) pupae to adults using two linear models (LMs). To account for sex differences, we excluded individuals that had an unknown sex or that failed to survive to the adult stage (n = 287). Explanatory variables included treatment, treatment date, larval mass, the interaction between treatment date and larval mass, sex, and the interaction between treatment date and sex. We evaluated treatment effects on mass (g) of (1) pupae and (2) adults using two LMs. Explanatory variables included treatment, treatment date, larval mass, the interaction between treatment date and larval mass, sex, and the interaction between treatment date and sex.
We explored female and male morphology individually using two PCAs with the following morphological measurements: abdomen length (mm), abdomen width (mm), forewing length (mm), and hindwing area (mm2). Prior to these analyses, we removed four females and two males with unmeasurable abdomens or wing deformities, three females with missing data values, and one male outlier with undue influence. Finally, we retained enough principal components to explain at least 80% of the variance for each PCA.
We analyzed treatment effects on lifetime fecundity of females that successfully mated with males in the same treatment. To evaluate fecundity of mated females, we used a negative binomial GLM with a log link function. The negative binomial GLM was used instead of the Poisson because of overdispersion. For this analysis, only treatment was included as an explanatory variable because of the low sample size of mated females.
We assessed treatment effects on survival and development time from eggs to pre-diapause larvae for the offspring produced by mated females. To examine offspring survival to pre-diapause larvae (n = 7504), we used a binomial generalized linear mixed model (GLMM) with a logit link function. In addition, to analyze offspring development time to pre-diapause larvae (n = 5470), we used a linear mixed model with an identity link function. For both analyses, we included treatment as a fixed effect, while the female that produced the offspring (ID) was applied as a random effect to account for heterogeneity in offspring characteristics across females (Bolker et al. 2009).
Oviposition behavior experiment
We examined treatment effects on oviposition behavior of Zerene silverspot females on treated early blue violets from the “Oviposition Behavior” experiment. To assess whether oviposition behavior was displayed or not (n = 375), we used a binomial GLMM with a logit link function. For this analysis, treatment (U, CA, and CN) and grow light (On and Off) were included as fixed effects, while female ID was applied as a random effect to account for repeated measures of individuals (Bolker et al. 2009).
Results
Herbicide exposure experiment
Larval and pupal survival and sex ratio
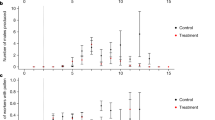
We did not detect negative treatment effects on larval survival to the pupal stage; however, we detected positive effects from treatments A and F. The 95% confidence intervals for the log odds of the effects of A and F, compared to U, excluded zero (Table 1). Estimates of larvae treated with A and F had a survival rate of 92.6% (95% CI = 80.1, 97.5) and 92.4% (95% CI = 79.8, 97.4), while untreated larvae had a survival rate of 75.9% (95% CI = 58.1, 87.7; Fig. 1a). Similar to larval survival, we did not detect treatment effects on pupal survival to the adult stage (Fig. 1b). None of the log odds of treatment effects, compared to U, had a 95% confidence interval that excluded zero (Table 1). Despite undetectable treatment effects, none of the untreated individuals died as pupae, whereas some fraction of treated individuals died in every treatment category. Finally, we found no evidence that any treatment resulted in a sex ratio different from 1:1 (Table S1).
Mean percent of a larval survival to pupation and b pupal survival to eclosion with 95% confidence intervals estimated by generalized linear models per treatment: U (n = 38 larvae and 28 pupae), A (n = 40 larvae and 34 pupae), N (n = 40 larvae and 33 pupae), C (n = 40 larvae and 35 pupae), CA (n = 40 larvae and 31 pupae), CN (n = 40 larvae and 32 pupae), F (n = 40 larvae and 34 pupae), FA (n = 40 larvae and 30 pupae), and FN (n = 40 larvae and 30 pupae)
Larval and pupal development time
We detected negative effects from treatments C and CA on larval development time to the pupal stage. The 95% confidence intervals for the coefficients of the effects of C and CA, compared to U, excluded zero (Table 2). Estimates of females treated with C and CA developed to pupae in 50.9 days (95% CI = 49.7, 52.0) and 50.8 days (95% CI = 49.5, 52.0), while untreated females developed in 53.7 days (95% CI = 52.4, 55.0; Fig. 2a). Estimates of males treated with C and CA developed to pupae in 45.7 days (95% CI = 44.5, 46.9) and 45.6 days (95% CI = 44.3, 46.9), while untreated males developed in 48.5 days (95% CI = 47.2, 49.8; Fig. 2a). For pupal development time to the adult stage, we detected negative effects from treatments CA and FA. The 95% confidence intervals for the coefficients of the effects of CA and FA, compared to U, excluded zero (Table 2). Estimates of females treated with CA and FA developed to adults in 15.2 days (95% CI = 14.8, 15.5) and 15.1 days (95% CI = 14.8 15.5), while untreated females developed in 15.7 days (95% CI = 15.4, 16.1; Fig. 2b). Estimates of males treated with CA and FA developed in 13.8 days (95% CI = 13.4, 14.2) and 13.8 days (95% CI = 13.4, 14.1), while untreated males developed in 14.4 days (95% CI = 14.0, 14.7; Fig. 2b).
