Abstract
Introduction
Single-shot devices have been developed to simplify pulmonary vein isolation (PVI). Randomized studies of the second-generation cryoballoon (CB 2nd) demonstrated excellent results. There are limited data comparing results of circular pulmonary vein ablation catheter (PVAC) with conventional RF ablation or CB for PVI.
Objective
Using a sequential registry cohort and a prospective randomized study, we aimed to compare the acute and long-term results of CB 2nd and PVAC Gold.
Methods
In the registry, consecutive patients with paroxysmal atrial fibrillation (AF) undergoing their first PVI were included. The preferred method used was PVAC Gold in 2014 and CB 2nd in 2015. Subsequently, a randomized study (PVAC vs. CB 2nd) was performed. Ablation success was measured as freedom of AF or atrial tachycardias (AT) off antiarrhythmic drugs.
Results
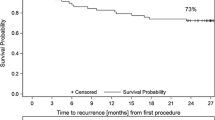
In the registry cohort, PVAC Gold was used in 60 patients and CB 2nd in 56 patients (age 66 ± 11 years, 52% male, LAD 43 ± 6). In the randomized study, 20 patients were treated with PVAC Gold and 22 with CB 2nd (age 67 ± 9; 43% men, LAD 40 ± 7 mm). During a mean follow up of 13.2 ± 3.6 months, success was 54% in PVAC Gold patients and 81% in CB 2nd cases (p = 0.001). In the randomized study 12 months success was 50% versus 86%, p < 0.05. Complications occurred rare in both groups.
Conclusions
Our registry data and the randomized study both suggest superiority of PVI using CB 2nd as compared with PVI using PVAC Gold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Complete pulmonary vein isolation (PVI) at the atrial level is the best documented target for catheter ablation of atrial fibrillation (AF) [1]. In paroxysmal AF, the long-term success of sole PVI is comparably high. Single-shot devices have been developed to simplify the PVI procedure. Several studies have documented that one of the single-shot devices, the cryoballoon (CB), can replicate the results of point-by-point radiofrequency (RF) ablation [2, 3]. This is true even when comparing second-generation techniques, such as CB ablation using Artic Front Advance and novel contact force guided RF ablation using 3D mapping [4, 5]. In paroxysmal AF cases, CB 2nd ablation results in an approximately 80% success rate [4,5,6]. The pulmonary vein ablation catheter (PVAC) is another tool that provides a simplified, fast method for achieving PVI. It consists of a circular mapping and ablation catheter and uses pulsed RF energy. The procedures can be performed very quickly and are associated with only minimal periprocedural risks [7,8,9]. Published success rates for the second-generation device of the PVAC (PVAC Gold) seem to be less optimal than those documented for CB 2nd [7,8,9,10]. The aim of our study was to compare for the first time procedural data, including complications and success rates, of the second-generation CB and PVAC devices. This involved the evaluation of a registry cohort followed by the performance of a randomized study comparing CB 2nd and PVAC-Gold for the ablation of paroxysmal AF. This represents the first direct comparison of acute and long-term results of the second-generation PVAC and CB techniques for treatment of paroxysmal AF.
2 Methods
2.1 Study design
The present study consisted of two parts. The first was a prospective, observational, single-center registry comparing outcomes of patients with paroxysmal AF who had undergone ablation procedures using CB 2nd or PVAC-Gold.
The second part of the study was a prospective, randomized, single center, pilot trial in patients undergoing a first ablation attempt for paroxysmal AF. Such patients were randomized to PVAC Gold or to CB 2nd ablation.
For the randomized pilot study, a cohort of 60 patients had been planned. A pre-specified interim analysis was performed after 12 months, at which point results were available for at least 15 patients in each group. Because of a clear difference in outcomes found during this interim analysis, we stopped the study prematurely for ethical reasons.
Both studies were approved by the Ethics Commission of the Landesärztekammer Thüringen, and all patients gave written, informed consent.
