Abstract
Encroachment by invasive plants is a leading threat to rare butterflies. Restoration plans increasingly recommend herbicides to control invasive plants within butterfly habitats. Few studies address the effects of these herbicides on at-risk butterflies. The effects of two graminicides (fluazifop-p-butyl and sethoxydim) and a surfactant (Preference®) were evaluated on Icaricia icarioides blackmorei and Pieris rapae. The effects on butterfly larvae were assessed by mimicking recommended timing and mixture rates of field applications. Differences in survival to adult eclosure, development time, biomass, sex ratio and adult morphology were assessed. Survival of P. rapae was reduced by 32% with sethoxydim and 21% with fluazifop-p-butyl. Wing size and pupal weights of P. rapae were reduced by herbicide treatments. Icaricia icarioides blackmorei experienced a 21% reduction in development time from the date of treatment to eclosure. These results highlight the importance of careful consideration in the use of herbicides in habitats harboring at-risk butterfly populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive plants are a leading threat to at-risk butterfly populations and are directly linked to the decline of 33 butterfly and skipper species in the US (New et al. 1995; Wilcove et al. 1998). As a result, invasive plant control is recommended in over 80% of conservation plans for butterflies currently listed as threatened or endangered under the US Endangered Species Act (Schultz et al. 2008). Herbicides are an increasingly popular option for vegetation management in butterfly habitats (Schultz et al. 2008). However, limited toxicological screenings and uncertainty about ecological risks to non-target insects warrants further investigation (Freemark and Boutin 1995; Pratt et al. 1997; Colborn and Short 1999). As herbicide use increases in natural areas, managers need to address the potential likelihood that non-target organisms will be exposed to herbicides including spray drift, direct overspray (dermal exposure) and ingestion (oral exposure). Designing conservation strategies for sites that harbor rare butterflies is challenging because management actions to control invasive species must maximize their impact on problematic species while limiting their impact on native species, especially threatened and endangered species.
Control of invasive plants is of particular interest in western Oregon and Washington, USA, where habitat loss and introduction of invasive plants are linked to the decline of several at-risk butterfly species. These include Fender’s blue (Icaricia icarioides fenderi, US endangered, USFWS 2009), Oregon silverspot (Speyeria zerene hippolyta, US threatened, USFWS 2009), Taylor’s checkerspot (Euphydryas editha taylori, US candidate, USFWS 2009), Mardon skipper (Polites mardon, US candidate, USFWS 2009), Valley silverspot (Speyeria zerene bremneri, US species of concern WDFW 2009), and Puget blue (Icaricia icarioides blackmorei, Washington state species of concern, WDFW 2009).
Several key issues arise concerning the potential impacts of herbicides on non-target butterflies. First, studies of terrestrial invertebrates are generally set in an agricultural context and focus on herbicidal effects on beneficial invertebrates that are important for biocontrol or pollination or focus on invertebrates considered pest species which impact crop yield (e.g. Brust 1990; Haughton et al. 2001; Verhoef and Brussaard 1990). However, the results may not generalize to conservation or ecological impact to non-target species such butterflies due to differences in foraging, life history, habitat selection or other details of species’ ecology (Longley and Sotherton 1997).
Second, although herbicides are designed to kill plants, studies indicate that terrestrial invertebrates exhibit adverse effects from formulations including 2,4,5-T (reduced fecundity and lifespan, Eijsackers 1978), endothall (increased mortality, reduced pupal weight, Brown 1987); sethoxydim and fluazifop-p-butyl (altered pupal weight and fecundity, Agnello et al. 1986b), diclofop (altered pheromone production, Eliyahu et al. 2003) and atrazine (altered fecundity and instar duration, Al-Assiuty and Khalil 1996). In contrast, other studies suggest ingested or absorbed herbicides may have negligible effects on non-target organisms when toxins are rapidly eliminated from animal systems or exposure times are reduced (Tatum 2004).
Third, results generated from traditional toxicity tests for one invertebrate species may not be comparable for other invertebrate species (Stark et al 2004). Conventional toxicity screening methods in the US include acute toxicity LC50 or LD50 tests (concentration or dose that kills 50% of a population) and are generally performed on a single terrestrial insect species. In the US, the honeybee (Apis mellifera) is the surrogate test species for acute toxicity screenings which generally involve single exposure or exposure over a short period of time (US Environmental Protection Agency 2009; Tatum 2004). Thus, the underlying assumptions are that toxicology results on A. mellifera transfer to all insect orders, that adverse effects are determined from single dose or acute short-term screenings, and that exposure to fully developed adult organisms represent exposure effects to all life stages. In contrast, in Europe toxicity screenings include testing on multiple taxa of beneficial invertebrates such as predatory mites, spiders, flower bugs, lacewings, ladybird beetles, rove beetles, ground beetles, parasitoids, bees, and hoverflies (ECHCPD 2002; Boller et al. 2005). Because life history traits vary greatly among species, recovery from lethal or sub-lethal effects varies between species. Life history traits that play a role in recovery include survivorship, development time, number of generations produced annually and number of offspring produced per generation (Stark et al. 2004). Sub-lethal effects from toxicants such as reduced reproductive potential, changes in morphology, alterations in sex ratio and development rates can have long-term generational effects (Stark and Banks 2003). Simulated models by Stark et al. (2004) indicate that a 50% reduction in survivorship and fecundity would delay the population growth of some arthropod species but not others.
Fourth, herbicides applied to control target invasive plant species may compromise the nutrient quality of non-target plants, and indirectly influence growth and development of feeding larvae (Agnello et al 1986a). Inadequate larval food resources affect adult morphology, survivorship and fecundity in Lepidoptera (Tammaru et al. 1996; Boggs and Freeman 2005).
