Abstract
Purpose
Complete pulmonary vein isolation (PVI) is the best documented target for catheter ablation, and different technologies have shown comparable outcomes. The multielectrode phased-RF/duty cycled (PhRF/DC) pulmonary vein ablation catheter (PVAC) and its second generation (PVAC-GOLD) have shown promising clinical results in single and multicenter experiences. Our aim is to assess and compare the safety and efficacy in the real clinical practice among two generations of circular PhRF/DC catheters by performing PVI in patients suffering from recurrent atrial fibrillation (AF).
Methods
Eighty-four AF patients treated with PVAC and 64 with PVAC-GOLD were prospectively followed in five Italian cardiology centers in the mainframe of the 1STOP-ClinicalService project.
Results
Fluoroscopic and total procedure time were significantly different in the two groups. In particular, in the PVAC-GOLD group, the mean fluoroscopic time was 22.8 ± 12.7 min vs 31.6 ± 18.9 in the PVAC group (p = 0.002), and the mean total procedure duration was 117.6 ± 36.0 vs 147.4 ± 40.6, in the PVAC-GOLD group and the PVAC group, respectively (p = 0.001). Only two out of 148 patients reported a peri-procedural complication. Over 20.9 ± 12.0 months of follow-up, AF recurrence occurred in 58 patients. Kaplan-Meier freedom from AF recurrence did not differ between the two groups (64.1 ± 10% in the PVAC group vs 68.2 ± 9% in the PVAC-GOLD group at 1 year, p = ns).
Conclusions
In our multicenter analysis, AF ablation using two generations of circular PhRF/DC catheters is safe and effective. No difference was observed in terms of safety and efficacy of the AF ablation between the two catheters, with the mean procedural time being shorter in the PVAC-GOLD group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Several clinical trials have shown that pulmonary vein isolation (PVI) is an effective therapy for the treatment of paroxysmal atrial fibrillation (AF). [1,2,3,4] The multielectrode phased RF/duty cycled (PhRF/DC) pulmonary vein ablation catheter (PVAC) and its second generation (PVAC-GOLD) have shown promising clinical results in single and multicenter experiences. [5,6,7,8] Procedural performance, times, and overall freedom from AF recurrence are important issues for healthcare providers and patients. [9] Since 2012, five cardiology centers in Italy have been prospectively collecting clinical and procedural data from patients treated with the Medtronic PhRF/DC system. Clinical and technical information is stored in a cardiovascular data repository created to monitor catheter performances and patient outcomes [http://clinicaltrials.gov/ct2/show/NCT01007474]. The aim of the present research was to compare acute procedural data and long-term freedom from AF recurrence in a multi-center series of patients treated with multielectrode PhRF/DC ablation catheter including either PVAC or PVAC-GOLD.
2 Methods
Since 2012, all consecutive patients who underwent PVI for recurrent symptomatic and drug refractory AF using multipolar a PhRF/DC Medtronic Catheter (PVAC or PVAC-GOLD, Medtronic, Minneapolis, MN, USA.) at any of the five Italian Cardiology centers participating in the Italian ClinicalService® framework [Clinical Trial Registration Information: http://clinicaltrials.gov/ct2/show/NCT01007474], One Shot TO Pulmonary vein isolation (1STOP) project, were prospectively followed according to each center’s clinical practice through standard in-hospital visits and were included in this analysis. The Clinical Service Project is a national medical care project aimed at evaluating and improving the use of medical therapies in clinical practice. The project consists of a shared environment for the collection, management, analysis, and reporting of data from patients in whom Medtronic therapies have been applied. An independent scientific committee of physicians prospectively identifies key clinical questions on a yearly basis for analysis and publication. A charter assigns the ownership of data to the centers and governs the conduct and relationship of the scientific committee and Medtronic. [10] The project was approved by each site’s Medical Ethics Committee or Medical Director and conforms to the principles outlined in the Declaration of Helsinki. Each patient provided informed consent for data collection and analysis.
Baseline and demographic data were collected during the baseline visit. The procedures were performed according to the clinical practice of each center and there is not a shared protocol for anticoagulation management. Despite this, intravenous heparin was used during all procedures to maintain an activated clotting time > 300 s. We did not collect data on cerebral MRI for the purpose of this research. Nevertheless, since 2012, we adopted the best practices suggested by studies and researches as the diligent sheath management and the exclusion of pairs 1–2 or 9–10 in order to avoid the use of bipolar energy when electrodes 1 and 10 were in close proximity. [11, 12]
Follow-up assessment visits were performed every 3 months or whenever AF related symptoms occurred in the patients. At each visit, an ECG and AF-related symptom assessment were performed. The realization of a 24-h dynamic ECG was done at the discretion of the referent/electrophysiologist (EP), according to the clinical practice. The use of the implantable continuous monitor (ICM) was based on the clinical decision of the EP.
