Abstract
Insect physiology is affected by the presence of toxins in the surrounding environment of insects as well as their food sources. The objective of this study was to determine the effect of heavy metal exposure to two low concentrations (50 μg/g and 150 μg/g) of lead (Pb) and zinc (Zn) through artificial diet to the larvae on biological parameters of Asian armyworm (Spodoptera litura Fabricius) (Lepidoptera: Noctuidae). Both Pb and Zn, even at low concentrations, had relatively high toxic effects on S. litura larvae (P < 0.01). S. litura larval weight and length suffered the maximum reduction when the larvae were fed on diet mixed with the high Pb concentration (150 μg/g) tested compared to the other treatments. At the same Pb concentration (150 μg/g), values of larva growth index, pupa growth index, immature growth index, standardized growth index, and fitness index were 4.66, 7.33, 7.82, 5.35, and 10.00 times lower, respectively, than those of control. At the same Zn concentration (150 μg/g), values of larval growth index, pupal growth index, immature growth index, standardized growth index, and fitness index were 5.61, 3.00, 3.04, 3.23, and 9.24 times lower, respectively, than those of control. The survival rate of S. litura larvae was also lower (12.5%) when the larvae were fed on diet mixed with Pb at 150 μg/g after 10 days of observation. Overall, the presence of those heavy metals in the environment, even at low concentrations, would exert an adverse impact on larvae development of this insect. From this point of view, findings could provide a basis for long-term evaluation of heavy metal risk and its impact on populations of important agricultural pests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of heavy metals in the environment creates great concern, because metals commonly deriving from industrialization, increased vehicle use, and modern farming practices contribute to environmental pollution (Atafar et al. 2010; Hamzeh et al. 2011; Liu et al. 2014). Indeed, natural ecosystems are adversely affected by common human interventions (Qadir and Malik 2009). For example, different types of metal-based pesticides, which are widely used to suppress common pests in crops, can lead to contamination of the environment (Verkleij 1993; Gimeno-García et al. 1996; Tariq et al. 2016). Moreover, common fertilization practices can also increase heavy metal concentration in soil in the long-term (Xu et al. 2018; Zhao et al. 2018). Also, the excessive amount of toxic metals in plantation sites not only contaminates soil but also can affect food quality and safety (Sharma et al. 2006; Muchuweti et al. 2006; Sharma et al. 2008).
Insects can accumulate heavy metals in their bodies when living in polluted areas (Heliövaara and Väisänen 1990; Zvereva et al. 2003). In those areas, insects can get into direct contact with contaminated soils in aquatic and terrestrial systems, through air pollution, and also through feeding on plants that have aggregated these materials. By feeding on insects with increased concentrations of these elements, parasites and predators can be also exposed (Vickerman et al. 2002). Metals entering the soil constitute a more lasting form of pollutants due to their accumulation in soil. Waste water from industries can contaminate growing crops when used for irrigation of vegetable crops. For example, municipal waste water is used to irrigate vegetable crops in District Sargodha of Pakistan (Ahmad et al. 2013b), which potentially causes bioaccumulation of heavy metals in biota, including food supplements, with potential harmful effects on the insect body. Heavy metals are not essential for plant growth, but they can be taken up by the roots and moved to the leaves of many plant species (Marschner 1983; Nagajyoti et al. 2010; Page and Feller 2015). In turn, several pests attack vegetable crops and can be directly or indirectly influenced by heavy metals (Heliövaara and Väisänen 1990; Maestri et al. 2010).
Insect behaviors play a significant role in the ecological interactions with plant species as well as with the abiotic environment, and therefore, these behaviors play a major role in the diversity of ecosystems (Fisher 1998; Campbell et al. 2012). Despite the crucial role of insects in most ecosystems, there is limited information on the impact of heavy metal contamination on the behavior of insects. Few insect species show tolerance against heavy metals at low levels, while most insects are negatively affected by metals, showing slower growth, development, and fertility (Xia et al. 2006). Heavy metals like Cd, Pb, and Zn showed detrimental effects on diamondback moth (Plutella xylostella) (Lepidoptera: Plutellidae) at concentrations near normal ranges found in plants (Coleman et al. 2005). Moreover, artificial diet with heavy metals (Zn and Pb) significantly decreased the survival and pupation rate of Plutella xylostella (Jhee et al. 2006), while both Cd and Pb, even at very low concentrations, had relatively high toxic effects on Spodoptera littoralis larvae (Eesa et al. 2017).