2.2 Patients
At our institution, the preferred method for PVI in paroxysmal AF in 2014 was PVAC Gold, but in 2015 it was CB 2nd. Each operator was experienced in the used method, meaning that each had performed > 50 procedures.
The inclusion criteria for the subsequent randomized study comparing both techniques were highly symptomatic paroxysmal AF and voluntarily given informed consent for both the randomization and the AF ablation procedure used. Exclusion criteria were persistent AF, left atrial (LA) thrombus, manifest hyperthyroidism, indication for heart valve surgery, cardiac surgery less than 3 months prior to the planned AF ablation, or a previous ablation attempt.
2.3 Ablation procedures
2.3.1 Pre-procedure
All patients underwent basic clinical and standard laboratory examinations to rule out hyperthyreodism or coagulopathy. Transthoracic and transesophageal echocardiography (TTE and TEE) were performed the day before the ablation procedure. No computed tomography or magnetic resonance imaging (MRI) scans of the LA and PV were performed prior to ablation. The ablations were performed under conscious sedation.
2.3.2 Ablation procedure using PVAC Gold
Femoral access was obtained for two sheaths. A decapolar coronary sinus (CS) catheter was introduced (Inquiry, St. Jude Medical, Saint Paul, MN, US). With fluoroscopy, pressure controlled transseptal puncture was performed using an SL1 sheath and BRK1 transseptal needle (St. Jude Medical, Saint Paul, MN, US). Thereafter heparin was administered to achieve an activated clotting time ≥ 300 s. The SL1 sheath was exchanged for the PVAC sheath (Medtronic, Minneapolis, MN, US), which was continuously flushed with heparinized saline. Angiography with selective contrast injection was performed to visualize the pulmonary vein ostia. Under continuous flushing, the PVAC Gold catheter (Medtronic, Minneapolis, MN, US) was introduced into the LA and then positioned at the PV antrum after wiring the PV (Fig. 1). Using the corresponding multichannel RF generator GENius R (Medtronic, Minneapolis, MN, US), RF energy was delivered simultaneously in a duty-cycled bipolar and unipolar fashion (2:1 ratio, maximum power 8 W for 1 min, temperature limit 60 °C). The PVAC catheter was rotated around the ostium of the PV, to deliver further energy applications until local signals disappeared. Using the PVAC electrodes slightly inside the PV and performing differential pacing, entrance- and exitblock was proven after the ablations. No further substrate modification techniques were applied. For the prospective randomized study, PVI entrance- and exitblock were tested again 20 min after the last ablation. Adenosine was not used to reveal possible dormant conduction after PVI.
2.3.3 Ablation procedure using CB 2nd
For the cryoballoon procedures, a 12F and a 7F introducer sheath (St. Jude Medical, Saint Paul, MN, US) were introduced into the femoral vein. A decapolar CS catheter (Inquiry, St. Jude Medical, Saint Paul, MN, US) was introduced. Transseptal puncture was performed fluoroscopically and with pressure guidance using an SL1 sheath (St. Jude Medical, Saint Paul, MN, US) and a BRK1 transseptal needle (St. Jude Medical, Saint Paul, MN, US). Heparin was administered to maintain an ACT ≥ 300 ms. The SL1 sheath was removed, and a 14F Flexcath Advance (Medtronic, Minneapolis, MN, US) sheath was introduced into the LA and flushed continuously with heparinized saline. The cryoballoon Arctic Front Advance 28 mm (Medtronic, Minneapolis, MN, US) was introduced into the LA using an Achieve catheter (Medtronic, Minneapolis, MN, US). Then, using the Achieve catheter as a guidewire, the CB was positioned at each PV antrum (Fig. 1). Occlusion of the PV ostium was confirmed with contrast injections, and the catheter was repositioned when occlusion was not optimal. At least two freeze cycles, each lasting 240 s, were performed at each vein. In each case, at least one bonus freeze was performed after reaching isolation. Phrenic nerve function was monitored by pacing the phrenic nerve from the superior vena cava (SVC) with the CS catheter and measuring the diaphragmatic compound motor action potentials using surface ECG leads as previously described [11]. The ablation procedure was considered finished when there was persistent bidirectional conduction block of the PV 20 min after bidirectional conduction block was first thought to be achieved. Adenosine was not used to unmask dormant conduction after PVI.