Mean days of a larval development to pupation and b pupal development to eclosion with 95% confidence intervals estimated by linear models by sex per treatment: U (n = 10 females and 18 males), A (n = 13 females and 21 males), N (n = 20 females and 13 males), C (n = 18 females and 17 males), CA (n = 15 females and 16 males), CN (n = 17 females and 15 males), F (n = 21 females and 13 males), FA (n = 15 females and 15 males), and FN (n = 17 females and 13 males)
Pupal and adult mass
We detected negative effects from treatments F and FA on mass in the pupal stage. The 95% confidence intervals for the coefficients of the effects of F and FA, compared to U, excluded zero (Table 3). Estimates of females treated with F and FA weighed 0.584 g (95% CI = 0.567, 0.602) and 0.578 g (95% CI = 0.559, 0.596) in the pupal stage, while untreated females weighed 0.612 g (95% CI = 0.592, 0.632; Fig. 3a), a reduction in mass of 4.6% and 5.6%, respectively. Estimates of males treated with F and FA weighed 0.457 g (95% CI = 0.438, 0.476) and 0.450 g (95% CI = 0.431, 0.469) in the pupal stage, while untreated males weighed 0.485 g (95% CI = 0.465, 0.504; Fig. 3a), a reduction in mass of 5.7% and 7.2%, respectively. For mass in the adult stage, we found no evidence of treatment effects (Fig. 3b). None of the coefficients of the treatment effects, compared to U, had a 95% confidence interval that excluded zero (Table 3).
Mean mass of a pupae and b adults with 95% confidence intervals estimated by linear models by sex per treatment: U (n = 10 females and 18 males), A (n = 13 females and 21 males), N (n = 20 females and 13 males), C (n = 18 females and 17 males), CA (n = 15 females and 16 males), CN (n = 17 females and 15 males), F (n = 21 females and 13 males), FA (n = 15 females and 15 males), and FN (n = 17 females and 13 males)
Adult morphology
For female and male morphology, we found that all herbicide and adjuvant treatments had overlapping 95% confidence ellipses with U (Fig. S1). For each analysis, we retained the first two principal components (PC1 and PC2) to explain approximately 80% of the variance for female and male morphology (Table S2). Finally, across both analyses, we found forewing length and hindwing area to be most correlated with PC1, while abdomen length and width were most correlated with PC2 (Table S2).
Female fecundity
For females that successfully mated, we did not detect treatment effects on fecundity (Fig. S2). None of the log odds of the treatment effects, compared to U, had a 95% confidence interval that excluded zero (Table S3).
Offspring survival and development time
We did not detect treatment effects on offspring survival to the pre-diapause larval stage (Fig. S3a). None of the log odds of the treatment effects, compared to U, had a 95% confidence interval that excluded zero (Table S4). Similar to offspring survival, we did not detect negative treatment effects on offspring development time to the pre-diapause larval stage; however, we detected a positive effect from treatments CA and CN. The 95% confidence interval for the coefficient of the effect of CA and CN, compared to U, excluded zero (Table S4). Estimates of offspring produced by females treated with CA developed to pre-diapause larvae in 12.0 days (95% CI = 11.3, 12.8) and 11.6 days (95% CI = 10.9, 12.3), respectively, while offspring produced by untreated females developed in 10.9 days (95% CI = 10.2, 11.7; Fig. S3b), a delay of 10.1% and 6.4%, respectively.
Oviposition behavior experiment
We did not detect treatment effects on oviposition behavior of Zerene silverspot females from the Oviposition Behavior experiment (Fig. S4). None of the log odds of the treatment effects, compared to U, had a 95% confidence interval that excluded zero (Table S5).
Discussion
Our results indicate two herbicides, clopyralid and fluazifop-P-butyl, and two adjuvants, Agri-Dex® and Nu-Film®-IR, have few detectable impacts on the Zerene silverspot, a surrogate subspecies for the Oregon silverspot. We found no evidence of negative treatment effects on larval and pupal survival, sex ratio, adult mass, or oviposition behavior. However, we detected small effects from some treatments on larval and pupal development time and pupal mass. Few Lepidoptera studies have compared direct non-target effects of fluazifop-P-butyl and Nu-Film®-IR (Russell and Schultz 2010; Schultz et al. 2016), while prior studies have not examined clopyralid or Agri-Dex® on Lepidoptera. Our results are consistent with these prior studies, which found limited detectable effects of fluazifop-P-butyl and Nu-Film®-IR on butterflies in the Pieris, Icaricia, and Euphydryas genera (Russell and Schultz 2010; Schultz et al. 2016).
Herbicides and adjuvants influenced development time in Zerene silverspots. We detected negative treatment effects from clopyralid both with and without Agri-Dex® on larval development time to pupation, and for both herbicides combined with Agri-Dex® on pupal development time to eclosion. In the larval stage, males developed faster than females, as expected in Lepidoptera (Fagerström and Wiklund 1982; Forsberg and Wiklund 1988; Fischer and Fiedler 2000); however, individuals treated with clopyralid both with and without Agri-Dex® experienced a faster larval development rate relative to untreated individuals (Fig. 2a). Similarly, in the pupal stage, males developed faster than females, but individuals treated with either herbicide combined with Agri-Dex® experienced a faster pupal development rate relative to untreated individuals. Stress can accelerate or decelerate development time in Lepidoptera (Horner et al. 2003; Walker et al. 2007; Russell and Schultz 2010; Huang et al. 2012; Hahn et al. 2014; Bush et al. 2018; Rabelo et al. 2020). For example, cotton leafworm (Spodoptera litura) larvae developed 14–19% faster when reared on food containing 25–50 mg/kg concentrations of copper compared to those reared on a non-copper diet, but larvae with 100–200 mg/kg concentrations of copper were not affected (Huang et al. 2012). In contrast, cotton leafworm larvae developed 37% and 18% slower when reared on food amended with a Bacillus thuringiensis pesticide, DiPel 2X, and Cry1Ac toxin, respectively, than those on an untreated diet (Walker et al. 2007). One obvious explanation for the inconsistencies in development time in these studies is a chemical-specific response. In our study, we suspect that the higher nitrogen content in early blue violets treated with clopyralid (Fig. S5) accelerated development time, as shown in previous Lepidoptera studies (Lavoie and Oberhauser 2004; Kerpel et al. 2006). For example, larvae of a neotropical butterfly (Heliconius erato phyllis) developed 10% faster when reared on passionflower (Passiflora suberosa) shoots from soils with 150–300 mg L−1 of nitrogen than those without a nitrogen addition (Kerpel et al. 2006).