Asian armyworm (Spodoptera litura Fabricius) (Lepidoptera: Noctuidae) is an economically important insect pest in the Asia-Pacific region and cause massive damage to agricultural crops (Gao et al. 2004). In Pakistan, S. litura is considered as a major insect pest of many crops (Saleem et al. 2008; Ahmad et al. 2013a). Previous research showed that exposure of S. litura to heavy metals had various toxic effects on this pest, especially Ni (Sun et al. 2008, 2010), Pb, and Zn (Xia et al. 2005; Shu et al. 2009, 2012a, b; Kafel et al. 2014). However, the above studies examined relatively high concentrations of those metals, typically above 50 mg, whereas lower rates were not tested. We hypothesize that many agricultural fields may accumulate concentrations of Pb and Zn that are high enough to alter the physiology of S. litura, but pertinent studies on the topic are lacking. Nevertheless, any negative effect of heavy metals on insect growth and survival could be utilized to test dual benefits of hyper-accumulator plants in controlling insect population and cleaning contaminated soils. Moreover, relevant findings could provide a basis for long-term evaluation of heavy metal risk and its impact on populations of important agricultural pests. In the light of the above, the current study tried to evaluate the impact of Pb and Zn at two concentrations (50 and 150 μg/g) on the growth, development, and survival of S. litura, for which no data exist in the literature.
Materials and methods
Insect culture
The egg batches of S. litura were collected from a tomato field near the University of Sargodha, Pakistan. Chickpea-based artificial diet (Seth and Sharma 2002) was provided to newly hatched larvae and the culture was maintained at 25–27 °C with 60–80% relative humidity (RH). The S. litura adults were released in rearing cages (40 cm × 40 cm × 40 cm) and were provided with 10% sugar solutions on absorbent cotton. The eggs laid by females were taken as a starting point for the first generation, while the second instar larvae of F2 generation (synchronized population) were used for further experiments.
Heavy metal source
The salts of Zn in the form of ZnCl2 and Pb in the form of Pb(NO3)2 were procured from commercial suppliers (Chemical Reagent Co Ltd., Shanghai, China and Wenzhou Chemical Materials Plant, Wenzhou, China, respectively). All chemical reagents were of analytical grade and used as received without further purification, unless otherwise noted.
Insect bioassay
Two concentrations of each heavy metal were added to the diet as shown in Table 1. The artificial diets containing heavy metals were fed to second instar larvae of S. litura. There were five treatments and each treatment replicated eight times. Two larvae were tested in each replicate totaling 16 larvae per treatment. Data for larval length (cm), weight (g), and survival rate were recorded at 24-h intervals for 10 days.
The growth and fitness parameters such as larval growth index, immature growth index, pupal growth index, standardized growth index, and fitness index of S. litura were measured with the formulas suggested by Pretorius (1976) and Itoyama et al. (1999) as below.
Detection of heavy metal in S. litura larval body
The heavy metals were detected as per method of Parven et al. (2009). The fifth instar larvae that were fed on artificial diet mixed with heavy metals were taken, washed with distilled H2O, and dried in an oven for 48 h at 65 °C. The dried larvae were crushed to a fine powder with an electrical grinder. The digestion of the above samples was performed according to the procedure defined by Cock et al. (1976) for the estimation of Zn and Pb accumulation in larval mass. One gram of the dried samples was transferred into a 100-mL conical flask followed by the addition of 10 mL of the tri-acid mixture (HNO3, HClO4, and H2SO4 at 5:2:1). The flasks were heated on a hot plate until fumes disappeared and the volume was reduced to 1.5 mL indicated by the clear colorless solution. After that, 1 mL of perchloric acid was placed to each flask to cool the solution. The sample was filtered with Whatman No. 1 filter paper after cooling and then diluted up to 250 mL using deionized distilled water in a volumetric flask. Accumulation of heavy metals (Zn and Pb) was detected by atomic absorption spectrophotometer (AA-6300 Series Polarized Zeeman, Hitachi, Japan), using the specific lamp for each specific metal at Hi-Tech Laboratory, Department of Pharmacy, University of Sargodha, Pakistan. First, standard solutions were used to calibrate the instrument and then a calibration curve was created. The actual concentration was estimated in the samples by establishing calibration curves.