2.4 Follow up
Patients in the registry cohort had clinic visits with 72-h Holter ECG at 3 and 12 months after PVI. The first 3 months were regarded as a blanking period. Specific antiarrhythmic drugs were usually stopped after the procedure; however, in cases where antiarrhythmic drugs were prescribed because of early recurrence, administration was stopped 3 months after ablation at the latest.
In the randomized study, follow-up clinic visits with 72-h Holter ECG were performed at 3, 6, and 12 months after the ablation procedure. Antiarrhythmic drugs were stopped at the time of PVI. In cases of early recurrence, specific antiarrhythmic drugs were allowed until 2 months post PVI.
In both the registry and in the randomized study, if a patient had typical AF symptoms but no ECG findings consistent with that, then triggered event recording was added to help detect AF.
The primary endpoint was freedom from AF or atrial tachycardia (AT) while off antiarrhythmic drugs at 12 months after the ablation. The secondary endpoints evaluated were freedom from AF or AT at 12 months, periprocedural complications, length of procedure, and fluoroscopy time.
2.5 Statistical analysis
Statistical analyses were performed with SPSS 24 (IBM) software. Clinical parameters are presented as mean values with standard deviations for continuous parameters when normally distributed and as median values with maximum and minimum values when not normally distributed. Categorical parameters are shown with percentages and frequencies.
Normal distribution of parameters was tested using the Kolmogorov–Smirnov test. The T test was used for comparisons of parameters that were metric and normally distributed. The Mann-Whitney U test was used for parameters that were not normally distributed, and the Fisher’s exact test was used for categorical parameters. A p value of < 0.05 was considered statistically significant.
3 Results
3.1 Baseline data for the registry cohort
In the registry cohort, 60 paroxysmal AF cases underwent PVAC Gold ablation, and 56 underwent CB 2nd ablation. Mean age was 66 ± 11, 52% were male, mean LVEF 60 ± 6%, mean LAD 43 ± 6 mm, and median BMI 28 (17–42) kg/m2. Baseline data for both groups are presented in Table 1. Only LAD was significantly different between the groups, p 0.012.
3.2 Procedural parameters and complications for the registry cohort
Procedure duration was significantly longer in the CB 2nd group, but there were no significant differences regarding fluoroscopy duration and dose area product (DAP) (Table 2).
In the CB group, one vascular access site complication occurred (1.8%), and two phrenic nerve palsies (3.6%) occurred. The phrenic nerve palsies resolved completely between the 3-month follow-up visit and 12 month follow up examination. We did not detect any stroke, transient ischemic attack, or esophageal fistula. No complications occurred in the PVAC Gold group (p = 0.08).
3.3 Long-term results in the registry cohort
The mean follow-up time was 13.2 ± 3.6 months. Freedom from recurrence of AF or AT was 63% in PVAC Gold patients and 83% in CB 2nd cases (0.010), and, while off antiarrhythmics, these values were 54% and 81%, respectively, (0.001).
3.4 Baseline data for the randomized study
In the randomized cohort, 20 patients were treated with PVAC Gold and 22 patients with CB 2nd (mean age 67 ± 9; 43% men, mean LVEF 61 ± 3%, LAD 40 ± 7 mm, median BMI 28 (20–36) kg/m2.) No significant differences regarding baseline clinical parameters were observed between the groups (Table 3).