Altered development time can cause long-term impacts on butterfly populations, such as population asynchrony (Lingren et al. 1988; Jones and Aihara-Sasaki 2001). We infer that, because herbicides are not homogenously applied to larvae and development time differs between treated and untreated individuals, the result may be increased asynchrony in adult emergence. Population asynchrony can exacerbate any Allee effects, such as lowered mating success, already occurring in small populations (Groom 1998; Calabrese and Fagan 2004; Gascoigne et al. 2009). There are only seven extant populations of the Oregon silverspot (including recent reintroduction efforts), with only one population greater than 200 butterflies in 2019 and four with less than 50 butterflies (USFWS 2020b). In laboratory-derived life tables for the koa seedworm (Cryptophlebia illepida), a 4- and 6-day delay of mating caused a decrease in population growth rate and resulted in asynchronous population cycling compared to a 1-day delay of mating (Jones and Aihara-Sasaki 2001). Mating success could be low in small populations, like Oregon silverspots, because encountering another adult is less frequent, which could be further reduced by population asynchrony. Under population asynchrony, females would encounter more older males, which has been found to decrease fecundity in Lepidoptera (Rogers and Marti 1997; Huang and Subramanyam 2003; Michereff et al. 2004; Dhillon et al. 2019). Finally, we observed that worn or aged males were rejected by females (C. F. Doll and K. C. King pers. obs., WSU), which could further lower mating success with increasing population asynchrony.
Herbicides and adjuvants affected Zerene silverspot mass in the pupal stage, but mass in the adult stage was not altered. We detected negative treatment effects from fluazifop-P-butyl both with and without Agri-Dex® on pupal mass. In the pupal stage, males weighed less than females, as expected in Lepidoptera (Fagerström and Wiklund 1982; Wiklund and Forsberg 1991); however, individuals treated with fluazifop-P-butyl both with and without Agri-Dex® were lighter than untreated individuals. Like development time, stress can affect mass in Lepidoptera (Horner et al. 2003; Walker et al. 2007; Russell and Schultz 2010; Huang et al. 2012; Stark et al. 2012; Hahn et al. 2014; Ali et al. 2019; Rabelo et al. 2020). For example, cotton leafworm larvae weighed 16–24% less when reared on food containing 25–200 mg/kg concentrations of copper compared to an untreated diet (Huang et al. 2012). In contrast, Behr’s metalmark (Apodemia virgulti) pupae weighed 15% more when treated with the herbicide triclopyr than did untreated individuals (Stark et al. 2012). Size reduction in Lepidoptera is associated with reduced fitness, reduced fecundity in females, and reduced reproductive success in males (Wiklund and Kaitala 1995; Jiménez-Pérez and Wang 2004; Calvo and Molina 2005; Boggs and Freeman 2005). Most studies show small-bodied females have reduced fecundity, but the effect of small-bodied males on female fecundity is unclear (Wiklund and Kaitala 1995; Jiménez-Pérez and Wang 2004; Calvo and Molina 2005; Boggs and Freeman 2005). Finally, although we detected effects from fluazifop-P-butyl both with and without Agri-Dex® on pupal mass, we did not detect treatment effects on adult mass. Similarly, triclopyr altered Behr’s metalmark mass in the pupal stage, but mass in the adult stage was not affected (Stark et al. 2012).
Our results indicate herbicides and adjuvants have negligible effects on Zerene silverspot survival, while in some cases treated individuals fared better than untreated ones. These results are consistent with other studies in which an effect of fluazifop-P-butyl and Nu-Film®-IR was not detected on survival to diapause across three checkerspot species (Euphydryas colon, E. editha, and E. phaeton), each reared on two host plant species (Schultz et al. 2016). In addition, an effect of fluazifop-P-butyl and the adjuvant Preference® was not detected on survival to eclosion in the Puget blue (Icaricia icarioides blackmorei), but reduced survival was detected in the cabbage white (Pieris rapae; Russell and Schultz 2010). In a study involving two herbicides and four pesticides, survival to pupation in monarch (Danaus plexippus) larvae reared on treated host plants was not different than survival of larvae reared on untreated host plants; however, reduced survival was detected from a high concentration of the pesticide azoxystrobin (Olaya-Arenas et al. 2020). Similarly, an effect of the herbicide dicamba on survival to pupation in the corn earworm (Helicoverpa zea) and painted lady (Vanessa cardui) was not detected (Bohnenblust et al. 2013). While herbicides and other pesticides can be harmful to insects (Eliyahu et al. 2003; Herbert et al. 2014; Saska et al. 2016; Pereira et al. 2018; Rainio et al. 2019), it appears survival in Lepidoptera is chemical- and species-specific.
In addition to detecting negligible effects of herbicides and adjuvants on Zerene silverspot survival, we found no evidence of treatment effects on other components associated with fecundity. Size in Lepidoptera can be a predictor for fecundity in females (Wiklund and Kaitala 1995; Jiménez-Pérez and Wang 2004; Calvo and Molina 2005; Boggs and Freeman 2005). For example, smaller-sized females were less fecund than larger females in the blueberry lappet (Streblote panda; Calvo and Molina 2005). Thus, because we did not detect treatment effects on adult mass, we suspect herbicides and adjuvants will not have a detectable impact on female fecundity. However, we only had a small sample of observations for estimating effects on fecundity. In addition, we did not detect treatment effects of early blue violets treated with clopyralid and adjuvants on Zerene silverspot oviposition behavior. Our results are consistent with other studies in which host plants treated with fluazifop-P-butyl and Nu-Film®-IR did not result in lower acceptance rates by silvery blue (Glaucopsyche lygdamus) females in sequential oviposition choice trials (Glaeser and Schultz 2014).