Statistical analysis
Data for larval length, weight, growth, and fitness indices, as well as heavy metals accumulation, were subjected to analysis of variance under a completely randomized design (CRD). The survival rate was calculated by the Kaplan-Meier test at a confidence level 95%. Means (all-pairwise comparisons) were compared with Tukey’s honestly significant difference (HSD) test at P < 0.05. Moreover, regression analysis was used to determine the relationship of each studied variable to the rates of Zn and Pb tested. All analyses were performed using SPSS (ver. 20.0) and Minitab (ver. 16.1) software.
Results
Larval length, weight, and survival rate
Heavy metals (Pb and Zn) significantly reduced larval length (F = 57.6; P < 0.01) and weight (F = 44.2; P < 0.01) of S. litura in a dose-dependent manner (Fig. 1). The maximum reduction of larval length (0.54 cm) and weight (0.06 g) was found in larvae fed on diet mixed with Pb (150 μg/g) as compared with control (1.94 cm and 0.27 g, respectively) (Fig. 1). Linear regression showed that Pb exerted more toxic effect on both traits than Zn (greater slopes of Pb than those of Zn), while the effect was more severe on larval length (Table 2).
A significant difference (χ2 = 10.223; P < 0.01) was found in the survival of S. litura larvae after exposure to heavy metals compared with control. The survival rate was higher (85–100%) in control than the heavy metal exposed treatments during all sampling days (Fig. 2). However, larvae survival was significantly reduced with the application of heavy metals. The low concentrations of Pb and Zn (50 μg/g) reduced significantly survival rate from the fourth day of exposure, while the high concentrations of Pb and Zn (150 μg/g) reduced significantly survival rate even from the second day of exposure (Fig. 2). The maximum reduction of survival rate occurred after exposure to the high rates of Pb and Zn (150 μg/g) at the end of the assessment period.
Growth and fitness indices
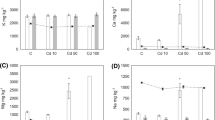
Heavy metals significantly affected (P < 0.01) larval growth, pupal growth, and immature growth of S. litura larvae in a dose-dependent manner (Fig. 3). The larval growth index was maximum (16.49) in control, while the lowest value (2.94) was observed when larvae were fed on Zn at 150 μg/g, which was statistically at par with Pb (3.54) when fed with the same concentration (150 μg/g). The pupal growth index of S. litura was maximum (15.45) in control, while the lowest value (2.11) was observed when larvae were fed on diet mixed with Pb at 150 μg/g, followed by the value 5.14 when larvae were fed on Zn at the same concentration (150 μg/g) (Fig. 3). Similarly, the immature growth index significantly decreased (1.05) in the case of Pb at 150 μg/g compared with control (8.25). Similar results were also found in the case of standardized growth and fitness index (Fig. 4). Control showed the highest values of standardized growth (0.054) and fitness index (2.33), while the lowest values (5.35 and 10.00 times lower than control) occurred with the high rate of Pb (150 μg/g). Linear regression showed that Pb exerted more toxic effect on most traits (greater slopes of Pb than those of Zn), except from LGI and FI, where Zn was found more toxic (Table 3). The effect of heavy metal exposure was more severe on LGI and PGI as compared with the other indices (Table 3).
Detection of heavy metal in larval body and feces
Heavy metal exposure significantly affected (P < 0.01) Zn and Pb accumulation in fifth instar larval body of S. litura. Atomic absorption spectrophotometry indicated that 2 μg/g of either ZnCl2 or Pb(NO3)2 were found in the body of untreated larvae (control) (Table 4). However, 120 μg/g of Pb(NO3)2 was detected in larvae feeding on either concentration of Pb(NO3)2, while the ZnCl2 content in larvae feeding on either concentration of ZnCl2 was zero. The Pb(NO3)2 content was zero in larvae feeding on both concentrations of ZnCl2, but ZnCl2 content was 120 μg/g in those larvae (Table 4). Similarly, heavy metal exposure significantly affected accumulation Zn and Pb (P < 0.01) in feces of fifth instar larvae of S. litura. Quantities of 2.5 μg/g of ZnCl2 and 1.5 μg/g of Pb(NO3)2 were found in feces of untreated larvae (control) (Table 4). However, a quantity of 200 μg/g of Pb(NO3)2 was detected in feces of larvae feeding on both concentrations of Pb(NO3)2, while ZnCl2 content was zero. Conversely, Pb(NO3)2 concentration was zero in feces of larvae feeding on both concentrations of ZnCl2, but ZnCl2 content was 200 μg/g in feces of those larvae (Table 4).