3.5 Procedural parameters and clinical complications in the randomized study
Procedure duration and left atrial dwell time (LA time) were slightly but not significantly shorter in the PVAC Gold group as compared with the CB 2nd. No significant differences were found for fluoroscopy time and DAP (Table 4). At the end of the ablation procedure, in the PVAC Gold group, 76/76 (100%) of PV were isolated, and in the CB 2nd group, 88/89 (99%) of PV were isolated at the end of the ablation procedure.
There were no complications in the PVAC Gold group, but there was one phrenic nerve palsy in the CB 2nd group, which completely resolved within 24 h (p = 0.329). No atrio-esophageal fistula occurred.
3.6 Esophageal lesions in the randomized study cohort
Esophagogastroscopy was performed within 72 h after the ablation procedure in 18 of the 20 (90%) PVAC Gold cases and in 21 of the 22 CB 2nd cases (95%). In the PVAC Gold group, there were no esophageal lesions found; however, in the CB 2nd group, one patient (4.5%) had an erosive esophageal lesion without clinical symptoms.
3.7 Long-term results in the randomized study
In the randomized study, 12 months freedom from AF or AT off antiarrhythmic medications was 55% in the PVAC Gold group vs. 91% in the CB 2nd group (p = 0.013).
4 Discussion
We report here the first direct comparison of the acute and long-term results of second-generation techniques of PVAC and CB for treatment of paroxysmal AF. We used both a registry cohort and a randomized study, and, in both, the procedure time was longer with the CB, although, in the randomized study this difference was not significant. There was a higher rate of periprocedural complications in the CB 2nd group, which was driven by phrenic nerve palsies, all of which completely resolved within 12 months. In terms of the primary endpoint, freedom from AF or AT while off of antiarrhythmic drugs at 12 months, a clear benefit was found for CB 2nd as opposed to PVAC Gold.
4.1 Procedural aspects of PVAC and CB
To the best of our knowledge, data comparing the second-generation techniques of PVAC and CB do not exist. The AF-COR study, which compared first-generation techniques, found a higher rate of complications in the CB group. The higher complication rate in the CB group was primarily driven by phrenic nerve palsies [12]. Similarly, we found complication rates for PVAC Gold versus CB 2nd in the registry of 0% vs 5.4%, respectively, and in the randomized study of 0% vs. 4.5%, respectively. Published complication rates range from 0 to 3% for PVAC Gold and from 1.8 to 18% for CB 2nd [7,8,9, 13,14,15].
The procedure and fluoroscopy times for our registry and randomized study are also comparable to those published earlier. Median procedure times in PVAC Gold cases have previously been reported to range from 83 to 112 min and mean fluoroscopy times from 15 to 23 min [7,8,9, 16]. For CB 2nd, the published procedural data vary widely, from 70 to 181 min for the procedure times and from 14 to 49 min for the fluoroscopy times [5, 13, 17,18,19]. However, the recent ICE-T trial, which was performed by a very experienced team, reported a mean procedure time of 89 min for an ablation protocol that included a bonus freeze. This procedure time is similar to ours, if one takes into consideration that we included a “wait time” to confirm that bidirectional conduction block was achieved. Mean fluoroscopy time was somewhat shorter (14 min) in the ICE-T trial. The procedure duration for CB 2nd cases in our randomized study was slightly, albeit insignificantly, longer than in PVAC Gold cases. Of note, with the evolution of newer techniques, such as the real-time pulmonary vein recordings used in the ICE-T trial, routine safety freezes can be avoided, and CB 2nd cases will be able to be done within similar procedure times as PVAC Gold cases [18].
4.2 Esophageal lesions
Silent esophageal lesions are considered a risk factor for esophageal fistula, a feared, life-threatening complication [20].