Finally, several studies suggest that biochemical pathways which cause herbicides to kill plants also have lethal effects on non-target insects. For example, plant growth regulators such as diclofop interfere with protein synthesis by inhibiting the activity of acetyl-coenzyme A carboxylase enzymes in monocotyledonous plants. The herbicide diclofop also disrupts pheromone synthesis in the noctuid moth (Helicoverpa armigera, Eliyahu et al. 2003). The herbicides atrazine and simazine, are designed to inhibit the photosynthetic process in plants. These same chemicals inhibit development rates and fecundity of springtails, reduce hatch rates of house flies, and inhibit metabolic rates of crickets (Borkovec et al. 1967; Chio and Sanborn 1977; Al-Assiuty and Khalil 1996), yet do not harm some beetle species (Brust 1990). Another herbicide formula, glufosinate-ammonium (GLA) is toxic to a skipper, Calpodes ethlius (Kutlesa and Caveney 2001). GLA is designed to inhibit the conversion of glutamate to glutamine, a required enzyme for both plants and animals. Caterpillars exposed to GLA died from glutamine depletion (Kutlesa and Caveney 2001).
Currently grass-specific herbicides are gaining favor due to their highly selective modes of action (Colborn and Short 1999; Tu et al. 2001; Fuhlendorf et al. 2002; Clark et al. 2004) and reported low toxicity to non-target insects (EXTOXNET 1996a, b). Selective chemical formulations disrupt key biochemical pathways in plants necessary for such functions as growth regulation, cell membrane formation, and protein synthesis (Walker et al. 1988). Susceptibility differs between plant species allowing for some herbicides to target grasses with intercalary meristemic cellular growth patterns and not impact grasses which lack this growth form. Many aggressive, non-native grasses in Oregon and Washington, USA, which have intercalary meristemic growth, are impacted by selective herbicides. Non-native grasses in these prairies include Brachypodium sylvaticum (false brome), Holcus lanatus (velvet grass), Festuca arundinacea (tall fescue) and Arrhenatherum elatius (tall oatgrass). The dominant native grass in these prairies, Festuca roemeri, has a growth form that is primarily a tussock bunchgrass and is unaffected (Clark et al. 2004; Dunwiddie and Delvin 2006). In addition, grass-specific herbicides do not affect broad leaf plants. Recent studies indicate that application of the grass-specific herbicide fluazifop-p-butyl reduces the presence of B. sylvaticum with no significant harm to native prairie forbs (Clark et al. 2004). Additionally, Dunwiddie and Delvin (2006) note a reduction in H. lanatus and A. elatius following sethoxydim herbicide applications while overall native species richness increases. Thus, these herbicides are effective in management of the native plant community where the dominant native grasses are bunchgrass fescues, but the impact of these herbicides on at-risk butterflies in these prairies is unknown.
The objectives of this study are to investigate the lethal and sub-lethal effects of herbicide exposure to developing butterfly larvae. Two butterfly species were evaluated to assess if herbicide effects are species-specific, or may generalize to multiple Lepidoptera species. Survival and development of Icaricia icarioides blackmorei and Pieris rapae are evaluated. Both species reside in prairies of the Pacific Northwest where butterfly larvae feeding on host plants are subject to herbicide spray or drift during applications. Two grass-specific herbicides are investigated, sethoxydim and fluazifop-p-butyl. Both herbicides are proposed for use within prairie habitats and have been evaluated for impacts to the native plant community in trial studies. The active ingredient in both chemicals is reported as having little or no toxic effect to insects (EXTOXNET 1996a, b). Based on known EPA toxicity screenings of the herbicides, the following hypotheses were tested: (1) Selective herbicides designed to interrupt cell growth in plants will have little or no impact on survival, sex ratio, or morphology of non-target butterflies, (2) Development time of butterflies exposed to selective herbicides during larval development will not be altered, and (3) Selective herbicides will not impact pupal and adult biomass.
Methods
Study organisms
Icaricia icarioides blackmorei is a Washington state species of concern and in the same species complex as I. i. fenderi (US endangered butterfly, USFWS 2009). It is abundant at sites which support populations and collection of eggs from a few dozen females has little impact on the population (Schultz, unpublished analyses). Both I. i. fenderi and I. i. blackmorei share similar life cycles, are univoltine and overwinter as diapause larvae. In early spring, post-diapause larvae feed on emerging perennial lupine host plants (Lupinus sulphureous kincaidii or L. laxiflorus for I.i. fenderi and L. albicaulis for I. i. blackmorei). Postemergence herbicides are designed to be applied in spring when invasive grasses are in their early growth stages. During early spring many butterfly species, including I. i. blackmorei, I. i. fenderi,S. z. hippolyta, S. z. bremneri and E. e. taylori, are in mid to late instar developmental stages (Schultz pers. obs., Potter, pers. comm., USFWS 2001). Using I. i. blackmorei in experimental trials allowed us to mimic the application of herbicides at the most likely life stage of exposure to assess the biological effects on survival, development, and growth.
Pieris rapae, is a non-native but established butterfly species in the Pacific Northwest. Pieris rapae eggs are easily purchased through commercial scientific laboratory supply houses which can be obtained year-round. These butterflies have multivoltine life cycles, complete in 3–6 weeks, allowing for faster assessment and data collection.
Rearing procedures
In May and June 2005, 60 I. i. blackmorei females were collected from Johnson Prairie, Washington and transported to a greenhouse at WSU Vancouver. Johnson Prairie is located on the Fort Lewis Military Reservation in Pierce County, WA (46°55′N 123°16′W). Using protocols developed in 2003–2004, individual females were placed in an enclosure containing the larval hostplant, Lupinus albicaulis. Enclosures were constructed with silk organza fabric and secured around a 3.8 l container and host plant. Females were housed in each enclosure for 1–3 days with a goal of collecting 50 eggs per female. While enclosed, females were misted daily with water and fed an artificial nectar source (Gatorade®, J. Daniels pers. comm.). Females were released at the capture site post-oviposition. Of the 60 females collected, 47 oviposited and produced viable larvae. Females on average laid 38 eggs.