For this analysis, the patient population was divided into two groups according to the type of PhRF/DC used: PVAC or PVAC-GOLD. Acute procedural success was defined as the complete PV electrical isolation confirmed by bidirectional block. Acute or peri-procedural complications were defined according to Calkins et al. survey. [1] We considered acute complications and/or peri-procedural complications to be any complications that occurred during the procedure until the discharge from the hospital.
The first 3 months after AF ablation was defined as a blanking period. Recurrence of AF was defined as the detection of at least 30 s of AF duration when assessed with ECG monitoring or at least 5 min when detected by ICM and confirmed by a physician, after the blanking period. [1, 2].
2.1 PVI PhRF/DC catheters
All patients underwent PVI using either the PVAC or PVAC-GOLD catheter. The catheters have been previously described. [13,14,15] In brief, they are nine French mapping and ablation over-the-wire multielectrode catheters delivering PhRF/DC energy at relatively low power. The second-generation multielectrode catheter, PVAC-GOLD, was designed to improve the delivery of PhRF/DC energy compared with the platinum PVAC [11] and to avoid interactions between electrodes 1 and 10. Moreover, gold electrodes have improved thermal conductivity versus platinum electrodes and so may offer advantages for this system in terms of temperature measurement accuracy and efficiency of energy delivery. [11, 14, 15] Energy was delivered via a multichannel, PhRF/DC generator (GENius, Medtronic, Inc., Carlsbad, CA, USA).
2.2 Statistical analysis
Baseline characteristics and clinical data have been summarized and compared between the two groups (PVAC vs PVAC-GOLD) using appropriate summary statistics. Variables on a continuous scale have been described as mean, standard deviation, median and interquartile range, minimum and maximum. Variables on a categorical scale were presented as counts and percentages. Summary statistics were reported with a maximum of two decimals, as appropriate. Continuous variables were compared using the t test or the non-parametric Mann-Whitney test, as appropriate. Normality of distribution was tested, calculating skewness and kurtosis values. Comparisons of categorical variables were performed by means of the Chi-square test or the Fisher exact test for extreme proportions, as appropriate.
The annual rates of events were reported (with the 95% Poisson confidence intervals) and compared between groups using the Poisson model or the negative binomial model to account for over dispersion of the data. Since the number of AF recurrences per patient during follow-up was not available, in the incidence analysis, the rate of event should be interpreted as the rate of patients with at least one AF recurrence.
3 Results
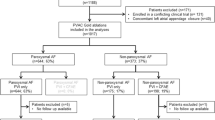
One hundred forty-eight patients underwent PVI ablation, 84 with a PVAC catheter, and 64 with a PVAC-GOLD catheter.
Baseline patient characteristics are described in Table 1 and are comparable between the two groups. Patient mean age was 57.9 years, and mean CHA2DS2-VASC was 1.73 + 1.30. The left atrium size was 41.5 ± 5.4 mm. Out of 148 patients, 116 (78.4%) suffered from paroxysmal AF and 32 from persistent AF. Out of 148 patients, 69 (46.6%) were implanted during the blanking period with an ICM, 36 (42.9%) in the PVAC group, and 33 (51.6%) in the PVAC-GOLD group. At baseline, 84% of patients were treated with antiarrhythmic (AAD) therapy, with only 12 patients per group not being treated with AADs.
3.1 Procedural and acute outcomes
Procedural duration times are reported in Table 1. Total procedure, fluoroscopy, and ablation times were 129 (110–150), 24 (17–34), and 20 (15–30) minutes, respectively. In the PVAC-GOLD group, procedure, fluoroscopy and ablation times were significantly shorter with respect to the PVAC group. Acute success rate was 97.5% in the whole population: 95.8% in the PVAC group and 99.6% in the PVAC-GOLD group. At the end of the procedure, 96.6% of patients were in sinus rhythm with no difference between the two groups.