Esophageal endoscopy detected no esophageal luminal lesions in the PVAC Gold group, but one erosive lesion was found in the CB 2nd group. The rate in PVAC cases is small compared with prior studies, but similar to what has been described by Zellerhoff et al. who, like us, also did not use an esophageal temperature probe to stop energy application when a critical temperature is reached [21]. Deneke et al. reported a rate of 18% in cases where the esophageal temperature rose to > 39 °C, but they found no esophageal lesions in cases done without a temperature probe. They speculated that the esophageal probe may act as an antenna which transfers energy into the esophagus [22]. Two studies have reported that in CB 2nd cases, luminal esophageal lesions were detected in 3 to 20% of cases using a temperature probe with or without a luminal esophageal temperature (LET) cutoff for ablation [22, 23]. The rate found in our study was very low without using a temperature probe. One possible explanation for this could be that the electrodes of the temperature probe prolong the cooling effect to the esophagus.
4.3 Outcome of first-generation techniques and second-generation techniques
Although multiple studies have reported first generation PVAC results, a meta-analysis by Andrade et al. identified only five studies with sufficiently reported data for 12 months post-procedure. At 1 year, the pooled estimate for freedom from recurrent AF was limited by significant heterogeneity [17]. In a European survey on efficacy and safety, Scharf et al. reported an initial procedure success rate in paroxysmal AF of 59% while off medication [24]. The long-term freedom from AF recurrence was similar in two large randomized trials that compared CB and point-by-point RF ablation, the gold standard [2, 3]. Freeze AF, which used only first-generation techniques, demonstrated a single-procedure freedom of recurrence of approximately 65% at 6 months post-procedure. [3]. In “FIRE and ICE,” first- and second-generation techniques were used with a 65% freedom from recurrences at 12 months [2].
Second-generation techniques of CB and point-by-point RF ablation (contact force measurement) seem to be associated with improved success rates of approximately 80–90% at 12 months [4, 5, 13, 15, 25, 26].
Published data with PVAC Gold is limited. Two studies showed comparable success rates between PVAC and PVAC Gold [7, 9]. One study comparing PVAC with PVAC Gold found freedom from AF recurrence not differing, with a rate of 68.2 ± 9% in the PVAC-Gold group at 1 year. Of the included patients, 70% were on antiarrhythmics [7]. These results suggest an inferiority when PVAC Gold is compared with CB 2nd. Our registry and randomized study results support this inference. In both the registry and randomized study, we found significantly better long-term outcomes after CB 2nd ablation as compared with the PVAC Gold procedure. The absolute success rates resemble those reported for studies on each of the methods [4, 5, 13, 15, 18, 26].
In our center, we observed very high rates of PV reconnection in redo procedures after ablation with PVAC Gold as compared with redo procedures after CB 2nd ablation. Published data on redo procedures confirm this finding and demonstrate PV reconnection rates of 18% after CB 2nd ablation and of more than 80% after PVAC Gold procedures [8, 27]. From a technical perspective, CB qualifies more as a “single-shot device” than PVAC Gold does. After confirming complete contact by the balloon with contrast injection, in most cases, one application leads to a contiguous lesion with bidirectional conduction block within 1 min. The use of a PVAC Gold catheter necessitates a median of six complementary RF applications to get a complete lesion, and continuity of the applied lesions is difficult to control [9].
Dormant PV conduction might occur more often in PVAC Gold procedures. Using intracardiac ultrasound maybe an option for attaining more targeted energy application. However, the additional use of a large sheath and expensive devices runs counter to the goal of simplified PVI procedures. The use of post-procedural adenosine as a final test to unmask PV conduction might have the potential to improve outcomes.
The possibility that the PVAC Gold electrodes have reduced diagnostic accuracy with respect to the confirmation of PVI is an unlikely explanation. Duytschaever et al. confirmed isolation after PVAC-guided PVI in 96% of PVs using a standard circular mapping catheter (Optima TM, St. Jude Medical, St. Paul, MN, USA) [28]. In the study of von Bary et al., in 94% of PV in which PVAC confirmed PVI, a standard circular mapping catheter also found isolation [29]. Recently, we documented a diagnostic accuracy of 100% for the Achieve 20 mm when compared with a standard circular mapping catheter (Optima TM, St. Jude Medical, St. Paul, MN, USA) [30]. Thus, reduced diagnostic accuracy of the PVAC Gold electrodes does not seem to explain sufficiently the lack of efficacy of PVAC Gold ablation.