Discussion
Many agricultural fields may accumulate concentrations of Pb and Zn that are high enough to alter the physiology of S. litura, but pertinent studies on the topic are lacking. In previous research, artificial diet with Zn and Pb significantly decreased the survival and pupation rate of Plutella xylostella (Jhee et al. 2006). In the current study, giving artificial diet enriched with low rates of Zn or Pb to S. litura showed a significant dose-dependent effect on growth and development parameters of this insect. Several researchers reported harmful effects of Zn on insect growth and development (Coleman et al. 2005; Sharaby et al. 2011; Bahadorani and Hilliker 2009; Al-Dhafar and Sharaby 2012; Kazemi-Dinan et al. 2014). However, Stolpe and Müller (2016) reported an increased aphid growth when fed on Zn-enriched diets, whereas Cd reduced the survival of aphids. In line with the results of our study, a recent study (Eesa et al. 2017) found that both Cd and Pb, even at very low concentrations, had relatively high toxic effects on S. littoralis larvae, with Cd being more toxic than Pb. Moreover, many morphological and structural changes were noticed in larvae and pupae of Drosophila melanogaster after 48 h of Pb acetate application (Haq et al. 2011).
Zn is an important element for animal nutrition (Maret 2005), but can negatively affect growth and development of organisms, especially insects, if its concentration exceeds physiological limits. As reported in the literature, Zn in insect food can destroy the midgut structure and influence the processes of digestion and absorption of food (Al-Dhafar and Sharaby 2012), thus causing starvation effects and reducing larval growth and survival. Reduction in growth and development due to Pb-enriched diet might be due to Pb toxic effects on the body of larvae, which decreases larval growth and development (Safaee et al. 2014). Locusts fed on food enriched with coumarin and Zn showed aversion to Zn that was developed by a post-ingestive mechanism involving associative learning (Behmer et al. 2005). Oxidative stress causing a consistent weakness of the larvae by starvation was reported as the mechanism of acute toxicity of metals on larvae, which probably decreased survival of the larvae (Leonard et al. 2004), with mortality rate being high especially in young larvae. A high mortality rate in young larvae was also found in other Lepidopterous insects and beetle larvae (Lapointe et al. 2004). Diet enriched with Zn or Pb increased mortality of Plutella xylostella larvae (Jhee et al. 2006), mortality of three herbivore species specialists on Brassicaceae (i.e., Pieris napi, Athalia rosae, and Phaedon cochleariae) (Kazemi-Dinan et al. 2014), and mortality of the generalist aphid Myzus persicae (Stolpe and Müller 2016).
Heavy metal contamination in our environment has become a major problem (Sun et al. 2007). These metals are immobilized in soil and can remain for long at the same place; thus, they can enter into organisms through the food chain (Qin et al. 2010). Previously, Lepidopterous insects have been used as bioindicators of environmental pollution near industrial and urban areas due to increased levels of heavy metals and carbon dioxide concentration (Azam et al. 2015). The occurrence of Cu, Cd, Fe, Ni, and SO4−2 ions and other materials used in fertilizers were studied in pupae of various Geometridae and Noctuidae species (Heliövaara and Väisänen 1990) and by monitoring the different life cycle and mortality rate in hatched larvae of butterflies (Nymphalidae) that feed on plants subjected to high carbon dioxide concentration (Fajer et al. 1989). The ecology, morphology, and physiology of insects are greatly affected by the presence of toxins, including heavy metals like Zn, Co, Ni, Pb, Cd, and Cr in their environment and food sources, as previously reported in earlier studies (Sorsa and Pfeifer 1973; Jeantet et al. 1977; Martoja et al. 1983), while the use of some insect groups as heavy metal indicators is examined up to nowadays (Nummelin et al. 2007; Azam et al. 2015).
