Abstract
Purpose
Pulmonary vein isolation with radiofrequency energy is widely used as a strategy for catheter ablation of atrial fibrillation (AF). Anatomically designed catheters have been developed to increase the efficiency of AF ablation procedures. The second-generation circular ablation catheter, PVAC GOLD, was re-designed to improve energy delivery and mitigate emboli. We investigated the procedural efficiency, biophysics, and chronic efficacy of PVAC GOLD in patients with AF.
Methods
We consecutively enrolled 40 patients (60 ± 11 years) with highly symptomatic, drug refractory AF. The first 20 patients were treated with the first-generation PVAC. The subsequent 20 patients were treated with the second-generation PVAC GOLD catheter. All patients were followed up at 3, 6, and 12 months.
Results
All 164 targeted PVs were successfully isolated. Ablations performed with PVAC GOLD showed a significant reduction in total number of ablations needed for PVI, fluoroscopy, and procedure times compared to PVAC (34.7 ± 7.0 vs. 27.0 ± 6.5; p = 0.009), fluoroscopy (29.5 ± 9.5 vs. 23.4 ± 7.0; p = 0.026), and procedure time (93.8 ± 18.9 vs. 83.1 ± 10.6; p = 0.033). PVAC GOLD showed improved biophysics including a reduction of low power ablations and an increase in mean effective energy delivery. At 12 months follow-up, AF recurrence rates were comparable in the two groups (35 vs. 30 %; p = 0.735). There were no adverse events.
Conclusions
The redesigned PVAC GOLD catheter demonstrates a reduction in radiofrequency ablation and procedure time and improved biophysics while maintaining chronic efficacy compared to the first-generation PVAC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atrial fibrillation is the most common arrhythmia encountered in clinical practice, accounting for approximately one third of all hospitalizations for cardiac rhythm disturbances [1, 2]. Pulmonary vein isolation (PVI) is currently the most well-accepted ablation strategy for patients with paroxysmal or persistent atrial fibrillation (AF) [3].
Initially, AF ablations with focal RF catheters were associated with long procedures and extensive RF applications. Anatomically designed, “single-shot” catheters were developed to reduce procedure times and RF applications. The circular, multi-electrode, pulmonary vein ablation catheter (PVAC) was one anatomically based catheter for PVI. The PVAC simultaneously delivers unipolar and bipolar energy to create contiguously transmural lesions. Studies using PVAC demonstrated promising chronic efficacy in paroxysmal AF patients [4–7]. However, early studies evaluating the incidence of asymptomatic cerebral emboli (ACE) after AF ablation showed an increased detection of ACE on MRI post-ablation with PVAC compared to focal RF and cryoballoon [8, 9].
Recently, Verma et al. demonstrated in the multi-center ERACE study that simple procedural changes including continuous therapeutic anti-coagulation and catheter management result in a low 1.7 % incidence of ACE post-ablation with PVAC [10]. Further, the second-generation phased RF catheter, PVAC GOLD, was developed to improve energy delivery using gold electrodes and removal of the tenth electrode to avoid the formation of emboli through the interaction of electrodes 1 and 10 [11–13]. The multi-center PRECISION GOLD trial was an acute study that demonstrated that the new PVAC GOLD catheter is associated with one of the lowest rates of ACE (2.1 %) for any AF ablation technology [14].
Recent pre-clinical work from Hocini et al. has demonstrated that PVAC GOLD electrodes maintaining a temperature ≥50 °C and ≥3 W of power for greater than 30 s have a 99 % positive predictive value for lesion transmurality [15].
This is the first study investigating procedural efficiency, RF biophysics, and chronic efficacy for the new PVAC GOLD catheter in comparison to the first-generation PVAC.