4.4 Limitations
A limitation for both our registry and randomized study is the lack of continuous ECG monitoring. However, when symptoms potentially caused by arrhythmias arose, the patients were provided with event-recorders. The main limitations are that procedures in the registry cohort were not completely standardized and that the randomized study was small. The analyses in the registry cohort are limited by the difference in LAD which indicates that the groups were not completely comparable. However the results of the randomized study resembled those of the registry cohort. We did not perform cerebral MRI to detect silent cerebral lesions. However, no neurological symptoms were observed in our study, and the clinical relevance of those lesions is unsettled [31].
5 Conclusion
Our study results provide further evidence of inferior long-term results with PVAC Gold versus CB 2nd for PVI. The success rates at 12 months post-procedure and off antiarrhythmics are comparable to those published separately for both of the methods. Ideally, a larger randomized study would be necessary to make a more definitive conclusion regarding the superiority of CB 2nd. However, in light of the large benefit of CB 2nd over PVAC Gold in both our registry and our randomized study, we have some ethical concerns regarding inclusion in such a study. Procedure time was slightly longer in CB 2nd cases in our trial; however, we anticipate that these differences will diminish given the shortened freeze time possible with real-time PV recordings. There is a higher rate of phrenic nerve palsies occurring in CB 2nd cases. Nevertheless, so long as the palsies are reversible within 24 h, this should not relevantly impact the use of CB 2nd for PVI.
References
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(11):1609–78. https://doi.org/10.1093/europace/euw295.
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–45. https://doi.org/10.1056/NEJMoa1602014.
Luik A, Radzewitz A, Kieser M, Walter M, Bramlage P, Hormann P, et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: the prospective, randomized, controlled, Noninferiority FreezeAF Study. Circulation. 2015;132(14):1311–9. https://doi.org/10.1161/circulationaha.115.016871.
Cardoso R, Mendirichaga R, Fernandes G, Healy C, Lambrakos LK, Viles-Gonzalez JF, et al. Cryoballoon versus radiofrequency catheter ablation in atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2016;27(10):1151–9. https://doi.org/10.1111/jce.13047.
Jourda F, Providencia R, Marijon E, Bouzeman A, Hireche H, Khoueiry Z, et al. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation-a prospective evaluation. Europace. 2015;17(2):225–31. https://doi.org/10.1093/europace/euu215.
He X, Chen Y, Zhou Y, Huang Y, He J. One-year clinical outcome of pulmonary vein isolation using the second-generation cryoballoon: a meta-analysis. Pacing Clin Electrophysiol. 2016;39(2):182–9. https://doi.org/10.1111/pace.12787.
Rovaris G, De Filippo P, Laurenzi F, Zanotto G, Bottoni N, Pozzi M, et al. Clinical outcomes of AF patients treated with the first and second-generation of circular mapping and ablation catheter: insights from a real world multicenter experience. J Interv Card Electrophysiol. 2017;50(3):245–51. https://doi.org/10.1007/s10840-017-0278-y.
Spitzer SG, Leitz P, Langbein A, Karolyi L, Scharfe F, Weinmann T, et al. Circumferential pulmonary vein isolation with second-generation multipolar catheter in patients with paroxysmal or persistent atrial fibrillation: procedural and one-year follow-up results. Int J Cardiol. 2017;241:212–7. https://doi.org/10.1016/j.ijcard.2017.04.035.
Weber S, Hoher M, Schultes D. First results and follow-up of a second-generation circular mapping and ablation catheter. J Interv Card Electrophysiol. 2016;47(2):213–9. https://doi.org/10.1007/s10840-016-0140-7.