Feeding efficiency is the ability of insects to ingest the food to the best of its potential. Our study demonstrated the effect of heavy metals on the feeding indices of S. litura larvae. The larval weight and length were reduced dramatically when the larvae were fed on diet mixed with the high concentration of either heavy metal compared with control (artificial diet only). A significant negative effect of Zn was previously found on larval mortality, pupal stage, and adult emergence of Heliothis virescens (F.) (Lepidoptera: Noctuidae) (Popham and Shelby 2006). Similarly, Baghban et al. (2014) observed reduced growth and mounting rate of cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) due to increased glycogen level by ZnCl2 application. Kafel et al. (2014) also noted that Zn decreased S. exigua larval weight.
The growth parameters of S. litura, such as larval growth index, pupal growth index, and immature growth index as well as standardized growth index and fitness index, were the lowest when larvae were fed on diet mixed with the high concentrations of either Pb or Zn. A previous study showed that larval survival rate, pupation percentage, and adult emergence were also decreased significantly with increasing the amount of heavy metal stress (Sun et al. 2007). Furthermore, Drosophila melanogaster (Diptera: Drosophilidae) larval survival rate was decreased significantly after feeding on diet mixed with Cd (Shirley and Sibly 1999). Comparatively, the high concentration of Pb reduced larval length and growth to the maximum extent compared with Zn. Our results keep up to those of Ying-Hua et al. (2012) who noted that Pb caused a significant effect on S. litura larval growth and reproduction. With an increase in Pb concentrations, body weight and survival rate of the larvae at different developmental stages (larvae, pupae, and adult) were reduced. The survival rate of S. litura larvae was the lowest when the larvae were fed on diet mixed with the high concentrations of either Pb or Zn, while the highest survival rate was observed in control. Our findings are in accordance with those of Popham and Shelby (2006) who reported that Zn negatively affected survival of immature stages of H. virescens and about 80% of the larvae failed to convert into pupae at 60 ppm concentration.
The accumulation of heavy metals in the insect body may hinder growth and development of these organisms. After application of Pb, the accumulation of heavy metals in larval body and feces was in the order Pb > Zn, while after application of Zn, the opposite trend (Zn > Pb) was observed. However, the accumulation of both heavy metals was found to be higher in feces compared to the larval body. According to Augustyniak et al. (2006), the excessive amount of Zn accumulated in grasshopper Chorthippus brunneus (Orthoptera: Acrididae) brain caused impairment in DNA structure. According to Huang et al. (2012), the feeding of S. litura on artificial diet mixed with Cu at 50 mg/kg may result in Cu accumulation in later larval stages (fourth and fifth instar), which provoked low rate of survival in the later larval instars during pupation period as well as adult emergence, eventually leading to other negative impacts on fertility and fecundity. Such accumulation of heavy metals may retard growth and development of insects. Our results clearly suggest that Pb and Zn, even at low rates, have potential to affect insect growth and survival, but the mechanism of these effects is still unknown. Therefore, future studies, probably at molecular level, might be useful towards documentation of the toxic mechanisms of heavy metals on this insect.
Conclusion
Understanding modification of insect behavior after exposure to heavy metals is necessary to assess the importance of heavy metal contamination. The current study evaluated the negative effects of low rates of Zn and Pb exposure through artificial diet on S. litura. Overall, the presence of those heavy metals in the environment, even at low concentrations, is capable of exerting a significant adverse impact on larval development of this insect. Findings highlight the threat to insect lives in the industrial area of Pakistan, where Pb and Zn concentrations are increasing and call upon measures for lessening environmental pollution in all levels (soil, air, and water) by enforcing implementation of pollution control laws. Moreover, findings could provide a basis for long-term evaluation of heavy metal risk and its impact on populations of important agricultural pests.