3.2 Safety outcomes
Only two patients out of 148 (1.4%) had an acute complication: one had a pneumothorax and the other had a hematoma. Both patients were in the PVAC-GOLD group. No deaths, ictus, or other major complications were reported as shown in the Table 2.
3.3 AF recurrence
The mean follow-up was 20.9 ± 12.0 months in the whole population. No patients were lost to follow-up. During the follow-up, 58 patients had a recurrence of AF, 40 (47.6%) in the PVAC group and 18 (28.1%) in the PVAC-GOLD group (p < 0.016). Freedom from AF recurrence is shown in Fig. 1, with no differences in the time to first AF recurrence between the two groups. At 12 months, the unadjusted freedom from AF recurrence was 64.1% (52.8–73.4%) and 68.2% (52.1–79.8%) in the PVAC and PVAC-GOLD groups, respectively. Among these patients, the 12-month unadjusted freedom from AF recurrence were 65.2% (95% CI 55.0–73.7%) and 64.8% (95% CI 43.2–79.9%) in paroxysmal and persistent AF patients, respectively. Moreover, the percentage of freedom from AF recurrence increased to 70% (95% CI 60.2–77.9%) in case of patients in AAD therapy.
A total of 7 (4.7%) re-ablations were recorded, involving 1 (1.6%) patient in the PVAC-GOLD group and 6 (7.1%) in the PVAC group. The rate *100 pt./years was 2.98 (95% CI 1.34–6.64) and 1.76 (95% CI 0.25–12.51) in the PVAC and PVAC GOLD groups, respectively (p = 0.32). At 12 months, 28.9% of patients were treated without AAD therapy, while at 24 months, the percentage increased to 37.4% with no difference between the two groups.
4 Discussion
PVI is effective in restoring and maintaining sinus rhythm in patients with symptomatic paroxysmal, persistent, and probably long-standing persistent AF, as a second-line treatment after failure of or intolerance to antiarrhythmic drug therapy [1, 2]. Indeed, one shot PhRF/DC PVI.
creates effective lesions to significantly modify the arrhythmic substrate while avoiding procedure-related complications.
4.1 Main findings
The main results of our analysis are as follows: (1) PVI using PVAC and PVAC-GOLD is feasible and safe with reduced procedural time and with low complication incidence to treat both paroxysmal and persistent drug refractory AF patients, (2) the use of the PVAC-GOLD catheter reduced procedural and fluoroscopy times without compromising the procedure safety, (3) the PVI ablation using both multielectrode circular mapping catheters is effective. This is the first analysis reporting long-term ablation-related outcomes comparing PVAC and PVAC-GOLD PVI from a multicenter, real clinical practice experience.
4.2 Safety and procedural data
Weber et al. reported the single-center comparison study of 40 patients, half treated with PVAC and half treated with PVAC-GOLD. The total procedure time from vascular access until removal of catheters was significantly reduced from 93.8 ± 18.9 min in the PVAC group to 83.1 ± 10.6 min in the PVAC-GOLD group. Furthermore, the total fluoroscopy time was reduced from 29.5 ± 9.5 min in the PVAC group to 23.4 ± 7.0 min in the PVAC-GOLD group. [16] In our experience, the total procedural time was 129 min (140 min in the PVAC group and 112 min in the PVAC GOLD group), which was longer than those of Weber et al. However, there was a significant time reduction between the two groups in favor of the PVAC-GOLD. The fluoroscopic times were comparable with those presented by Weber et al. The differences could be explained by the nature of the two different analyses: Weber reported a single-center study on 40 patients, while ours was a multicenter experience on 148 patients. Our data on procedural time confirmed those presented by Lietz et al., on 128 patients with their first PVAC-GOLD PVI. [17] In our analysis, the ablation time was significantly shorter in the PVAC-GOLD group (15 vs 28 min in the PVAC group). Contributing factors likely include the increased heat transfer of gold electrode material with more efficient cooling and deeper lesions, the 20° forward tilt, which provides more uniform contact with the PV antrum, and the use of the PhRF/DC generator. [11, 18] In fact, the software of the generator could give improved feedback on contact between the ablation catheter and PV and on the reached temperature to target an electrode-tissue interface temperature of 60 °C. [18, 19]