2 Methods
2.1 Study group
In this study, we included 40 consecutive patients with highly symptomatic and drug refractory AF presenting to our department for PVI. The patients were subdivided into two groups (PVAC and PVAC GOLD). The first 20 patients were treated with the first generation of the circular ablation catheter PVAC and utilized the same embolic lowering procedural changes identified in the ERACE study including using only electrodes 1–9 [10]. The subsequent 20 patients were treated with the second-generation PVAC GOLD catheter as it became commercially available. In both groups, the newest software version for the ablation generator was used (GENius ContactIQ software version 15.1). All 40 ablation procedures were performed over 4 months. All patients included in this study were treated unsuccessfully with at least one antiarrhythmic drug. All patients with paroxysmal AF had a history of recurrent arrhythmias of at least 6 months. Patients with persistent AF had received at least one cardioversion within 6 to 12 months to maintain SR. Written consent was obtained from all the patients, and the study protocol was approved by the institutional review board of Bayreuth Hospital.
2.2 Electrophysiological study
Electrophysiological studies and PVI were performed with patients in the fasting state. A transesophageal echocardiogram was performed in all patients before the procedure to rule out intracardiac thrombus. In all patients, conscious sedation with propofol was used during the procedures. All patients underwent ablation under continuous oral anticoagulation. Vascular access was obtained through femoral vein. An octapolar diagnostic catheter (Inquiry™, St. Jude Medical Inc., St. Paul, MN, USA) was positioned in the coronary sinus for recording electrograms and differential atrial pacing during the ablation procedure. After transseptal puncture, systemic anticoagulation was achieved with intravenous heparin to maintain an activated clotting time of ≥350 s. Direct selective angiography of all pulmonary veins was performed to obtain a geometric reference for positioning the PVAC catheter.
2.3 Pulmonary vein ablation catheter PVAC and PVAC GOLD
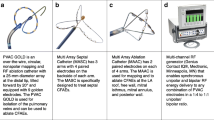
The PVAC is a nine French mapping and ablation catheter with a 25-mm circular electrode array (Fig. 1). The first-generation PVAC catheter was composed of ten 3-mm platinum electrodes with 3-mm spacing. The novel PVAC GOLD catheter is composed of nine 3-mm gold electrodes with 3.75-mm spacing. The tenth PVAC electrode was removed in order to eliminate an interaction between the first and tenth PVAC electrodes that has been shown to be a significant source of emboli with the first-generation PVAC [11].
Gold electrodes have improved thermal conductivity versus platinum electrodes and so may offer advantages for this system in terms of temperature measurement accuracy and efficiency of energy delivery. Additionally, the array of the PVAC GOLD catheter is tilted forward by 20° relative to the catheter shaft in order to improve electrode to tissue contact. Catheter navigation and positioning are supported by both bidirectional steering and over-the-wire technique. The circular array can be extended to assume a spiral configuration which allows for various tissue contact positions. Each electrode contains a thermocouple located adjacent to the electrode surface in contact with the endocardial tissue to maintain temperature accuracy.
2.4 Ablation generator and ablation
The GENius™ generator (Medtronic Inc., Minneapolis, MN, USA) is a multi-channel duty-cycled RF generator capable of independently delivering energy to the catheter electrodes. In contrast to standard ablation generators using RF energy delivered only in a unipolar circuit, the GENius generator is able to provide simultaneous bipolar as well as unipolar current from energized PVAC ablation electrodes. The phased RF technology has been previously described in detail elsewhere [4, 5]. All cases were performed using the GENius with ContactIQ software version 15.1.
2.5 Mapping and ablation protocol
A team of two very experienced physicians (>1000 PVAC cases) performed all 40 procedures together. The PVAC was forwarded to the left atrium via the steerable transseptal sheath and deployed in the left atrium. For catheter positioning, a 0.032ʺ guidewire was introduced into each targeted vein and the PVAC advanced over the wire to the antral region of the pulmonary vein (PV). The position of the PVAC was confirmed by biplane fluoroscopy and compared to the anatomical landmarks defined by previous PV angiography. For optimal tissue contact, positional changes of the PVAC were accomplished by rotating the array, using the steering mechanism of the catheter and the transseptal sheath. An optimal ablation position was always defined by local electrograms and fluoroscopic position.