References
Ahmad M, Ghaffar A, Rafiq M (2013a) Host plants of leafworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in Pakistan. Asian J Agric Biol 1:23–28
Ahmad K, Khan ZI, Jabeen H, Ashraf M, Shaheen M, Raza SH (2013b) Assessment of heavy metals and metalloids toxicity in buffaloes fed on forages irrigated with domestic wastewater in Bhalwal, Sargodha, Pakistan. Pakistan J Zool 45:1629–1637
Al-Dhafar ZM, Sharaby A (2012) Effect of zinc sulfate against the red palm weevil Rhynchophorus ferrugineus with reference to their histological changes on the larval midgut and adult reproductive system. J Agric Sci Technol A2:888–900
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi AH (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89
Augustyniak M, Juchimiuk J, Przybyłowicz WJ, Mesjasz-Przybyłowicz J, Babczyńska A, Migula P (2006) Zinc-induced DNA damage and the distribution of metals in the brain of grasshoppers by the comet assay and micro-PIXE. Comp Biochem Physiol C Toxicol Pharmacol 144:242–251
Azam I, Afsheen S, Zia A, Javed M, Saeed R, Sarwar MK, Munir B (2015) Evaluating insects as bioindicators of heavy metal contamination and accumulation near industrial area of Gujrat, Pakistan. Biomed Res Int 2015:942751
Baghban A, Sendi JJ, Zibaee A, Khosravi R (2014) Effect of heavy metals (Cd, Cu, and Zn) on feeding indices and energy reserves of the cotton bollworm Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). J Plant Prot Res 54:367–373
Bahadorani S, Hilliker AJ (2009) Biological and behavioral effects of heavy metals in Drosophila melanogaster adults and larvae. J Insect Behav 22:399–411
Behmer ST, Lloyd CM, Raubenheimer D, Stewart-Clark J, Knight J, Leighton RS, Harper FA, Smith JAC (2005) Metal hyperaccumulation in plants: mechanisms of defence against insect herbivores. Funct Ecol 19:55–66
Campbell AJ, Biesmeijer JC, Varma V, Wäckers FL (2012) Realising multiple ecosystem services based on the response of three beneficial insect groups to floral traits and trait diversity. Basic Appl Ecol 13:363–370
Cock J, Yoshida S, Forno DA (1976) Laboratory manual for physiological studies of rice. International Rice Research Institute, Manila
Coleman CM, Boyd RS, Eubanks MD (2005) Extending the elemental defense hypothesis: dietary metal concentrations below hyperaccumulator levels could harm herbivores. J Chem Ecol 31:1669–1681
Eesa NM, El-Sherif H, El-Sayed WM, Abd El-Monem DH (2017) Bioefficacy of cadmium and lead on cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae) larvae. Inv Reprod Dev 61:27–33
Fajer ED, Bowers MD, Bazzaz FA (1989) The effects of enriched carbon dioxide atmospheres on plant-insect herbivore interactions. Science 243:1198–1201
Fisher BL (1998) Insect behavior and ecology in conservation: preserving functional species interactions. Ann Entom Soc Am 91:155–158
Gao C, Bei Y, Chen T, Gu X (2004) On factors causing the outbreak of Spodoptera litura (Fabricius). Acta Agric Univ Zhejiangensis 16:332–335
Gimeno-García E, Andreu V, Boluda R (1996) Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pollut 92:19–25
Hamzeh MA, Aftabi A, Mirzaee M (2011) Assessing geochemical influence of traffic and other vehicle-related activities on heavy metal contamination in urban soils of Kerman city, using a GIS-based approach. Environ Geochem Health 33:577–594
Haq R, Farhanullah Khan M, Haq E (2011) Adverse effect of lead acetate on Drosophila melanogaster. J Basic Appl Sci 7:157–163
Heliövaara K, Väisänen R (1990) Heavy-metal contents in pupae of Bupalus piniarius (Lepidoptera: Geometridae) and Panolis flammea (Lepidoptera: Noctuidae) near an industrial source. Environ Entomol 19:481–485
Huang D, Kong J, Seng Y (2012) Effects of the heavy metal Cu2+ on growth, development, and population dynamics of Spodoptera litura (Lepidoptera: Noctuidae). J Econ Entomol 105:288–294
Itoyama K, Kawahira Y, Murata M, Tojo S (1999) Fluctuations of some characteristics in the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae) reared under different diets. Appl Entomol Zool 34:315–321