In our experience, only two patients experienced a peri-procedural complication: one patient had a pneumothorax, while in the second patient, a hematoma in the right groin occurred. No other major complications were reported. Concerns have been raised about the safety of the PVAC system after an increased incidence of acute silent cerebral micro-embolisms (ASCE) in comparison with irrigated tip RF ablation or cryoablation was reported [20,21,22]. PVAC-GOLD was projected to minimize the risk of interaction between electrodes 1 and 10 [23, 24], one of the causes contributing to ASCE formation. The ERACE trial demonstrated that PVAC procedural changes led to significant reductions in occurrence of ASCE (1.7%) [23, 25, 26] and the PRECISION Gold Study confirmed this result in 51 patients treated with PVAC-GOLD [11]. Spitzer et al. reported on 384 paroxysmal and persistent AF patients, only 4 (1%) with relevant early complications requiring intervention: 3 early complications occurred in the group of patients with paroxysmal AF and 1 posterior cerebral infarction without long-term consequences occurred in the group of persistent AF patients. This two-center registry confirmed the safety of PVI performed with PVAC-GOLD. [26]
4.3 Efficacy data
Our findings on the efficacy of PVI ablation are in line with the results of other groups. [26, 27] In particular, Weber et al. reported that at 12 months, AF recurrence rates were similar between the PVAC (7/20) and PVAC-GOLD (6/20) groups (35 and 30%, respectively; p = 0.735). [16] Recently, Lepillier et al. have reported a single-center experience on 77 paroxysmal AF patients treated with PVAC. After a mean follow-up of 55 ± 11 months, and after a single procedure, the success rate (absence of symptomatic AF) was 70.1%. [28] In our experience, at 12 months, the unadjusted freedom from AF recurrence was 64.1 and 68.2% in the PVAC and PVAC-GOLD groups, respectively. It is, however, important to point out some methodological differences between the two experiences: (a) the patients evaluated by Lepillier et al. suffered from paroxysmal AF, whereas 21.6% of our patients had persistent or long persistent AF; (b) in our analysis, 46.6% of patients were continuously monitored using ICM to find AF recurrence, while the remaining 53.4% and patients studied by Lepillier et al. were followed with a standard approach consisting of 3-month follow-ups with a clinical exam and ECG and the realization of a 24-h Holter ECG done at the discretion of the referent. [28]
Recently, the use of implantable loop recorders to detect AF recurrence rates has been evaluated in different studies: the accuracy of rhythm detection is more effective with ILR monitoring compared to intermittent rhythm monitoring [29–31]. Lately, Spitzer et al. reported data on 198 paroxysmal and 186 persistent AF patients undergoing multipolar phased bipolar RF ablation using PVAC-GOLD. Rates of freedom from recurrence of AF after a single PVAC-GOLD ablation procedure, including redoprocedures, were 68.3% with paroxysmal AF and 58.6% with persistent AF remaining in sinus rhythm and off drugs after 6 months, showing that PVAC-GOLD appeared to be favorable in patients both with paroxysmal and persistent AF. [26] Largely, our patients were treated with AAD therapy both at baseline and in the follow-up. At 24 months only, 38% of patients were not treated with AADs. Goldenberg et al., in a meta-analysis of published controlled trials comparing temporary AAD therapy after atrial fibrillation ablation with no AAD therapy, suggested that AAD usage did not substantially reduce overall recurrence of AF after ablation in the mean follow-up period of 8 months. [32, 33] Our research reflecting the clinical practice of participating centers showed that the AAD usage is common even after the ablation of AF. Further studies are needed to assess AAD protocols and correlation with the presence of AF recurrence.
In the present analysis, despite the similar rate of AF recurrence between the two groups, the re-procedure rates were different. One of the reasons could be the difference in duration of AF episodes, or the frequency of AF episodes. In this analysis, we have studied only the recurrence of the first AF episode and not the duration of the episodes, or the subsequent AF episodes.
5 Limitations
This was not a randomized comparison, so the limitations of observational studies applied to our research. Nevertheless, possible biases are mitigated by the fact that data were collected prospectively, the analysis was designed before the dataset was opened, and research endpoints were pre-specified.
6 Conclusions
Our experience suggests that PVAC and PVAC-GOLD procedures are effective for long-term follow-up, with reduced time of procedure and time of fluoroscopy and low peri-procedural complications. Our results confirm a low occurrence of AF with PhRF/DC systems and a better efficiency when PVAC-GOLD was used.
References
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and followup, definitions, endpoints, and research trial design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e21.
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–5.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; https://doi.org/10.1093/eurheartj/ehw210.
Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66.