Isolation of large PVs or common ostium exceeding the diameter of the PVAC was achieved by repositioning the guidewire into different PV branches (commonly upper and lower branch) to achieve optimal and stable catheter position.
RF energy was usually delivered for 60 s per application with a target temperature of 60 °C for circular as well as segmental ablations. When electrode temperature did not rise above 50 °C within 20 s or the power was below 2 W, this electrode was switched off. If the majority of selected electrodes did not reach target temperature or had low energy levels, the application was discontinued to improve position. In most cases, all electrode pairs were activated during the first applications to create overlapping lesions rings. After these first applications, residual potentials were ablated using selected electrode pairs.
Ablation success was defined by disappearance of all PV signals. Further testing of PV isolation was performed by the use of adenosine at the end of the procedure to reveal dormant PV-LA conduction.
Before commencing ablation in the right superior PV, high-voltage stimulation was applied to consecutive electrodes. On instances of right phrenic nerve capture, the catheter was repositioned and pacing resumed prior to RF application.
2.6 Evaluation of ablation performance
In addition to number of RF ablations, procedure, and fluoroscopy time, the following biophysical parameters were measured:
2.6.1 Low power ablations (≤3 W)
The low power analysis counts the number of electrodes with ≤3 W out of the total number of activated electrodes during ablations.
2.6.2 Effective contact (s)
Effective contact is the cumulative time within each 60-s ablation where each electrode reached a temperature ≥50 °C and power ≥3 W. In the analysis, the number of electrodes with effective contact greater than 30 s is counted for each ablation.
2.6.3 Effective energy
Effective energy is defined as the total energy (J) delivered for when each electrode meets the Effective Contact criteria.
2.7 Post-ablation management
All patients were instructed to continue oral anticoagulation for at least 3 months after ablation. Patients with a CHA2DS2-VASC score ≥2 were continued on oral anticoagulation throughout the study. Patients were followed up in our outpatient clinic at 3, 6, and 12 months. Ablation success was defined as freedom from atrial fibrillation/atrial flutter/atrial tachycardia as determined by 7-day Holter monitoring (no episodes ≥30 s) and additional anamnesis with respect to rhythm disturbances.
2.8 Statistical analysis
Data are presented as mean ± SD or as percentages. Differences between groups were determined by t test, Fisher’s exact test, or test of two proportions. A p value of <0.05 was considered significant.
3 Results
The complete characteristics of the two study groups are given in (Table 1). PV isolation was performed in 40 consecutive patients, 15 were female. Mean patient age was 60.8 ± 11.1 years (range 30–79 years). Coronary artery disease (CAD) was present in 15 patients (37.5 %), a history of hypertension in 32 patients (80 %), and diabetes in 11 patients (27.5 %). The baseline characteristics did not significantly differ in between these two groups of consecutive patients (Table 1). The median left atrial diameter was 39.4 ± 8.8 mm (range 26–54 mm). Left atrial enlargement was documented by echocardiography in 17.5 % of patients. Twenty-three patients had paroxysmal AF and 17 patients had persistent AF. There were two left-sided common trunks (one in each group), two LA-roof-veins (one in each group), and two right-sided middle veins (both in PVAC group). All 164 targeted PVs could be isolated successfully using only the PVAC or PVAC GOLD catheter.