To ensure abundant host plant biomass, newly hatched larvae were distributed among host plants with a maximum of five larvae per plant during pre-diapause development. Larvae were transferred to new plants with camel hair brushes and handling time was limited. To minimize the possible transfer of pathogens, brushes were cleaned using a light mixture of soapy water and bleach (active ingredient sodium hypochlorite 6.0%) between transfers. Parental identification was retained for each offspring to support random assignment between treatments. Larvae were reared on live host plants until reaching diapause. Using techniques developed in a previous study (Schultz et al. 2009), larvae developed to diapause stage were placed in diapause tubes, 3 cm pieces of plastic tubing lined and capped with silk organza, with a maximum of three larvae per tube. Diapause tubes were placed in inverted clay pots and put in a protected outdoor location. Pots were placed on a wood palette on the North side of a building under plastic covers which diverted rain and snow. Climate in Vancouver, WA is mild, with average summer temperatures about 20°C and average winter temperatures about 4°C (NOAA accessed April 2008).
Between April 10 and 14, 2006 post-diapause larvae were returned to the greenhouse where up to five sibling larvae were placed on individual lupine host plants with fitted enclosures. Larvae were observed for 2 weeks before randomizing individuals between treatments to assure that larvae being placed into treatments had broken diapause and were exhibiting normal eating behavior.
Pieris rapae eggs were purchased from Carolina Biological Supply Company, Burlington, NC. This is a generalist species which feeds on a variety of Brassica species. Upon receipt of the P. rapae eggs, separate batches were placed in 60 ml translucent plastic cups (hereafter small plastic cups) containing green cabbage (Brassica oleracea var. capitata) leaves as a larval food source. To avoid cannibalism, newly hatched larvae were dispersed among several cups for the duration of the hatch period. Larvae were transferred using camel hair brushes as above. At 4 days post receipt of P. rapae eggs, all viable larvae that completed hatching were placed on live mustard plants (Brassica rapa) in 3.8 l containers fitted with fabric enclosures. Larvae were reared for 10 days to an average stage of 3rd and 4th instar before being randomized between treatments.
Experimental design
One surfactant and two grass-specific herbicides were tested in two mixture formulations: herbicide and water and herbicide mixed with a surfactant. The two graminicides used in experiments were fluazifop-p-butyl (24.5% active ingredient, commercial name Fusilade®, manufacture: Syngenta, Greensboro, North Carolina) and sethoxydim (18% active ingredient, commercial name Poast®, manufacture: BASF, Research Triangle Park, North Carolina). Fluazifop-p-butyl is a selective postemergence herbicide in the aryloxyphenoxypropionate group of pesticides and is applied in agriculture crops and non-crop areas. Sethoxydim is also a postemergent grass-specific herbicide, classified in the cyclohexanedione group of chemical herbicides and is labeled for primary use in agricultural areas and for non-crop grass suppression. The mode of action for both herbicides is the effective inhibition of lipid synthesis, a necessary component of cell membrane formation and plant growth (Walker et al. 1988; Luo et al. 2001).
Preference® is a soy-based nonionic surfactant with the principal functioning agents alkylphenol polyethoxylate and soybean-based fatty acids. Surfactants are common additives in herbicide mixtures designed to promote efficacy by increasing penetration and droplet spread of water-based herbicide spray applications (Gauvrit and Cabanne 1993).
To test for direct exposure and to simulate the effects of direct overspray with ground application, an R&D Precision CO2 powered backpack sprayer (R&D Sprayers, Opelousas, Louisiana) with a hand held wand and flat fan 8002VS nozzle set at a spray pressure of 40 psi was used. After tank was thoroughly cleaned, procedural controls were treated with water. For herbicide applications, the maximum labeled spot spray recommended rates were used for each mixture (fluazifop-p-butyl, 0.75 oz/gal; sethoxydim, 1.9 oz/gal; surfactant, 1 oz/gal). Thus, we added in 2.4 ml of fluazifop-p-butyl, 6 ml of sethoxydim, and when mixed with the surfactant, 3 ml Preference® was added in spray mixture bottles containing 400 ml of water.
The experiment with I. i. blackmorei included a randomized block design with three blocks. Three climate controlled greenhouse bays at the WSU Vancouver campus served as blocks for this study. The six treatments were randomized within each block. Due to small scale environmental differences within each bay, plants were rotated on a weekly basis in an effort to minimize variation within each block. Treatments included a procedural control (C), an untouched control group (UC), fluazifop-p-butyl mixed with water only (F), fluazifop-p-butyl plus surfactant and water (F+), a treatment with surfactant and water only (A), and sethoxydim plus surfactant (S+). The procedural control group (C) acted as a placebo treatment, receiving the same manipulation as the other experimental groups however, the chemical was omitted. The untouched control (UC) group received no experimental manipulation. This experimental design was used with the goal of distinguishing between the effect of herbicide exposure and the effect of the experimental procedure (Dytham 2003).
Each treatment of C, UC, F, F+, and A within each block contained 25 randomly selected larvae (n = 75). The sethoxydim plus surfactant trial (S+) contained 11, 11 and 12 larvae per block (n = 34). A seventh treatment group using a sethoxydim and water mixture was omitted due to limited number of larval specimens.
The experiment with P. rapae included a randomized design in a single greenhouse bay. Numbers of treated larvae within each treatment included C (n = 45), UC (n = 47), F+ (n = 47), P+ (n = 47), and A (n = 47).
Herbicide applications
To mimic most likely exposure in field conditions of herbicide exposure to larvae by direct contact, residue and feeding on herbicide treated plants, randomly selected active I. i. blackmorei larvae were placed on individual lupine plants prior to administering herbicide treatments. Each lupine plant had approximately 4 months of growth and had not been previously used for larval rearing. The treatments were designed to correspond with timing of field herbicide application as recommended by the chemical label. The timing of larval emergence from diapause corresponded with wild I. i. blackmorei populations and is the most likely life stage of herbicide exposure in the wild. According to herbicide application recommendations, larvae would most likely be exposed to herbicides during spring applications corresponding with the 3rd instar larval stage.