We note several limitations of our study. One limitation was associated with a low sample size of mated females in the “Herbicide Exposure” experiment, which limited inference about the effects of treatments on fecundity. Second, we used the Zerene silverspot as a surrogate subspecies for the Oregon silverspot. This subspecies is closely related to the Oregon silverspot, sharing many life history characteristics, but the limitation of a surrogate species for fully understanding important biological and ecological aspects of a rare species is well documented (Banks et al. 2010; Henry et al. 2019). For example, an ecologically similar species, the Appalachian brown (Lethe appalachia), which shares the habitat of the federally endangered Saint Francis’ satyr (Neonympha mitchellii francisci), was not a suitable surrogate species because their differences in resource use, habitat selection, behavior, and survival produced differences in relative abundances following restoration (Henry et al. 2019). Third, a greenhouse study, such as this one, only provides a quantitative estimate of direct effects on butterflies in the wild. Our study cannot estimate indirect effects of these management actions on butterflies, such as changes in the plant community (Pearson et al. 2016; Bennion et al. 2020). While we acknowledge these limitations, we believe working with a closely related species under greenhouse conditions is an important step in testing novel and potential management strategies that may pose risks to a declining species.
Our study provides evidence of limited direct effects of herbicides and adjuvants on Zerene silverspots. Field studies are needed to fully evaluate direct and indirect effects of treatments on the butterfly population. A reasonable next step would be to design field-based protocols to apply these treatments in the field and to monitor effects on Oregon silverspot populations. Only with a field study will it be possible to estimate the potential benefits of these management strategies relative to their potential costs. In addition to the Oregon silverspot, 24 out of 26 recovery plans for butterflies currently listed as threatened or endangered in the U.S. recommend invasive plant management (USFWS 2020a), including two other S. zerene subspecies: the Behren’s silverspot and Myrtle’s silverspot (Hammond and McCorkle 1983; Sims 2017). Among these recovery plans, at least 13 have reported herbicide use, but none of them have used clopyralid, while only two have used fluazifop-P-butyl (LaBar and Schultz 2012; Bennion et al. 2020). Our results do not suggest any serious risks of using clopyralid and fluazifop-P-butyl with one of two adjuvants, Agri-Dex® and Nu-Film®-IR, in Oregon silverspot occupied habitat, yet suggest the need for more thorough evaluations of their costs and benefits in the field, and throughout the butterfly’s life cycle.
Data availability
Data are available online at Doll CF, Converse SJ, James AB, Schultz CB (2021) Data on Zerene silverspot butterfly and early blue violet responses to herbicide treatments from 2018–2019 greenhouse experiments: U.S. Geological Survey data release, https://doi.org/10.5066/P9R9W2JM.
References
Agnello AM, van Duyn JW, Bradley JR (1986) Influence of postemergence herbicides on populations of bean leaf beetle, Cerotoma trifurcata (Coleoptera: Chrysomelidae), and corn earworm, Heliothis zea (Lepidoptera: Noctuidae), in soybeans. J Econ Entomol 79:261–265. https://doi.org/10.1093/jee/79.1.261
Ali S, Ullah MI, Saeed MF, Khalid S, Saqib M, Arshad M, Afzal M, Damalas CA (2019) Heavy metal exposure through artificial diet reduces growth and survival of Spodoptera litura (Lepidoptera: Noctuidae). Environ Sci Pollut Res Int 26:14426–14434. https://doi.org/10.1007/s11356-019-04792-0
Anderson MJ, Arnold M, Barclay E, Myers L, Shepherdson D, Sullivan E (2010) Oregon silverspot butterfly husbandry manual. Oregon Zoo, Portland
Ansley RJ, Castellano MJ (2006) Strategies for savanna restoration in the southern Great Plains: effects of fire and herbicides. Restor Ecol 14:420–428. https://doi.org/10.1111/j.1526-100X.2006.00150.x
Augustine KE, Kingsolver JG (2017) Biogeography and phenology of oviposition preference and larval performance of Pieris virginiensis butterflies on native and invasive host plants. Biol Invasions 20:413–422. https://doi.org/10.1007/s10530-017-1543-9
Banks JE, Ackleh AS, Stark JD (2010) The use of surrogate species in risk assessment: using life history data to safeguard against false negatives. Risk Anal 30:175–182. https://doi.org/10.1111/j.1539-6924.2009.01349.x
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bennion LD, Ferguson JA, New L, Schultz CB (2020) Community-level effects of herbicide-based restoration treatments: structural benefits but at what cost? Restor Ecol 28:553–563. https://doi.org/10.1111/rec.13118
Bierzychudek P, Warner K (2015) Modeling caterpillar movement to guide habitat enhancement for Speyeria zerene hippolyta, the Oregon silverspot butterfly. J Insect Conserv 19:45–54. https://doi.org/10.1007/s10841-014-9741-6