Comparison of procedural parameters between the second-generation PVAC GOLD and the first-generation PVAC showed a significant improvement in procedural efficiency. The median number of RF energy application for successful isolation of all PVs was reduced from 34.7 ± 7.0 in the first-generation PVAC group to 27.0 ± 6.5 in the PVAC GOLD group including all circumferential and segmental ablations (Table 2). All PVs, except the left inferior, were associated with a significant decrease in RF energy applications. The improved procedural efficiency was also reflected by a reduction in total RF duration from 32.1 ± 6.5 min with PVAC to 24.8 ± 6.7 min with PVAC GOLD (Fig. 2a). Due to the reduced number of ablations and ablation duration, the total procedure time from vascular access until removal of catheters was significantly reduced from 93.8 ± 18.9 min in the PVAC group to 83.1 ± 10.6 min in the PVAC GOLD group (Table 2). Furthermore, the total fluoroscopy time was reduced from 29.5 ± 9.5 min in the PVAC group to 23.4 ± 7.0 min in the PVAC GOLD group (Table 2). No char or thrombus formation was observed on any of the catheters used in this study.
Improved RF biophysics with PVAC GOLD. a Ablations with PVAC GOLD showed a significant decrease in the number of electrodes with low power. b Mean effective energy of PVAC GOLD was significantly greater than the mean effective energy of PVAC. c No difference in the percentage of electrodes with >30 s of effective contact between PVAC GOLD and PVAC. d Mean RF duration was significantly decreased with PVAC GOLD compared to PVAC
Analysis of the RF applications show that the number of electrodes which only reached a power ≤3 W during ablation was significantly reduced from 6 % using the PVAC to 2 % using the PVAC GOLD (Fig. 2b). Analysis of the RF biophysics shows a significant 9.7 % increase in effective energy (130 J) with PVAC GOLD compared to PVAC (Fig. 2c). Evaluation of the electrodes with effective contact showed no difference between PVAC and PVAC GOLD (Fig. 2d).
3.1 Follow-up
All patients completed a 12-month follow-up visit, including a 7-day Holter to evaluate AF recurrence. At 12 months, AF recurrence rates were similar between the PVAC (7/20) and PVAC GOLD (6/20) groups (35 and 30 %, respectively; p = 0.735). During the follow-up, none of the patients developed left atrial flutter or left atrial tachycardia after AF ablation.
3.2 Safety
No procedure-related complications were observed during the patients’ hospital stay. There were no access-related bleeding complications and no cardiac effusion or tamponade or phrenic nerve palsy. At the time of hospital discharge, all patients were free of any neurological symptoms.
4 Discussion
This is the first study reporting chronic clinical outcomes with the novel PVAC GOLD catheter. The main findings of this study are that the redesigned PVAC GOLD catheter is associated with improved procedural efficiency and RF biophysics while maintaining similar efficacy compared to the first-generation PVAC.
Our clinical follow-up data for PVAC and PVAC GOLD are in a line with results of other groups reporting about the clinical outcomes with the first-generation PVAC catheter. These include three randomized comparisons of PVAC versus a conventional electro-anatomic approach [16–18]. All three of these studies demonstrate no difference between the two techniques in freedom from AF at follow-up, but all show statistically significant reductions in procedure time and fluoroscopy time with PVAC. Other head-to-head comparisons of PVAC versus the conventional approach demonstrate equivalent freedom from AF at the 3-year follow-up [19] and 5-year follow-up [20] but also improved procedural efficiency with PVAC. The present study demonstrates that the procedural efficiency with PVAC GOLD is further improved over PVAC. The use of PVAC GOLD resulted in significantly fewer RF applications and significantly less procedure and fluoroscopy time. These improvements in procedural efficiency may be related to the thermal properties of gold electrode material versus platinum [13, 21].