Jeantet AY, Ballan-Dufrancais C, Martoja R (1977) Insects resistance to mineral pollution. Importance of spherocrystal in ionic regulation. Rev Ecol Biol Sol 14:563–582
Jhee EM, Boyd RS, Eubanks MD (2006) Effectiveness of metal-metal and metal-organic compound combinations against Plutella xylostella: implications for plant elemental defense. J Chem Ecol 32:239–259
Kafel A, Rozpedek K, Szulińska E, Zawisza-Raszka A, Migula P (2014) The effects of cadmium or zinc multigenerational exposure on metal tolerance of Spodoptera exigua (Lepidoptera: Noctuidae). Environ Sci Pollut Res 21:4705–4715
Kazemi-Dinan A, Thomaschky S, Stein RJ, Krämer U, Müller C (2014) Zinc and cadmium hyperaccumulation act as deterrents towards specialist herbivores and impede the performance of a generalist herbivore. New Phytol 202:628–639
Lapointe SL, Weathersbee AA III, Doostdar H, Mayer RT (2004) Effect of dietary copper on larval development of Diaprepes abbreviatus (Coleoptera: Curculionidae). Florida Entomol 87:25–29
Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37:1921–1942
Liu Y, Su C, Zhang H, Li X, Pei J (2014) Interaction of soil heavy metal pollution with industrialisation and the landscape pattern in Taiyuan city, China. PLoS One 9:e105798
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68:1–13
Maret W (2005) Zinc coordination environments in proteins determine zinc functions. J Trace Elem Med Biol 19:7–12
Marschner H (1983) General introduction to the mineral nutrition of plants. In: Lauchii A, Bieleskir L (eds) Inorganic plant nutrition. Springer Verlag, New York, pp 5–60
Martoja R, Bouquegneau JM, Verthe C (1983) Toxicological effects and storage of cadmium and mercury in an insect Locusta migratoria (Orthoptera). J Invert Pathol 42:17–32
Muchuweti M, Birkett JW, Chinyanga E, Zvauya R, Scrimshaw MD, Lester JN (2006) The heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: implications for human health. Agric Ecosyst Environ 112:41–48
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nummelin M, Lodenius M, Tulisalo E, Hirvonen H, Alanko T (2007) Predatory insects as bioindicators of heavy metal pollution. Environ Pollut 145:339–347
Page V, Feller U (2015) Heavy metals in crop plants: transport and redistribution processes on the whole plant level. Agronomy 5:447–463
Parven N, Bashar MA, Quraishi SB (2009) Bioaccumulation of heavy and essential metals in trophic levels of the pond ecosystem. J Bangladesh Acad Sci 33:131–138
Popham HJ, Shelby KS (2006) Uptake of dietary micronutrients from artificial diets by larval Heliothis virescens. J Insect Physiol 52:771–777
Pretorius LM (1976) Laboratory studies on the development and reproductive performance of Heliothis armigera (Hubn.) on various food plants. J Entomol Soc South Afr 39:337–343
Qadir A, Malik RN (2009) Assessment of an index of biological integrity (IBI) to quantify the quality of two tributaries of river Chenab, Sialkot, Pakistan. Hydrobiologia 621:127–153
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65
Safaee S, Fereidoni M, Mahdavi-Shahri N, Haddad F (2014) Effects of lead on the development of Drosophila melanogaster. Period Biol 116:259–265
Saleem MA, Ahmad M, Ahmad M, Aslam M, Sayyed AH (2008) Resistance to selected organochlorine, organophosphate, carbamates and pyrethroid insecticides in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. J Econ Entomol 101:1667–1675
Seth RK, Sharma VP (2002) Growth, development, reproductive competence and adult behaviour of Spodoptera litura (Lepidoptera: Noctuidae) reared on different diets. In: Bloem S, Carpenter JE, Hendrichs J (eds) Evaluation of Lepidoptera population suppression by radiation induced sterility. IAEA-TECDOC-1283, Vienna, pp 15–28
Sharaby A, EL-Hawari F, Ibrahim SA (2011) Some inorganic salts for production of sterile adults of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J Agric Sci Technol 1:728–733
Sharma RK, Agrawal M, Marshall F (2006) Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull Environ Contam Toxicol 77:312–318