Blake RJ, Woodcock BA, Westbury DB, Sutton P, Potts SG (2011) New tools to boost butterfly habitat quality in existing grass buffer strips. J Insect Conserv 15:221–232. https://doi.org/10.1007/s10841-010-9339-6
Boggs CL, Freeman KD (2005) Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia 144:353–361. https://doi.org/10.1007/s00442-005-0076-6
Bohnenblust E, Egan JF, Mortensen D, Tooker J (2013) Direct and indirect effects of the synthetic-auxin herbicide dicamba on two Lepidopteran species. Environ Entomol 42:586–594. https://doi.org/10.1603/EN13021
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol (Amst) 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Buckingham DA, Linders M, Landa C, Mullen L, LeRoy C (2016) Oviposition preference of endangered Taylor’s checkerspot butterflies (Euphydryas editha taylori) using native and non-native hosts. Northwest Sci 90:491–497. https://doi.org/10.3955/046.090.0409
Bush DS, Siegel JP, Berenbaum MR (2018) Accelerated development and toxin tolerance of the navel orangeworm Amyelois transitella (Lepidoptera: Pyralidae) in the presence of Aspergillus flavus. J Chem Ecol 44:1170–1177. https://doi.org/10.1007/s10886-018-1027-0
Calabrese JM, Fagan WF (2004) Lost in time, lonely, and single: reproductive asynchrony and the Allee effect. Am Nat 164:25–37. https://doi.org/10.1086/421443
Calvo D, Molina JM (2005) Fecundity-body size relationship and other reproductive aspects of Streblote panda (Lepidoptera: Lasiocampidae). Ann Entomol Soc Am 98:191–196. https://doi.org/10.1603/0013-8746(2005)098[0191:FSRAOR]2.0.CO;2
De Moya RS, Savage WK, Tenney C, Bao X, Wahlberg N, Hill RI (2017) Interrelationships and diversification of Argynnis Fabricius and Speyeria Scudder butterflies. Syst Entomol 42:635–649. https://doi.org/10.1111/syen.12236
Dennehy C, Alverson ER, Anderson HE, Clements DR, Gilbert R, Kaye TN (2011) Management strategies for invasive plants in Pacific Northwest prairies, savannas, and oak woodlands. Northwest Sci 85:329–351. https://doi.org/10.3955/046.085.0219
Dhillon MK, Tanwar AK, Hasan F (2019) Fitness consequences of delayed mating on reproductive performance of Chilo partellus (Swinhoe). J Exp Zool A 331:161–167. https://doi.org/10.1002/jez.2249
Doll CF, Converse SJ, James AB, Schultz CB (2021) Data on Zerene silverspot butterfly and early blue violet responses to herbicide treatments from 2018–2019 greenhouse experiments: U.S. Geological Survey data release. https://doi.org/10.5066/P9R9W2JM
Dow (2003) Transline MSDS. Dow AgroSciences, Indianapolis
Durkin PRD (2014) Scoping/screening level risk assessmenton on fluazifop-P-butyl. Syracuse Environmental Research Associates Inc, New York
Eliyahu D, Applebaum S, Rafaeli A (2003) Moth sex-pheromone biosynthesis is inhibited by the herbicide diclofop. Pestic Biochem Physiol 77:75–81. https://doi.org/10.1016/S0048-3575(03)00101-9
Fagerström T, Wiklund C (1982) Why do males emerge before females? Protandry as a mating strategy in male and female butterflies. Oecologia 52:164–166. https://doi.org/10.1007/BF00363830
Fischer K, Fiedler K (2000) Sex-related differences in reaction norms in the butterfly Lycaena tityrus (Lepidoptera: Lycaenidae). Oikos 90:372–380. https://doi.org/10.1034/j.1600-0706.2000.900218.x
Florens FBV, Mauremootoo JR, Fowler SV, Winder L, Baider C (2010) Recovery of indigenous butterfly community following control of invasive alien plants in a tropical island’s wet forests. Biodivers Conserv 19:3835–3848. https://doi.org/10.1007/s10531-010-9930-x
Forsberg J, Wiklund C (1988) Protandry in the green-veined white butterfly, Pieris napi L. (Lepidoptera; Pieridae). Funct Ecol 2:81. https://doi.org/10.2307/2389464
Gallien L, Altermatt F, Wiemers M, Schweiger O, Zimmermann NE (2017) Invasive plants threaten the least mobile butterflies in Switzerland. Divers Distrib 23:185–195. https://doi.org/10.1111/ddi.12513
Gascoigne J, Berec L, Gregory S, Courchamp F (2009) Dangerously few liaisons: a review of mate-finding Allee effects. Popul Ecol 51:355–372. https://doi.org/10.1007/s10144-009-0146-4
Gauvrit C, Cabanne F (1993) Oils for weed control: uses and mode of action. Pestic Sci 37:147–153. https://doi.org/10.1002/ps.2780370207
Glaeser RM, Schultz CB (2014) Characterizing a contentious management tool: the effects of a grass-specific herbicide on the silvery blue butterfly. J Insect Conserv 18:1047–1058. https://doi.org/10.1007/s10841-014-9714-9
Groom MJ (1998) Allee effects limit population viability of an annual plant. Am Nat 151:487–496. https://doi.org/10.1086/286135
Hahn M, Geisthardt M, Brühl CA (2014) Effects of herbicide-treated host plants on the development of Mamestra brassicae L. caterpillars. Environ Toxicol Chem 33:2633–2638. https://doi.org/10.1002/etc.2726
Hammond PC, McCorkle DV (1983) The decline and extinction of Speyeria populations resulting from human environmental disturbances (Nymphalidae: Argynninae). J Res Lepidoptera 22:217–224
Hanula JL, Horn S (2011) Removing an exotic shrub from riparian forests increases butterfly abundance and diversity. For Ecol Manag 262:674–680. https://doi.org/10.1016/j.foreco.2011.04.040