Comparison of RF biophysics through analysis of generator files demonstrates that the PVAC GOLD delivered energy more efficiently than did PVAC. On the GENius generator, power ≥3 W and temperature >50 °C are visually displayed as green bars and referred to as “Effective Contact.” In this study, we did not observe a difference between the two catheters in this aspect, potentially because electrodes with low temperature or low power were switched off by the software algorithm or manually by the physicians. However, we did observe that more power was delivered during ablations with PVAC GOLD (“Effective Energy”). This may explain the reduction in RF applications required for PVI in our study. We also observed a significant decrease in low-power ablations with PVAC GOLD versus PVAC (2 vs. 6 %). Decreased rates of low-power ablations have been shown to reduce acute PV reconnection (with adenosine challenge) and improve clinical AF recurrence at follow-up [22]. In our series of patients, PVAC GOLD was associated with a decrease in low-power ablations yet resulted in similar freedom from AF at 12 months as PVAC. This may be due to the limited sample size and mid-term follow-up.
There have been concerns over safety with the PVAC catheter, particularly with respect to reports of asymptomatic cerebral embolism and neurological complications. The ERACE trial demonstrated that PVAC procedural changes, in particular avoiding close proximity of electrodes 1 and 10, led to significant reductions in occurrence of ACE (1.7 %) [10]. The PVAC GOLD catheter has only nine electrodes which avoids this potential source of emboli. Further, results of the PRECISION GOLD study show a low 2.1 % rate of ACE with the novel PVAC GOLD [14]. In the current study, no significant adverse events using either the PVAC or PVAC GOLD catheter were reported.
5 Study limitations
The study has a relatively low sample size as it was designed to compare the performance of the second-generation PVAC GOLD to the first-generation PVAC.
Also, this study was not randomized because these two catheters (first- and second-generation PVAC) were not commercially available at the same time; however, all procedures were consecutive and performed by the same operators. Finally, we did not perform post-procedure imaging to assess the prevalence of ACE or asymptomatic PV stenosis in this study. Future studies are warranted to assess the longer-term effectiveness and safety of this second-generation catheter.
6 Conclusion
The PVAC GOLD system allows a reduction in radiofrequency ablation time, higher energy delivery, fewer low power ablations, and improved biophysical efficiency when compared to the first-generation PVAC system. This measureable effects during the procedure did not improve the AF recurrence rate at the 6-month follow-up. Further studies are needed to rule out the long-term effect of improved ablation parameters with regard to freedom from AF.
References
Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–34.
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632–96.
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812–22.
Boersma LV, Wijffels MC, Oral H, Wever EF, Morady F. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm. 2008;5:1635–42.
Fredersdorf S, Weber S, Jilek C, Heinicke N, von Bary C, Jungbauer C, et al. Safe and rapid isolation of pulmonary veins using a novel circular ablation catheter and duty-cycled RF generator. J Cardiovasc Electrophysiol. 2009;20:1097–101.
von Bary C, Weber S, Dornia C, Eissnert C, Fellner C, Latzin P, et al. Evaluation of pulmonary vein stenosis after pulmonary vein isolation using a novel circular mapping and ablation catheter (PVAC). Circ Arrhythm Electrophysiol. 2011;4(5):630–6.
Wieczorek M, Hoeltgen R, Akin E, Salili AR, Oral H, Morady F. Results of short-term and long-term pulmonary vein isolation for paroxysmal atrial fibrillation using duty-cycled bipolar and unipolar radiofrequency energy. J Cardiovasc Electrophysiol. 2010;21(4):399–405.
Gaita F, Leclercq JF, Schumacher B, Scaglione M, Toso E, Halimi F, et al. Incidence of silent cerebral thrombomembolic lesions after atrial fibrillation ablation may change according to technology used: comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon. J Cardiovasc Electrophysiol. 2011;2:961–8.
Herrera Siklódy C, Deneke T, Hocini M, Lehrmann H, Shin DI, Miyazaki S, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol. 2011;58(7):681–8.
Verma A, Debruyne P, Nardi S, Deneke T, DeGreef Y, Spitzer S, et al. L. Evaluation and reduction of asymptomatic cerebral embolism in ablation of atrial fibrillation, but high prevalence of chronic silent infarction: results of the evaluation of reduction of asymptomatic cerebral embolism trial. Circ Arrhythm Electrophysiol. 2013;6:835–42.