After each application plants were left to dry to the point of no observable mist droplets or for a minimum of 15 min before replacing the enclosure and returning plants and larvae to the greenhouse. To prevent cross contamination between herbicide treatments, potted plants were grouped into trays by treatment and bottom watered for the duration of the study. For P. rapae, herbicide application rates and overspray procedures used in the I. i. blackmorei trials were replicated with one exception. To mimic worst case scenario and to ensure exposure, individual larvae were placed in small plastic cups for each treatment. To administer the spray, the nozzle was held approximately 1 m above the treatment group, making a single overspray pass with the appointed chemical. Simultaneously, the treatment group larval host plant was sprayed with the appointed treatment chemical.
Sampling and assessment
The endpoints considered for analysis were percent survival, sex ratio, development time from date of treatment to pupal and adult life stages, biomass, and adult morphology upon eclosure. Survival from date of treatment to eclosure and development time from treatment date to pupae and adult life stages were recorded. Larvae were observed every 2 days to record mortality and feeding behavior (a binary variable: feeding or not feeding) as an indicator of acute toxicity of each chemical or chemical mixture. As larvae approached pupation, larvae were monitored daily to record pupation date and initial pupal weight. Upon eclosure newly emerged adults were placed in glassine envelopes and cooled to arrest movement, then weighed, sexed and photographed for morphological measurements. Morphological measurements included left and right forewing area, abdomen length and maximum width (measured on the ventral side) and head to thorax length (measured on the ventral side). Morphology measurements serve as an indirect indicator of reproductive fitness. Morphology photos were measured using a computerized morphometric analysis program (ImageJ http://rsbweb.nih.gov).
Statistical analysis
Percent survival and sex ratio were assessed using Chi-square (χ2) tests. Development time, pupal and adult biomass were analyzed using Analysis of Variance (ANOVA) with post-hoc Tukey tests to assess pairwise significance. Morphological data were analyzed by discriminant function analysis to determine differences between correlated morphological measurements including wing area, abdomen width and total body length as well as univariate analysis of these variables. These analyses produced similar results (Russell 2008), so only univariate analyses are presented below. All data were log-transformed prior to analysis to meet assumptions of normality and equal variances. P. rapae and I. i. blackmorei data were analyzed separately. Preliminary analyses indicated no differences between procedural control and untouched control for percent survival, sex ratio, development time and morphology of either species (Russell unpublished analyses). Therefore, the untouched control groups were omitted in results which follow. Data were analyzed with Minitab Statistical Software.
Results
Survival and sex ratio
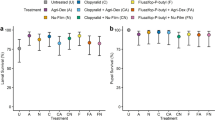
Herbicides are associated with lower survival for P. rapae but not I. i. blackmorei (P. rapae: χ2 = 27.77, df = 3, P < 0.001; I. i. blackmorei: χ2 = 7.21, df = 4, P = 0.206, Fig. 1). For P. rapae, herbicide treated larvae experienced significantly lower survival than the control group. Those treated with sethoxydim(+) were particularly impacted, with a 32% decrease in survival compared to the control group while those treated with fluazifop-p-butyl(+) experienced 21% decrease in survival (Fig. 1a). Treatments are not associated with sex ratios for either species (P. rapae: χ2 = 2.23, df = 3, P = 0.694; I. i. blackmorei: χ2 = 1.57, df = 4, P = 0.905, Table 1).
Development time
Treatments impacted development time for I. i. blackmorei, however, this trend was not observed for P. rapae. Development time for P. rapae larvae, from date of treatment to eclosure was 15.6 ± 1.4 days with no significant difference between treatment and control groups and no significant interaction between treatments and sex (Fig. 2a). For I. i. blackmorei test results indicate that both sexes eclosed earlier in all treatment groups compared to the control (treatment effect F 5,142 = 29.77, P < 0.001, sex effect F 1,142 = 38.70, P < 0.001, Fig. 2b). No interaction between treatment and sex was observed for I. i. blackmorei (F 5,142 = 0.30, P = 0.914). Fluazifop-p-butyl treated larvae eclosed 10.7 ± 6.5 days earlier than control, followed by sethoxydim(+) 9.9 ± 5.6, fluazifop-p-butyl(+) 8.9 ± 5.8 and surfactant 6.9 ± 6.4 days earlier than the control mean (Table 1b).
Development times from date of treatment to pupation were comparable to those for development to eclosure for both species (Table 1).
Pupal and adult biomass
Female P. rapae treated with sethoxydim(+) experienced an average 10% reduction in pupal weight and were on average 21.2 mg lighter than the control group (F 4,77 = 5.33, P < 0.001, Table 1a, Fig. 3a). Male P. rapae treated with surfactant experienced an average 8% reduction in pupal weight and were on average 16.3 mg lighter than the control group (F 4,86 = 2.98, P = 0.023,Table 1a). Treatments did not affect pupal biomass for either sex of I. i. blackmorei (F 5,139 = 0.67, P = 0.644, Fig. 3b, Table 1b). Biomass of male P. rapae pupae was heavier than females (F 1,163 = 44.6, P < 0.001, Fig. 3a) but there were no differences between the sexes for I. I blackmorei (F 1,139 = 0.38, P = 0.54) and there were no interactions between treatment and sex for either treatment (F 5,139 = 2.09, P = 0.07).
Treatments did not affect adult biomass for either species (P. rapae: F 4,166 = 0.61, P = 0.655; I. i. blackmorei: F 5,142 = 0.83, P = 0.529, Table 1).
Morphology
Wing area was significantly smaller in two treatment groups for female P. rapae with a 10% reduction in wing size in fluazifop-p-butyl(+) treatments and a 14% reduction in sethoxydim(+) treatments (F 4,73 = 6.57, P < 0.001, Table 2a). Abdomen width for females treated with the surfactant alone increased by 21% over the control group (F 4,74 = 7.35, P < 0.001, Table 2a) while total body length was similar across treatment groups (Table 2a). Male P. rapae exhibited a 9% reduction in total wing area in the sethoxydim(+) treatment group (F 4,80 = 4.20, P = 0.004, Table 2a) while total body length and abdomen width was similar across treatments (Table 2a).