Henry E, Brammer-Robbins E, Aschehoug E, Haddad N (2019) Do substitute species help or hinder endangered species management? Biol Conserv 232:127–130. https://doi.org/10.1016/j.biocon.2019.01.031
Herbert LT, Vázquez DE, Arenas A, Farina WM (2014) Effects of field-realistic doses of glyphosate on honeybee appetitive behaviour. J Exp Biol 217:3457–3464. https://doi.org/10.1242/jeb.109520
Horner TA, Dively GP, Herbert DA (2003) Development, survival and fitness performance of Helicoverpa zea (Lepidoptera: Noctuidae) in MON810 Bt field corn. J Econ Entomol 96:914–924. https://doi.org/10.1603/0022-0493-96.3.914
Huang F, Subramanyam B (2003) Effects of delayed mating on reproductive performance of Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). J Stored Prod Res 39:53–63. https://doi.org/10.1016/S0022-474X(02)00018-8
Huang D, Kong J, Seng Y (2012) Effects of the heavy metal Cu2+ on growth, development, and population dynamics of Spodoptera litura (Lepidoptera: Noctuidae). J Econ Entomol 105:288–294. https://doi.org/10.1603/ec11163
Huntzinger M (2003) Effects of fire management practices on butterfly diversity in the forested western United States. Biol Conserv 113:1–12. https://doi.org/10.1016/S0006-3207(02)00356-7
James DG (2008) Comparative studies on the immature stages and developmental biology of five Argynnis spp. (Subgenus Speyeria) (Nymphalidiae) from Washington. J Lepidopterists’ Soc 61:61–66
James DG, Nunnallee D (2011) Life histories of Cascadia butterflies. Oregon State University Press, Corvallis
James DG, Pelham JP (2011) Observations on the seasonal biology and apparent migration of Argynnis (Speyeria) coronis (Nymphalidae) in central Washington. J Lepidopterists’ Soc 65:249–255. https://doi.org/10.18473/lepi.v65i4.a4
Jiménez-Pérez A, Wang Q (2004) Effect of body weight on reproductive performance in Cnephasia jactatana (Lepidoptera: Tortricidae). J Insect Behav 17:511–522. https://doi.org/10.1023/B:JOIR.0000042538.19559.09
Jones VP, Aihara-Sasaki M (2001) Demographic analysis of delayed mating in mating disruption: a case study with Cryptophelbia illepida (Lepidoptera: Tortricidae). J Econ Entomol 94:785–792. https://doi.org/10.1603/0022-0493-94.4.785
Keeler MS, Chew FS, Goodale BC, Reed JM (2006) Modelling the impacts of two exotic invasive species on a native butterfly: top-down vs. bottom-up effects. J Anim Ecol 75:777–788. https://doi.org/10.1111/j.1365-2656.2006.01098.x
Kerpel SM, Soprano E, Moreira GRP (2006) Effect of nitrogen on Passiflora suberosa L. (Passifloraceae) and consequences for larval performance and oviposition in Heliconius erato phyllis (Fabricius) (Lepidoptera: Nymphalidae). Neotrop Entomol 35:192–200. https://doi.org/10.1590/s1519-566x2006000200006
Kopper BJ, Shu S, Charlton RE, Ramaswamy SB (2001) Evidence for reproductive diapause in the fritillary Speyeria idalia (Lepidoptera: Nymphalidae). Ann Entomol Soc Am 94:427–432. https://doi.org/10.1603/0013-8746(2001)094[0427:EFRDIT]2.0.CO;2
Kral-O’Brien KC, Limb RF, Hovick TJ, Harmon JP (2018) Compositional shifts in forb and butterfly communities associated with Kentucky bluegrass invasions. Rangel Ecol Manag 72:301–309. https://doi.org/10.1016/j.rama.2018.10.003
Kutlesa NJ, Caveney S (2001) Insecticidal activity of glufosinate through glutamine depletion in a caterpillar. Pest Manag Sci 57:25–32. https://doi.org/10.1002/1526-4998(200101)57:1%3c25::AID-PS272%3e3.0.CO;2-I
LaBar CC, Schultz CB (2012) Investigating the role of herbicides in controlling invasive grasses in prairie habitats: effects on non-target butterflies. Nat Areas J 32:177–189. https://doi.org/10.3375/043.032.0207
Lavoie B, Oberhauser KS (2004) Compensatory feeding in Danaus plexippus (Lepidoptera: Nymphalidae) in response to variation in host plant quality. Environ Entomol 33:1062–1069. https://doi.org/10.1603/0046-225X-33.4.1062
Lingren PD, Warner WB, Henneberry TJ (1988) Influence of delayed mating on egg production, egg viability, mating, and longevity of female pink bollworm (Lepidoptera: Gelechiidae). Environ Entomol 17:86–89. https://doi.org/10.1093/ee/17.1.86
Luo X-Y, Sunohara Y, Matsumoto H (2004) Fluazifop-butyl causes membrane peroxidation in the herbicide-susceptible broad leaf weed bristly starbur (Acanthospermum hispidum). Pestic Biochem Physiol 78:93–102. https://doi.org/10.1016/j.pestbp.2003.10.002
MacDonald NW, Dykstra KM, Martin LM (2019) Restoration of native-dominated plant communities on a Centaurea stoebe-infested site. Appl Veg Sci 22:300–316. https://doi.org/10.1111/avsc.12427
Martínez IM, Lumaret JP, Cruz MR (2001) Suspected side effects of a herbicide on dung beetle populations (Coleoptera: Scarabaeidae). C R Acad Sci III Sci Vie 324:989–994. https://doi.org/10.1016/s0764-4469(01)01384-1
McCorkle DV, Hammond PC (1988) Biology of Speyeria zerene hippolyta (Nymphalidae) in a marine-modified environment. J Lepidopterists’ Soc 42:184–195
McHugh A, Bierzychudek P, Greever C, Marzulla T, Van Buskirk R, Binford G (2013) A molecular phylogenetic analysis of Speyeria and its implications for the management of the threatened Speyeria zerene hippolyta. J Insect Conserv 17:1237–1253. https://doi.org/10.1007/s10841-013-9605-5