Wasmer K, Krusemann D, Leitz P, Guner F, Pott C, Zellerhoff S, et al. Lower rate of left atrial tachycardia after pulmonary vein isolation with PVAC versus irrigated-tip circumferential antral ablation. Heart Rhythm. 2016;13(8):1596–601. https://doi.org/10.1016/j.hrthm.2016.02.017.
Franceschi F, Dubuc M, Guerra PG, Khairy P. Phrenic nerve monitoring with diaphragmatic electromyography during cryoballoon ablation for atrial fibrillation: the first human application. Heart Rhythm. 2011;8(7):1068–71. https://doi.org/10.1016/j.hrthm.2011.01.047.
Malmborg H, Lonnerholm S, Blomstrom P, Blomstrom-Lundqvist C. Ablation of atrial fibrillation with cryoballoon or duty-cycled radiofrequency pulmonary vein ablation catheter: a randomized controlled study comparing the clinical outcome and safety; the AF-COR study. Europace. 2013;15(11):1567–73. https://doi.org/10.1093/europace/eut104.
Jiang J, Li J, Zhong G, Jiang J. Efficacy and safety of the second-generation cryoballoons versus radiofrequency ablation for the treatment of paroxysmal atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2017;48(1):69–79. https://doi.org/10.1007/s10840-016-0191-9.
Leitz P, Guner F, Wasmer K, Foraita P, Pott C, Dechering DG, et al. Data on procedural handling and complications of pulmonary vein isolation using the pulmonary vein ablation catheter GOLD(R). Europace. 2016;18(5):696–701. https://doi.org/10.1093/europace/euv355.
Miyamoto K, Doi A, Hasegawa K, Morita Y, Mishima T, Suzuki I, et al. Multicenter study of the validity of additional freeze cycles for cryoballoon ablation in patients with paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2019;12(1):e006989. https://doi.org/10.1161/circep.118.006989.
De Greef Y, Dekker L, Boersma L, Murray S, Wieczorek M, Spitzer SG, et al. Low rate of asymptomatic cerebral embolism and improved procedural efficiency with the novel pulmonary vein ablation catheter GOLD: results of the PRECISION GOLD trial. Europace. 2016;18(5):687–95. https://doi.org/10.1093/europace/euv385.
Andrade JG, Dubuc M, Rivard L, Guerra PG, Mondesert B, Macle L, et al. Efficacy and safety of atrial fibrillation ablation with phased radiofrequency energy and multielectrode catheters. Heart Rhythm. 2012;9(2):289–96. https://doi.org/10.1016/j.hrthm.2011.09.009.
Chun KR, Stich M, Furnkranz A, Bordignon S, Perrotta L, Dugo D, et al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm. 2017;14(4):495–500. https://doi.org/10.1016/j.hrthm.2016.12.014.
Squara F, Zhao A, Marijon E, Latcu DG, Providencia R, Di Giovanni G, et al. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: a multicentre European evaluation. Europace. 2015;17(5):718–24. https://doi.org/10.1093/europace/euv060.
Halbfass P, Pavlov B, Muller P, Nentwich K, Sonne K, Barth S, Hamm K, Fochler F, Mugge A, Lusebrink U, Kuhn R, Deneke T. Progression from esophageal thermal asymptomatic lesion to perforation complicating atrial fibrillation ablation: a single-center registry. Circ Arrhythm Electrophysiol. 2017;10(8). https://doi.org/10.1161/circep.117.005233.
Zellerhoff S, Lenze F, Ullerich H, Bittner A, Wasmer K, Kobe J, et al. Esophageal and mediastinal lesions following multielectrode duty-cycled radiofrequency pulmonary vein isolation: simple equals safe? Pacing Clin Electrophysiol. 2016;39(4):316–20. https://doi.org/10.1111/pace.12797.