Treatments did not impact morphological characteristics of either male or female I. i. blackmorei (P > 0.10 for all comparisons, Table 2b).
Discussion
Control of non-native species is critical to habitat maintenance for a wide diversity of at-risk Lepidoptera (Schultz et al 2008). Invasive plants alter the structure of butterfly habitat, compete with key host and nectar plants and alter butterfly behavior (Severns 2008). In the Pacific Northwest, selective herbicide use is one of the most effective tools to reduce non-native plants while limiting impacts on native plant species of concern (Stanley et al. 2009). Studies investigating the impacts of these herbicides on at-risk Lepidoptera are absent and limit the use of these herbicides in many natural areas. In the current study we observe noteworthy effects of post-emergent herbicides on Lepidoptera, in contrast with expected toxicological screening data. Our results raise concern about applications in habitats with at-risk butterflies. Larvae are susceptible to exposure via direct contact, indirect contact from residues on plant surfaces, and ingestion of host plant material sprayed with a herbicide formula (Jepson 1989) and susceptibility to herbicide exposure differs between individual insect species and for species exposed at different life stages (Stark and Banks 2003). In the Pacific Northwest many at-risk butterfly species are in early to mid larval stages in the spring, the main feeding and development period for butterflies (Scott 1986). This critical development period also coincides with recommended timing of post-emergent herbicide applications in this region.
Under US Environmental Protection Authority (EPA) guidelines, standardized toxicity thresholds use adult bees to set the following criteria: highly toxic (acute LD50 less than 2 μg/bee); moderately toxic (acute LD50 2–10.99 μg/bee); slightly toxic (acute LD50 11–100 μg/bee); and practically non-toxic (acute LD50 more than 100 μg/bee; EPA 1992). EPA rates both chemicals in our study as practically non-toxic with LD50 values of 200 μg/bee. Based on laboratory studies, the International organization for biological and integrated control of noxious animals and plants (IOBC) rates sethoxydim as harmful (T) to the parasitoid Trichogramma cacoeciae, moderately harmful (M) to the predatory mite Typholodromus pyri and the flower bug Anthocoris nemoralis, and harmless (N) for three other taxa of terrestrial invertebrates tested (Boller et al. 2005). IOBC rates fluaziflop-butyl as (T) for the hoverfly Syrphus corollae, (M) for T. pyri, and (N) for four other taxa which were tested (Boller et al. 2005). We note that in standardized toxicity screenings on honey bees, exposures are done with topic application methods and may not be indicative of the manner in which invertebrates are exposed to herbicides in the field. In contrast, we expose butterfly larvae to realistic spray applications in which larvae experience herbicides via dermal and oral pathways, procedures which are markedly different than standard toxicological screenings. In addition, methods we use for P. rapae may intensify the treatment effect because it was intended to mimic maximum exposure in the field.
In field application mixtures, fluazifop-p-butyl and sethoxydim concentrates are mixed with water resulting in dilutions beyond the non-toxic threshold of 100 μg/bee. This study indicates that response to herbicide exposure differs between herbicide formula and between species such that sethoxydim(+) and fluazifop-p-butyl(+) have lethal and sub-lethal effects on P. rapae and sub-lethal effects on I. i. blackmorei. Survivorship is a direct indicator of acute toxicity (an exposure in which the dose is delivered in a single event, Girard 2005). In contrast to traditional toxicology screenings which measure acute toxicity and mortality over short time periods, generally a few hours to a few days (Stark and Banks 2003), we observe lethal effects of two herbicides for P. rapae over periods of days to weeks. These data parallel the pupal biomass trends. Low pupal weights correlate with reduced survivorship (Boggs and Freeman 2005), reduced fecundity and reproductive success (Tammaru et al. 1996; Delisle and Hardy 1997).
Results of previous studies indicate that some herbicides are directly toxic to invertebrates following dermal or oral exposure. For example, 2,4,5-T, an herbicide used in forest and nature reserves in The Netherlands, is toxic to springtails (Onychiurus quadriocellatus) upon direct contact (Eijsackers 1978). Coccinella larvae treated with 2,4-D experience higher mortality than untreated controls and surviving treated larvae are smaller than untreated individuals in the same stage of development (Adams 1960). Fifth instar caterpillars of the skipper butterfly C. ethlius die when fed GLA treated host plants (Kutlesa and Caveney 2001). The butterfly larvae in our study were subjected to both direct dermal exposure from overspray and indirect exposure from plant residue or feeding suggesting that P. rapae larvae are sensitive to at least one of these routes of exposure.
At least two mechanisms may lead to reduced pupal weights as a result of herbicide exposure. First, studies suggest that herbicides alter plant physiology, which negatively impacts the diet of herbivorous insects. Some non-target plants exposed to postemergent specific herbicides have reduced sugar, nitrogen and protein levels after herbicide exposure (Asare-Boamah and Fletcher 1982; Peregoy and Glenn 1984). Further, Agnello et al. (1986a) observe that Epilachna varivestis (Coleoptera: Coccinellidae) larvae reared on fluazifop-butyl treated soybeans (Glycine max L.) had lower pupal weights compared to larvae feeding on lima beans (Phaseolus lunatus L.) treated with the same chemical. However, sethoxydim treatments have no effect on Coccinellid pupal weight (Agnello et al. 1986a). In our study decreased pupal weights are observed in treatment groups of P. rapae but not in treatment groups of I. i. blackmorei larvae. One possible explanation for these observed differences is a species-specific plant response between the hostplants in this study, Lupinus albicaulis and Brassica rapa, when exposed to selective herbicides. Alternatively, differences in species-specific results may stem from differences in our protocols.