Mönnig G, Eckardt L. Multielectrode Pulmonary Vein Ablation Catheter (PVAC®). Current data on results and risks. Herzschrittmacherther Elektrophysiol. 2014;25(4):236–40.
De Greef Y, Buysschaert I, Schwagten B, Stockman D, Tavernier R, Duytschaever M. Duty-cycled multi-electrode radiofrequency vs. conventional irrigated point-by-point radiofrequency ablation for recurrent atrial fibrillation, comparative 3-year data. Europace. 2014;16(6):820–5.
Gal P, Aarntzen AE, Smit JJ, Adiyaman A, Misier AR, Delnoy PP, et al. Conventional radiofrequency catheter ablation compared to multi-electrode ablation for atrial fibrillation. Int J Cardiol. 2014;176(3):891–5.
Nardi S, Argenziano L, Cappato R, de Martino G, Esposito C, Scaglione M, et al. Ablation of paroxysmal and persistent atrial fibrillation with multielectrode phased radiofrequency duty-cycled catheters: long-term results from a large cohort of patients. J Cardiovasc Med (Hagerstown). 2013;14(12):879–85.
Bhatt N, Turakhia M, Fogarty TJ. Cost-effectiveness of cardiac radiosurgery for atrial fibrillation: implications for reducing health care morbidity, utilization, and costs. Cureus. 2016;8(8):e720.
Padeletti L, Curnis A, Tondo C, Lunati M, Porcellini S, Verlato R, et al. Pulmonary vein isolation with the Cryoballoon technique: feasibility, procedural outcomes, and adoption in the real world: data from one shot technologies TO pulmonary vein isolation (1STOP) project. Pacing Clin Electrophysiol. 2016; https://doi.org/10.1111/pace.12975.
De Greef Y, Dekker L, Boersma L, Murray S, Wieczorek M, Spitzer SG, Davidson N, Furniss S, Hocini M, Geller JC, Csanádi Z; PRECISION GOLD investigators. Low rate of asymptomatic cerebral embolism and improved procedural efficiency with the novel PVAC GOLD: results of the PRECISION GOLD trial. Europace 2016;18(5):687–695. https://doi.org/10.1093/europace/euv385.
Deneke T, Shin DI, Balta O, Bünz K, Fassbender F, Mügge A, et al. Postablation asymptomatic cerebral lesions: long-term follow-up using magnetic resonance imaging. Heart Rhythm. 2011;8(11):1705–11.
Lau M, Hu B, Werneth R, Sherman M, Oral H, Morady F, et al. A theoretical and experimental analysis of radiofrequency ablation with a multielectrode, phased, duty-cycled system. Pacing Clin Electrophysiol. 2010;33(9):1089–100.
Fredersdorf S, Weber S, Jilek C, Heinicke N, von Bary C, Jungbauer C, et al. Safe and rapid isolation of pulmonary veins using a novel circular ablation catheter and duty-cycled RF generator. J Cardiovasc Electrophysiol. 2009;20:1097–101.
von Bary C, Weber S, Dornia C, Eissnert C, Fellner C, Latzin P, et al. Evaluation of pulmonary vein stenosis after pulmonary vein isolation using a novel circular mapping and ablation catheter (PVAC). Circ Arrhythm Electrophysiol. 2011;4(5):630–6.
Weber S, Höher M, Schultes D. First results and follow-up of a second-generation circular mapping and ablation catheter. J Interv Card Electrophysiol. 2016;47(2):213–9.
Leitz P, Güner F, Wasmer K, Foraita P, Pott C, Dechering DG, et al. Data on procedural handling and complications of pulmonary vein isolation using the pulmonary vein ablation catheter GOLD. Europace. 2016;18:696–701.
Haines DE, Stewart MT, Dahlberg S, Barka ND, Condie C, Fiedler GR, et al. Microembolism and catheter ablation I: a comparison of irrigated radiofrequency and multielectrode phased radiofrequency catheter ablation of pulmonary vein ostia. Circ Arrhythm Electrophysiol. 2013;6:16–22.
Scharf C, Boersma L, Davies W, Kanagaratnam P, Peters NS, Paul V, et al. Ablation of persistent AF using multielectrode catheters and duty-cycled radiofrequency energy. J Am Coll Cardiol. 2009;54:1450–6.
Gaita F, Leclercq JF, Schumacher B, Scaglione M, Toso E, Halimi F, et al. Incidence of silent cerebral thromboembolic lesions after atrial fibrillation ablation may change according to technology used: comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon. J Cardiovasc Electrophysiol. 22:961–8.