Sharma RK, Agrawal M, Marshall FM (2008) Heavy metal (Cu, Zn, Cd and Pb) contamination of vegetables in urban India: a case study in Varanasi. Environ Pollut 154:254–263
Shirley MD, Sibly RM (1999) Genetic basis of a between-environment trade-off involving resistance to cadmium in Drosophila melanogaster. Evolution 53:826–836
Shu Y, Gao Y, Sun H, Zou Z, Zhou Q, Zhang G (2009) Effects of zinc exposure on the reproduction of Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Ecotoxicol Environ Saf 72:2130–2136
Shu Y-H, Du Y, Wang J-W (2012a) Effects of lead stress on the growth and reproduction of Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Chin J Appl Ecol 23:1562–1568
Shu Y, Zhang G, Wang J (2012b) Response of the common cutworm Spodoptera litura to zinc stress: Zn accumulation, metallothionein and cell ultrastructure of the midgut. Sci Total Environ 438:210–217
Sorsa M, Pfeifer S (1973) Effects of cadmium on development time and prepupal puffing pattern of Drosophila melanogaster. Hereditas 75:273–277
Stolpe C, Müller C (2016) Effects of single and combined heavy metals and their chelators on aphid performance and preferences. Environ Toxicol Chem 35:3023–3030
Sun H, Shu Y, Tang W, Wang Q, Zhou Q, Zhang G (2007) Nickel accumulation and its effects on the survival rate of Spodoptera litura Fabricius under continuous nickel stress. Chin Sci Bull 52:1957–1963
Sun HX, Zhou Q, Tang WC, Shu YH, Zhang GR (2008) Effects of dietary nickel on detoxification enzyme activities in the midgut of Spodoptera litura Fabricius larvae. Chin Sci Bull 53:3324–3330
Sun HX, Xia Q, Tang WC, Zhou Q, Zhang GR, Dang Z (2010) Effects of dietary nickel on apoptosis of hemocytes of Spodoptera litura (Fabricius) larvae. Chin Sci Bull 55:390–397
Tariq SR, Shafiq M, Chotana GA (2016) Distribution of heavy metals in the soils associated with the commonly used pesticides in cotton fields. Scientifica 2016:7575239
Verkleij JA (1993) The effects of heavy metal stress on higher plants and their use as biomonitors. Plant as bioindicators: indicators of heavy metals in the terrestrial environment. VCH, New York, pp 415–424
Vickerman DB, Young JK, Trumble JT (2002) Effect of selenium-treated alfalfa on development, survival, feeding and oviposition preferences of Spodoptera exigua (Lepidoptera: Noctuidae). Environ Entomol 31:953–959
Xia Q, Sun H, Hu X, Shu Y, Gu D, Zhang G (2005) Apoptosis of Spodoptera litura larval hemocytes induced by heavy metal zinc. Chin Sci Bull 50:2856–2860
Xia Q, Hu X, Shu Y, Sun H, Zhang G, Gu D (2006) Survival and development of | sl Microplitis bicoloratus Chen on larvae of Spodoptera litura Fabricius stressed by heavy metal zinc. Acta Entomol Sin 49:387–392
Xu Y, Tang H, Liu T, Li Y, Huang X, Pi J (2018) Effects of long-term fertilization practices on heavy metal cadmium accumulation in the surface soil and rice plants of double-cropping rice system in Southern China. Environ Sci Pollut Res 25:19836–19844
Ying-Hua S, Yan D, Jian-Wu W (2012) Effects of lead stress on the growth and reproduction of Spodoptera litura Fabricius (Lepidoptera: Noctuidae). J Appl Ecol 23:1562–1568 (in Chinese with English abstract)
Zhao S, Qiu S, He P (2018) Changes of heavy metals in soil and wheat grain under long-term environmental impact and fertilization practices in North China. J Plant Nutr 41:1970–1979
Zvereva E, Serebrov V, Glupov V (2003) Activity and heavy metal resistance of non specific esterases in leaf beetle, Chrysomela lapponica from polluted and unpolluted habitats. Comp Biochem Physiol C Toxicol Pharmacol 135:383–391
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Giovanni Benelli
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, S., Ullah, M.I., Saeed, M.F. et al. Heavy metal exposure through artificial diet reduces growth and survival of Spodoptera litura (Lepidoptera: Noctuidae). Environ Sci Pollut Res 26, 14426–14434 (2019). https://doi.org/10.1007/s11356-019-04792-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04792-0