Second, some herbicide residues have been found to have a repellent effect in which larvae stop feeding or reduce feeding rates on herbicide exposed plants (Agnello et al. 1986a; Brust 1990). For example, food consumption, growth rate, pupation and eclosure decline after exposure and ingestion of herbicide treated plants in studies on cotton leafworm (Spodoptera littoralis, Abo El-Ghar 1994; Meisner et al. 1987). Further, the level of feeding deterrence diminished over time and is dependent on the period of exposure and chemical concentration. In another greenhouse study, simazine and atrazine has short term repellent effects on carabid beetles, (Coleoptera: Carabidae, Brust 1990). Although both chemicals in our study are rapidly absorbed through leaf surfaces, transport and metabolism can take days to weeks. Tu et al. (2001) report that fluazifop-p-butyl takes 2–4 weeks to completely metabolize within a plant and residues of the metabolized form, fluazifop acid, can remain in the plant up to 45 days after treatment.
Larvae ingesting enzyme inhibitors may also experience adverse effects. The mode of action in both sethoxydim and fluazifop-p-butyl is the inhibition of acetyl CoA carboxylase, a necessary enzyme required for cell membrane formation and plant growth (Walker et al. 1988; Luo et al. 2001). This same enzyme is essential for lipid and protein synthesis in insects (Goldring and Read 1993). Acetyl CoA carboxylase enzymes trigger juvenile hormone production in insects which play a vital role in the growth, development, and reproduction (Goldring and Read 1993; Eliyahu et al. 2003; Noriega et al. 2006). In feeding experiments, Popham and Chippendale (1996) observe that the inhibition of acetyl CoA in the southern corn borer (Diatraea grandiosella, Lepidoptera:Pyralidae) is deleterious to newly hatched larvae and impacts size and development of later instar larvae.
Adult morphology, weight and body size are important indicators of fitness and stress in insects. Size reduction is associated with reduced fitness, reduced fecundity in females and reduced reproductive success in male butterflies (Wiklund and Kaitala 1995; Boggs and Freeman 2005). Morphometric analysis of adult butterflies in our study indicate a strong response to herbicides by P. rapae.
Development time is an important ecological trait and can be influenced by stress. Accelerated larval development is associated in food stress studies of the gypsy moth (Lymantria dispar) and a tropical fruit-feeding butterfly, Bicyclus anynana (Leonard 1968; Bauerfeind and Fischer 2005). Herbicides significantly influence development time of I. i. blackmorei larvae. Both sexes of I. i. blackmorei larvae exposed to chemical treatments reach pupation and eclose earlier than control individuals. Although eclosure response followed expected patterns for butterflies in the Lycaenidae family which favor protrandry, a mechanism in which male butterflies eclose earlier than females, early eclosure in both males and females may have broad and detrimental impacts for species with short flight seasons and seasonal plant dependence. Because reproductive success is associated with timing of adult emergence and fecundity (Cushman et al. 1994), changes on development time are of particular interest in butterfly species with short flight and breeding seasons such as I. i. blackmorei. Springtime applications coincide with early to mid instar larval growth stages for many at-risk butterfly species in the Pacific Northwest and flights seasons for many of these species are 3–6 weeks. Herbicide induced alterations of development timing may have long term population level impacts on species which have specialized physiological requirements.
Our study aims to aid managers with developing strategies to control invasive plants while limiting impacts on at-risk butterflies. Although we are not able to separate effects from oral and dermal exposure, the focus of this experiment was to assist managers in understanding the maximum potential effects of these herbicides in the field. Organisms in the field experience herbicides through multiple pathways and therefore specific mechanisms are often of less immediate interest. Of greater interest are differences in species-specific and life stage responses and differences between chemical formulations because these details can be used to design strategies which minimize herbicide effects. For example, timing of herbicide applications may be critical in decreasing any adverse effects to developing larvae or adult butterflies. Applications in late summer and early fall, post flight season and during larval diapause, may reduce effects of both oral exposure and dermal exposure. Species such as I. i. fenderi and I. i. blackmorei cease feeding in early summer when their host plants senesce and diapause larvae retreat within the ground litter (Scott 1986). Results from our study suggest that selective herbicides with similar modes of action have different effects on different butterfly species. These results parallel the species-specific results observed by Stark et al. (2004) and suggest that limiting screening to a single terrestrial insect may be insufficient in evaluating risk of these chemicals in natural areas. Both fluazifop-p-butyl and sethoxydim chemical formulae target grasses through inhibition of lipid synthesis. The EPA considers both formulations as having low toxicity to invertebrates at field recommended rates. However, in our study this is not the case for at least some Lepidoptera. Additional studies are needed to assess influences of herbicides such as fluazifop-p-butyl and sethoxydim on butterflies and their associated hostplants. These studies will assist managers in designing conservation treatments which reduce invasive plants in a manner that minimizes risks to at-risk butterfly populations.