Herrera Siklody C, Deneke T, Hocini M, Lehrmann H, Shin DI, Miyazaki S, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol. 2011;58:681–8.
Mönnig G, Eckardt L. Multielectrode Pulmonary Vein Ablation Catheter (PVAC®). Herzschrittmacherther Elektrophysiol. 2014;25:236–40.
Zellerhoff S, Ritter MA, Kochhauser S, Dittrich R, Kobe J, Milberg P, et al. Modified phased radiofrequency ablation of atrial fibrillation reduces the number of cerebral microembolic signals. Europace. 2014;16:341–6.
Wieczorek M, Hoeltgen R, Brueck M. Does the number of simultaneously activated electrodes during phased RF multielectrode ablation of atrial fibrillation influence the incidence of silent cerebral microembolism? Heart Rhythm. 2013;10:953–9.
Verma A, Debruyne P, Nardi S, Deneke T, DeGreef Y, Spitzer S, et al. ERACE investigators. Evaluation and reduction of asymptomatic cerebral embolism in ablation of atrial fibrillation, but high prevalence of chronic silent infarction, results of the evaluation of reduction of asymptomatic cerebral embolism trial. Circ Arrhythm Electrophysiol. 2013;6(5):835–42.
Spitzer SG, Leitz P, Langbein A, Karolyi L, Scharfe F, Weinmann T, et al. Circumferential pulmonary vein isolation with second-generation multipolar catheter in patients with paroxysmal or persistent atrial fibrillation: Procedural and one-year follow-up results. Int J Cardiol. 2017; https://doi.org/10.1016/j.ijcard.2017.04.035.
Bulava A, Hanis J, Sitek D, Osmera O, Karpianus D, Snorek M, et al. Catheter ablation for paroxysmal atrial fibrillation: a randomized comparison between multielectrode catheter and point-by-point ablation. Pacing Clin Electrophysiol. 2010;33:1039–46.
Bittner A, Monnig G, Zellerhoff S, Pott C, Köbe J, Dechering D, et al. Randomized study comparing duty-cycled bipolar and unipolar radiofrequency with point-by-point ablation in pulmonary vein isolation. Heart Rhythm. 2011;8:1383–90.
Lepillier A, Copie X, Lascault G, Paziaud O, Piot O. A 5-year clinical follow-up after duty-cycled phased RF ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2016;48(3):327–31.
Pecha S, Aydin MA, Ahmadzade T, Hartel F, Hoffmann B, Steven D, et al. Implantable loop recorder monitoring after concomitant surgical ablation for atrial fibrillation (AF): insights from more than 200 continuously monitored patients. Heart Vessel. 2016;31(8):1347–53.
Charitos EI, Stierle U, Ziegler PD, Baldewig M, Robinson DR, Sievers HH, et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012;126(7):806–14.
Brachmann J, Morillo CA, Sanna T, Di Lazzaro V, Diener HC, Bernstein RA, et al. Uncovering Atrial Fibrillation Beyond Short-Term Monitoring in Cryptogenic Stroke Patients: Three-Year Results From the Cryptogenic Stroke and Underlying Atrial Fibrillation Trial. Circ Arrhythm Electrophysiol. 2016;9(1):e003333. https://doi.org/10.1161/CIRCEP.115.003333.
Goldenberg GR, Burd D, Lodzinski P, Stabile G, Udell JA, Newman D, et al. Antiarrhythmic therapy as an adjuvant to promote post pulmonary vein isolation success, a meta-analysis. J Interv Card Electrophysiol. 2016;47:171–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
The project was approved by each site’s Medical Ethics Committee or Medical Director and conforms to the principles outlined in the Declaration of Helsinki. Each patient provided informed consent for data collection and analysis.
Competing interests
The authors declare that they have no conflicts of interest.
Funding
This research was performed within the framework of the Italian ClinicalService, a project funded by Medtronic Italia, an affiliate of Medtronic Inc. No other funding sources were involved in the research.
Additional information
Rights and permissions
About this article
Cite this article
Rovaris, G., De Filippo, P., Laurenzi, F. et al. Clinical outcomes of AF patients treated with the first and second-generation of circular mapping and ablation catheter: insights from a real world multicenter experience. J Interv Card Electrophysiol 50, 245–251 (2017). https://doi.org/10.1007/s10840-017-0278-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-017-0278-y