Michereff MFF, Vilela EF, Filho MM, Nery DMS, Thiebaut JT (2004) Effects of delayed mating and male mating history on the reproductive potential of Leucoptera coffeella (Lepidoptera: Lyonetiidae). Agric For Entomol 6:241–247. https://doi.org/10.1111/j.1461-9555.2004.00227.x
Moranz RA, Fuhlendorf SD, Engle DM (2014) Making sense of a prairie butterfly paradox: the effects of grazing, time since fire, and sampling period on regal fritillary abundance. Biol Conserv 173:32–41. https://doi.org/10.1016/j.biocon.2014.03.003
Morghan KJR, Leger EA, Rice KJ (2003) Clopyralid effects on yellow starthistle (Centaurea solstitialis) and nontarget species. Weed Sci 51:596–600. https://doi.org/10.1614/0043-1745(2003)051[0596:CEOYSC]2.0.CO;2
Moroń D, Skórka P, Lenda M, Kajzer-Bonk J, Mielczarek Ł, Rożej-Pabijan E, Wantuch M (2018) Linear and non-linear effects of goldenrod invasions on native pollinator and plant populations. Biol Invasions 21:947–960. https://doi.org/10.1007/s10530-018-1874-1
Olaya-Arenas P, Hauri K, Scharf ME, Kaplan I (2020) Larval pesticide exposure impacts monarch butterfly performance. Sci Rep 10:14490. https://doi.org/10.1038/s41598-020-71211-7
Pearson DE, Ortega YK, Runyon JB, Butler JL (2016) Secondary invasion: the bane of weed management. Biol Conserv 197:8–17. https://doi.org/10.1016/j.biocon.2016.02.029
Pelham JP (2021) A catalogue of the butterflies of the United States and Canada. Butterflies of America. https://www.butterfliesofamerica.com/US-Can-Cat.htm. Accessed 12 Aug 2021
Pereira JL, Galdino TVS, Silva GAR, Picanço MC, Silva AA, Corrêa AS, Martins JC (2018) Effects of glyphosate on the non-target leaf beetle Cerotoma arcuata (Coleoptera: Chrysomelidae) in field and laboratory conditions. J Environ Sci Health B 53:447–453. https://doi.org/10.1080/03601234.2018.1455363
Pyle RM, LaBar CC (2018) Butterflies of the Pacific Northwest. Timber Press Inc, Portland
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rabelo MM, Matos JML, Orozco-Restrepo SM, Paula-Moraes SV, Pereira EJG (2020) Like parents, like offspring? Susceptibility to Bt toxins, development on dual-gene Bt cotton, and parental effect of Cry1Ac on a nontarget Lepidopteran pest. J Econ Entomol 113:1234–1242. https://doi.org/10.1093/jee/toaa051
Rainio MJ, Margus A, Lehmann P, Helander M, Lindström L (2019) Effects of a glyphosate-based herbicide on survival and oxidative status of a non-target herbivore, the Colorado potato beetle (Leptinotarsa decemlineata). Comp Biochem Physiol C 215:47–55. https://doi.org/10.1016/j.cbpc.2018.09.005
Rasband WS (2018) ImageJ. U.S. National Institutes of Health, Bethesda
Rogers CE, Marti OG (1997) Once-mated beet armyworm (Lepidoptera: Noctuidae): effects of age at mating on fecundity, fertility, and longevity. Environ Entomol 26:585–590. https://doi.org/10.1093/ee/26.3.585
Russell C, Schultz CB (2010) Effects of grass-specific herbicides on butterflies: an experimental investigation to advance conservation efforts. J Insect Conserv 14:53–63. https://doi.org/10.1007/s10841-009-9224-3
Saska P, Skuhrovec J, Lukáš J, Chi H, Tuan S-J, Honěk A (2016) Treatment by glyphosate-based herbicide alters life history parameters of the rose-grain aphid Metopolophium dirhodum. Sci Rep 6:27801. https://doi.org/10.1038/srep27801
Schultz CB, Ferguson JA (2020) Demographic costs and benefits of herbicide-based restoration to enhance habitat for an endangered butterfly and a threatened plant. Restor Ecol 28:564–572. https://doi.org/10.1111/rec.13102
Schultz CB, Russell C, Wynn L (2008) Restoration, reintroduction, and captive propagation for at-risk butterflies: a review of British and American conservation efforts. Isr J Ecol Evol 54:41–61. https://doi.org/10.1560/IJEE.54.1.41
Schultz CB, Zemaitis JL, Thomas CC, Bowers MD, Crone EE (2016) Non-target effects of grass-specific herbicides differ among species, chemicals and host plants in Euphydryas butterflies. J Insect Conserv 20:867–877. https://doi.org/10.1007/s10841-016-9920-8
Silvernail IS (2017) Nestucca Bay National Wildlife Refuge, Cannery Hill prairie restoration: 2016 report and updated restoration plan. Institute for Applied Ecology, Corvallis
Sims SR (1984) Reproductive diapause in Speyeria (Lepidoptera: Nymphalidae). J Res Lepidoptera 23:211–216
Sims SR (2017) Speyeria (Lepidoptera: Nymphalidae) conservation. Insects 8:45–57. https://doi.org/10.3390/insects8020045
Sims SR, Shapiro AM (2014) Interspecific variation in size, diapause intensity, and moisture responses of first-instar Speyeria (Lepidoptera: Nymphalidae) larvae. Ann Entomol Soc Am 107:163–169. https://doi.org/10.1603/AN13099
Sims SR, Shapiro AM (2016) Reproductive strategies and life history evolution of some California Speyeria (Nymphalidae). J Lepidopterists’ Soc 70:114–120. https://doi.org/10.18473/lepi.70i2.a6
Singer MC (1982) Quantification of host preference by manipulation of oviposition behavior in the butterfly Euphydryas editha. Oecologia 52:224–229. https://doi.org/10.1007/BF00363841
Singer MC, Vasco D, Parmesan C, Thomas CD, Ng D (1992) Distinguishing between “preference” and “motivation” in food choice: an example from insect oviposition. Anim Behav 44:463–471. https://doi.org/10.1016/0003-3472(92)90056-F