References
Abo El-Ghar GA (1994) Effects of herbicides on consumption, growth and food utilization by cotton leafworm Spodoptera littoralis (Boisd.) larvae. Anzeiger für Schädlingskunde, Pflanzenschutz. Umweltschutz 67:143–146
Adams JB (1960) Effects of spraying 2, 4,-D amine on Coccinellid larvae. Can J Zool 38:285–288. doi:10.1139/z60-035
Agnello AM, Bradley JR Jr, Van Duyn JW (1986a) a) Plant-mediated effects of postemergence herbicides on Epilachna varivestis (Coleoptera: Coccinellidae). Environ Entomol 15:216–220
Agnello AM, VanDuyn JW, Bradley JR (1986b) Influence of postemergence herbicides on populations of bean leaf beetle, Cerotoma trifurcata (Coleoptera: Chysomelidae), and corn earworm, Heliothis zea (Lepidoptera: Noctuidae), in soybeans. J Econ Entomol 79:261–265
Al-Assiuty AIM, Khalil MA (1996) Effects of the herbicide atrazine on Entomobrya musatica (Collembola) in field and laboratory experiments. Appl Soil Ecol 4:139–146. doi:10.1016/0929-1393(96)00107-2
Asare-Boamah NK, Fletcher RA (1982) Phytotoxicity of sethoxydim on corn seedlings. p. 90. Abstracts. Weed Science Society of America, Champaign, Ill
Bauerfeind SS, Fischer K (2005) Effects of food stress and density in different life stages on reproduction in a butterfly. Oikos 111:514–524. doi:10.1111/j.0030-1299.2005.13888.x
Boggs CL, Freeman KD (2005) Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia 144:353–361. doi:10.1007/s00442-005-0076-6
Boller EF, Vogt H, Ternes P, Malavolta C (2005) Working document on selectivity of pesticides. International organization for biological and integrated control of noxious animals and plants (IOBC) http://www.iobc.ch/toolbox.html#6
Borkovec AJ, LaBrecque GC, DeMilo AB (1967) S-Triazine herbicides as Chemosterilants of house flies. J Econ Entomol 60:893–894
Brown JJ (1987) Toxicity of herbicides thiobencarb and endothall when fed to laboratory-reared Trichoplusia ni (Lepidoptera: Noctuidae). Pestic Biochem Physiol 27:97–100. doi:10.1016/0048-3575(87)90100-3
Brust GE (1990) Direct and indirect effects of four herbicides on the activity of Carabid beetles (Coleoptera: Carabidae). Pestic Sci 30:309–320. doi:10.1002/ps.2780300308
Chio H, Sanborn JR (1977) Atrazine inhibition of carbofuran metabolism in the house cricket. J Econ Entomol 70:544–546
Clark D, Blakeley-Smith M, Hammond P, Johnson D, Kaye T, Kelpsas B, Pfund F, Vomocil M, Wilson M (2004) Control of Brachypodium sylvaticum and restoration of rare native upland prairie habitat at butterfly meadows, Benton County. Final Report to Oregon State Weed Board and Oregon Department of Agriculture, Salem
Colborn T, Short P (1999) Pesticide use in the US and policy implications: a focus on herbicides. Toxicol Ind Health 15:241–276. doi:10.1177/074823379901500121
Cushman JH, Boggs CL, Weiss SB, Murphy DD, Harvey AW, Ehrlich PR (1994) Estimating female reproductive success of a threatened butterfly: influence of emergence time and host plant phenology. Oecologia 99:194–200. doi:10.1007/BF00317101
Delisle J, Hardy M (1997) Male larval nutrition influences the reproductive success of both sexes of the spruce budworm, Choristoneura fumiferana (Lepidoptera: Torricidae). Funct Ecol 11:451–463. doi:10.1046/j.1365-2435.1997.00114.x
Dunwiddie P, Delvin E (2006) Preliminary prairie restoration study finds sethoxydim reduces exotics without harming natives (Washington). Ecol Res 24:54
Dytham C (2003) Choosing and using statistics: a biologist guide. Blackwell Science, Malden
Eijsackers H (1978) Side effects of the herbicide 2, 4, 5-T on reproduction, food consumption, and moulting of the springtail Onychiurus quadriocellatus Gisin (Collembola). Z Angew Entomol 85:341–360
Eliyahu D, Applebaum S, Rafaeli A (2003) Moth sex-pheromone biosynthesis is inhibited by the herbicide diclofop. Pestic Biochem Physiol 77:75–81. doi:10.1016/S0048-3575(03)00101-9
Environmental Protection Agency (2009) Code of federal regulations. Title 40, Vol 22:158.630. From the US government printing office via GPO access 40 CFR 158
European Commission Health & Consumer Protection Directorate (ECHCPD) (2002) Guidance document on terrestrial ecotoxicology under council directive 91/414/EEC http://ec.europa.eu/food/plant/protection/evaluation/guidance/wrkdoc09_en.pdf
EXTOXNET (1996a) Fluazifop-p-butyl. Pesticide information profiles. Extension toxicology network. http://extoxnet.orst.edu/pips/fluazifo.htm
EXTOXNET (1996b) Sethoxydim. Pesticide information profiles. Extension toxicology network. http://extoxnet.orst.edu/pips/sethoxyd.htm
Freemark K, Boutin C (1995) Impacts of agricultural herbicide use on terrestrial wildlife in temperate landscapes: a review with special reference to North America. Agric Ecosyst Environ 52:67–91. doi:10.1016/0167-8809(94)00534-L
Fuhlendorf SD, Engle DM, Arnold DC, Bidwell TG (2002) Influence of herbicide application on forb and arthropod communities in North American tallgrass prairies. Agric Ecosyst Environ 92:251–259. doi:10.1016/S0167-8809(01)00291-2
Gauvrit C, Cabanne F (1993) Oils for weed control: uses and mode of action. Pestic Sci 37:147–153. doi:10.1002/ps.2780370207
Girard JE (2005) Principles of environmental chemistry. Jones and Bartlett, Sudbury, p 379
Goldring JP, Read JS (1993) Insect acetyl-CoA carboxylase activity during the larval, pupal, and adult stages of insect development. Comp Biochem Physiol 106:855–858. doi:10.1016/0305-0491(93)90041-3
Haughton AJ, Bell JR, Wilcox A, Boatman ND (2001) The effect of the herbicide glyphosate on non-target spiders: part I. Direct effects on Lepthyphantes tenuis under laboratory conditions. Pest Manag Sci 57:1003–1036
Jepson PC (1989) The temporal and spatial dynamics of pesticide side effects on non-target invertebrates. Pesticides and non-target invertebrates. Intercept, Wimborne, pp 95–128
Kutlesa NJ, Caveney S (2001) Insecticidal activity of glufosinate through glutamine depletion in a caterpillar. Pest Manag Sci 57:25–32. doi:10.1002/1526-4998(200101)57:1<25::AID-PS272>3.0.CO;2-I