Deneke T, Bunz K, Bastian A, Pasler M, Anders H, Lehmann R, et al. Utility of esophageal temperature monitoring during pulmonary vein isolation for atrial fibrillation using duty-cycled phased radiofrequency ablation. J Cardiovasc Electrophysiol. 2011;22(3):255–61. https://doi.org/10.1111/j.1540-8167.2010.01916.x.
Furnkranz A, Bordignon S, Bohmig M, Konstantinou A, Dugo D, Perrotta L, et al. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm. 2015;12(2):268–74. https://doi.org/10.1016/j.hrthm.2014.10.033.
Scharf C, Ng GA, Wieczorek M, Deneke T, Furniss SS, Murray S, et al. European survey on efficacy and safety of duty-cycled radiofrequency ablation for atrial fibrillation. Europace. 2012;14(12):1700–7. https://doi.org/10.1093/europace/eus188.
Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Y, et al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. Europace. 2018;20(Fi_3):f419–27. https://doi.org/10.1093/europace/eux376.
Su W, Orme GJ, Hoyt R, Baker J, Compton S, Fellows C, et al. Retrospective review of Arctic front advance cryoballoon ablation: a multicenter examination of second-generation cryoballoon (RADICOOL trial). J Interv Card Electrophysiol. 2018;51(3):199–204. https://doi.org/10.1007/s10840-018-0335-1.
Westra SW, van Vugt SPG, Sezer S, Evertz R, Hemels ME, Beukema RJ, et al. Second-generation cryoballoon ablation for recurrent atrial fibrillation after an index cryoballoon procedure: a staged strategy with variable balloon size. J Interv Card Electrophysiol. 2019;54(1):17–24. https://doi.org/10.1007/s10840-018-0418-z.
Duytschaever M, Anne W, Papiashvili G, Vandekerckhove Y, Tavernier R. Mapping and isolation of the pulmonary veins using the PVAC catheter. Pacing Clin Electrophysiol. 2010;33(2):168–78. https://doi.org/10.1111/j.1540-8159.2009.02609.x.
von Bary C, Fredersdorf-Hahn S, Heinicke N, Jungbauer C, Schmid P, Riegger GA, et al. Comparison of PV signal quality using a novel circular mapping and ablation catheter versus a standard circular mapping catheter. J Interv Card Electrophysiol. 2011;31(2):131–9. https://doi.org/10.1007/s10840-011-9546-4.
Schade A, Schumacher B, Dietrich JW, Langbein A, Groschup G, Koucky K, et al. Stand-alone mapping using different transluminal mapping catheters--an accurate and safe way to isolate all pulmonary veins with the cryoballoon? J Interv Card Electrophysiol. 2015;42(1):33–41. https://doi.org/10.1007/s10840-014-9957-0.
von Bary C, Deneke T, Arentz T, Schade A, Lehrmann H, Eissnert C, et al. Silent cerebral events as a result of left atrial catheter ablation do not cause neuropsychological sequelae--a MRI-controlled multicenter study. J Interv Card Electrophysiol. 2015;43(3):217–26. https://doi.org/10.1007/s10840-015-0004-6.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Ablation procedures were performed by A. Schade and F. Steinborn for the randomized study, and additionally R. Simeoni and F.M. Malur for the registry. Material preparation and data collection was performed by P. Seidl, C. Böttcher, A. Sommermeier, and V. Mattea. Analysis was prepared by P. Seidl and F. Steinborn. The first draft of the manuscript was written by P. Seidl and A. Schade. Critical revisions of the article were done by R. Surber, P.C. Schulze, H. Lapp, and L. Costello-Boerrigter. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Both studies were approved by the Ethics Commission of the Landesärztekammer Thüringen.
Statement of informed consent
All patients gave written, informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seidl, P., Steinborn, F., Costello-Boerrigter, L. et al. Pulmonary vein isolation using second-generation single-shot devices: not all the same?. J Interv Card Electrophysiol 60, 521–528 (2021). https://doi.org/10.1007/s10840-020-00751-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00751-9