Stark JD, Chen XD, Johnson CS (2012) Effects of herbicides on Behr’s metalmark butterfly, a surrogate species for the endangered butterfly, Lange’s metalmark. Environ Pollut 164:24–27. https://doi.org/10.1016/j.envpol.2012.01.011
Sterling TM, Hall JC (1997) Mechanism of action of natural auxin and the auxinic herbicides. In: Roe RM, Burton JD, Kuhr RJ (eds) Herbicide activity: toxicology, biochemistry and molecular biology. IOS Press, Amsterdam, pp 111–136
Tu M, Hurd C, Randall JM (2001) Weed control methods handbook: tools & techniques for use in natural areas. The Nature Conservancy
USFWS (2009) Myrtle’s silverspot butterfly (Speyeria zerene myrtleae) 5-year-review: summary and evaluation. U.S Fish and Wildlife Service
USFWS (2016) Recovery plan for the Behren’s silverspot butterfly (Speyeria zerene behrensii). U.S. Fish and Wildlife Service, Sacramento
USFWS (2020a) Environmental conservation online system. Species Reports. https://ecos.fws.gov/ecp/. Accessed 15 Nov 2020
USFWS (2020b) Oregon silverspot butterfly (Speyeria zerene hippolyta) 5-year review: summary and evaluation. U.S. Fish and Wildlife Service, Newport
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Vogel JA, Debinski DM, Koford RR, Miller JR (2007) Butterfly responses to prairie restoration through fire and grazing. Biol Conserv 140:78–90. https://doi.org/10.1016/j.biocon.2007.07.027
Wagner DL, Van Driesche RG (2010) Threats posed to rare or endangered insects by invasions of nonnative species. Annu Rev Entomol 55:547–568. https://doi.org/10.1146/annurev-ento-112408-085516
Walker KA, Ridley SM, Lewis T, Harwood JL (1988) Fluazifop, a grass-selective herbicide which inhibits acetyl-CoA carboxylase in sensitive plant species. Biochem J 254:307–310. https://doi.org/10.1042/bj2540307
Walker GP, Cameron PJ, MacDonald FM, Madhusudhan VV, Wallace AR (2007) Impacts of Bacillus thuringiensis toxins on parasitoids (Hymenoptera: Braconidae) of Spodoptera litura and Helicoverpa armigera (Lepidoptera: Noctuidae). Biol Control 40:142–151. https://doi.org/10.1016/j.biocontrol.2006.09.008
Warren AD, Davis KJ, Strangeland EM, Pelham JP, Willmott KR, Grishin NV (2017) Illustrated lists of American butterflies. Butterflies of America. http://butterfliesofamerica.com/. Accessed 13 Jul 2021
Watts C, Ranson H, Thorpe S, Cave V, Clarkson B, Thornburrow D, Bartlam S, Bodmin K (2015) Invertebrate community turnover following control of an invasive weed. Arthropod Plant Interact 9:585–597. https://doi.org/10.1007/s11829-015-9396-6
Wiklund C, Forsberg J (1991) Sexual size dimorphism in relation to female polygamy and protandry in butterflies: a comparative study of Swedish Pieridae and Satyridae. Oikos 60:373–381. https://doi.org/10.2307/3545080
Wiklund C, Kaitala A (1995) Sexual selection for large male size in a polyandrous butterfly: the effect of body size on male versus female reproductive success in Pieris napi. Behav Ecol 6:6–13. https://doi.org/10.1093/beheco/6.1.6
Zhang X, Miyashita T (2018) Effects of local and landscape factors on the abundance of an endangered multivoltine butterfly at riverbanks. Entomol Sci 21:133–141. https://doi.org/10.1111/ens.12291
Zhang J, Cong Q, Shen J, Opler PA, Grishin NV (2020) Genomic evidence suggests further changes of butterfly names. Taxon Rep Int Lepidoptera Surv 8:41
Acknowledgements
We thank P. C. Hammond and D. V. McCorkle for providing larvae, I. Silvernail (Institute for Applied Ecology) and D. Selk (Woodland Park Zoo) for offering seeds, and M. Dommer (Sun Gro® Horticulture) for donating potting soil. We are also grateful to K. C. King, C. C. Thomas, C. Jason, K. Key, K. M. Summerhill, C. Perez, H. R. Coker, C. E. Irwin, A. B. James, B. J. Plumhoff, R. L. Viera, A. E. Petriyenko, C. N. Dougher, J. T. Shi, M. Hymas, A. B. Thomas, K. A. Reddick, J. V. Arriens, R. E. Bonoan, S. K. Bussan, and J. E. Louman (Washington State University) for assistance in the greenhouse, and with collecting experimental data; L. New and C. B. Edwards (Washington State University) for advice on statistical analyses; and R. G. Chuck, A. Walker, and B. Flanders (U.S. Fish and Wildlife Service) for facilitating this project. Funding provided by the U.S. Geological Survey Science Support Program (facilitated by the Washington Cooperative Fish and Wildlife Research Unit), the U.S. Fish and Wildlife Service in-kind, and the Carl H. Elling Endowment through Washington State University was critical to the success of this work. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Funding
This work was funded by the U.S. Geological Survey Science Support Program (facilitated by the Washington Cooperative Fish and Wildlife Research Unit), the U.S. Fish and Wildlife Service in-kind, and the Carl H. Elling Endowment through Washington State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doll, C.F., Converse, S.J. & Schultz, C.B. Non-target effects of herbicides on the Zerene silverspot butterfly, a surrogate subspecies for the threatened Oregon silverspot butterfly. J Insect Conserv 26, 1–15 (2022). https://doi.org/10.1007/s10841-021-00355-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-021-00355-2