Leonard DE (1968) Effects of density of larvae on the biology of the gypsy moth, Porthetria dispar. Entomol Exp Appl 11:291–304. doi:10.1007/BF00333753
Longley M, Sotherton NW (1997) Factors determining the effects of pesticides upon butterflies inhabiting arable farmland. Agric Ecosyst Environ 61:1–12. doi:10.1016/S0167-8809(96)01094-8
Luo X, Matsumoto H, Usui K (2001) Comparison of physiological effects of fluazifop-butyl and sethoxydim on oat (Avena sativa L.). Weed Biol Manag 1:120–127. doi:10.1046/j.1445-6664.2001.00019.x
Meisner J, Lifshitz N, Ascher KRS (1987) Antifeedant properties of herbicides against Spodoptera littoralis larvae (Lepidoptera: Noctuidae), with special reference to pronamide. J Econ Entomol 80:724–727
National Oceanic and Atrmospheric Administration (NOAA) www.noaa.gov. Accessed April (2008)
New TR, Pyle RM, Thomas JA, Thomas CD, Hammond PC (1995) Butterfly conservation management. Annu Rev Entomol 40:57–83. doi:10.1146/annurev.en.40.010195.000421
Noriega FG, Ribeiro JMC, Koener JF, Valenzuela JG, Hernandez-Martinez S, Pham VM, Feyereisen R (2006) Comparative genomics of insect juvenile hormone biosynthesis. Insect Biochem Mol Biol 36:366–374. doi:10.1016/j.ibmb.2006.01.013
Peregoy RS, Glenn S (1984) Protein and nucleic acid synthesis in corn and soybean as affected by fluazifop, pp. 78–79. Abstracts. Weed Science Society of America, Champaign, Ill
Popham HJR, Chippendale GM (1996) Effect of a hypolipidemic agent on the growth and development of the southwestern corn borer, Diatraea grandiosella. Comp Biochem Physiol 115C:247–249
Pratt JR, Melendez AE, Barreiro R, Bowers NJ (1997) Predicting the ecological effects of herbicides. Ecol Appl 7:1117–1124. doi:10.1890/1051-0761(1997)007[1117:PTEEOH]2.0.CO;2
Russell C (2008) Investigating the use of herbicides to control invasive grasses: effects on at-risk butterflies. MS Thesis. Washington State University
Schultz CB, Russell C, Wynn L (2008) Restoration, reintroduction and captive propagation efforts for at-risk butterflies: a review of British and American conservation efforts. Isr J Ecol Evol 54:41–61. doi:10.1560/IJEE.54.1.41
Schultz CB, Dzurisin JD, Russell C (2009) Captive rearing of Puget blue butterflies (Icaricia icarioides blackmorei) and implications for conservation. J Insect Conserv. doi:10.1007/s10841-008-9174-1
Scott JA (1986) The butterflies of North America: a natural history and field guide. Stanford University Press, Stanford
Severns PM (2008) Exotic grass invasion impacts fitness of an endangered prairie butterfly, Icaricia icarioides fenderi. J Insect Conserv 12:651–661. doi:10.1007/s10841-007-9101-x
Stanley AG, Kaye TN, Dunwiddie PW (2009) Multiple treatment combinations and seed additions increase abundance and diversity of native plants in Pacific Northwest prairies. Restor Ecol (in press)
Stark JD, Banks JE (2003) Population-level effects of pesticides and other toxicants on arthropods. Annu Rev Entomol 48:505–519. doi:10.1146/annurev.ento.48.091801.112621
Stark JD, Banks JE, Vargas RI (2004) How risky is risk assessment? The role that life history strategies play in susceptibility of species to stress. Proc Natl Acad Sci USA 101:732–736. doi:10.1073/pnas.0304903101
Tammaru T, Kaitaniemi P, Ruohomäki K (1996) Realized fecundity in Epirrita autumnata (Lepidoptera: Geometridae): relation to body size and consequences to population dynamics. Oikos 77:407–416. doi:10.2307/3545931
Tatum VL (2004) Toxicity, transport, and fate of forest herbicides. Wildl Soc Bull 32:1042–1048. doi:10.2193/0091-7648(2004)032[1042:TTAFOF]2.0.CO;2
Tu M, Hurd C, Randall JM (2001) Weed control methods handbook: tools and techniques for use in natural areas. The Nature Conservancy, http://tncweeds.ucdavis.edu, Version: April 2001
USFWS 2009. Threatened and endangered species list. US fish and wildlife service (USFWS). http://ecos.fws.gov/tess_public/
Verhoef HA, Brussaard L (1990) Decomposition and nitrogen mineralization in natural and agro-ecosystems: the contribution of soil animals. Biogeochemistry 11:175–211. doi:10.1007/BF00004496
Walker KA, Ridley SM, Lewis T, Harwood JL (1988) Fluazifop, a grass-selective herbicide which inhibits acetyl-CoA carboxylase in sensitive plant species. Biochem J 254:307–310
WDFW 2009. Species of Concern in Washington State. Washington department of fish and wildlife http://wdfw.wa.gov/wildlife/management/endangered.html
Wiklund C, Kaitala A (1995) Sexual selection for large male size in a polyandrous butterfly: the effect of body size on male versus female reproductive success in Pieris napi. Behav Ecol 6:6–13. doi:10.1093/beheco/6.1.6
Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E (1998) Quantifying threats to imperiled species in the US. Bioscience 48:607–615. doi:10.2307/1313420
Acknowledgments
We give special thanks to John Stark and Steve Sylvester for their comments, technical assistance, and manuscript review. Many individuals contributed in laboratory support, field work and data collection, including Lora Martinez, Brenda Green, Crystal Hazen, Sara Hansen, and, Kristine Casteel. Leslie Rossmell, Caitlin LaBar and Loni Beyer provided additional feedback during this project. This work was supported by US Fish and Wildlife Service Oregon Field Office, WSU Myer’s Endowment, a Joan Mosenthal DeWind Award for Graduate Research and Conservation on the Lepidoptera from the Xerces Society for Invertebrate Conservation to C. Russell, and a WSU Robert Lane Fellowship in Environmental Studies to C. Russell. In addition we thank two anonymous reviewers and the journal editor for comments which substantially improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russell, C., Schultz, C.B. Effects of grass-specific herbicides on butterflies: an experimental investigation to advance conservation efforts. J Insect Conserv 14, 53–63 (2010). https://doi.org/10.1007/s10841-009-9224-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-009-9224-3