Haines DE, Stewart MT, Dahlberg S, Barka ND, Condie C, Fiedler GR, et al. Microembolism and catheter ablation I: a comparison of irrigated radiofrequency and multielectrode phased radiofrequency catheter ablation of pulmonary vein Ostia. Circ Arrhythm Electrophysiol. 2013;6(1):16–22.
Haines DE, Stewart MT, Barka ND, Kirchhof N, Lentz LR, Reinking NM, et al. Microembolism and catheter ablation II: effects of cerebral microemboli injection in a canine model. Circ Arrhythm Electrophysiol. 2013;6(1):23–30.
Haines DE, Strunk AR, Novichenok A, Kirchhof N, Stewart MT. The biophysics of passive convective cooling during catheter ablation with gold versus platinum electrodes and multielectrode phased radiofrequency energy delivery. J Cardiovasc Electrophysiol. 2015.
De Greef Y, Dekker L, Boersma L, Murray S, Wieczorek M, Spitzer SG, et al. Low rate of asymptomatic cerebral embolism and improved procedural efficiency with the novel PVAC GOLD: Results of the PRECISION GOLD trial. Europace. 2016.
Hocini M, Kirchhof NA, Condie C, Stewart MT. Strong correlation between RF biophysics, contact assessment, and lesion quality in a temperature-controlled, multi-electrode ablation system. Europace. 2014; 16(suppl 2);Abstract 136_80.
Bulava A, Hanis J, Sitek D, Osmera O, Karpianus D, Snorek M, et al. Catheter ablation for paroxysmal atrial fibrillation: a randomized comparison between multielectrode catheter and point-by-point ablation. Pacing Clin Electrophysiol. 2010;33:1039–46.
Bittner A, Monnig G, Zellerhoff S, Pott C, Kobe J, Dechering D, et al. Randomized study comparing duty-cycled bipolar and unipolar radiofrequency with point-by-point ablation in pulmonary vein isolation. Heart Rhythm. 2011;8:1383–90.
McCready J, Chow AW, Lowe MD, Segal OR, Ahsan S, de Bono J. Safety and efficacy of multipolar pulmonary vein ablation catheter vs. irrigated radiofrequency ablation for paroxysmal atrial fibrillation: a randomized multicentre trial. Europace. 2014;16(8):1145–53.
De Greef Y, Buysschaert I, Schwagten B, Stockman D, Tavernier R, Duytschaever M. Duty-cycled multi-electrode radiofrequency vs. conventional irrigated point-by-point radiofrequency ablation for recurrent atrial fibrillation: comparative 3-year data. Europace. 2014;16(6):820–5.
Gal P, Aarntzen AE, Smit JJ, Adiyaman A, Misier AR, Delnoy PP, et al. Conventional radiofrequency catheter ablation compared to multi-electrode ablation for atrial fibrillation. Int J Cardiol. 2014;176(3):891–5.
Lewalter T, Weiss C, Spencker S, Jung W, Haverkamp W, Willems S. Gold vs. platinum-iridium tip catheter for cavotricuspid isthmus ablation: the AURUM 8 study. Europace. 2011;13(1):102–8.
De Greef Y, Tavernier R, Schwagten B, De Keulenaer G, Stockman D, Duytschaever M. Impact of radiofrequency characteristics on acute pulmonary vein reconnection and clinical outcome after PVAC ablation. J Cardiovasc Electrophysiol. 2013;24(3):290–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written consent was obtained from all the patients, and the study protocol was approved by the institutional review board of Bayreuth Hospital.
Rights and permissions
About this article
Cite this article
Weber, S., Höher, M. & Schultes, D. First results and follow-up of a second-generation circular mapping and ablation catheter. J Interv Card Electrophysiol 47, 213–219 (2016). https://doi.org/10.1007/s10840-016-0140-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-016-0140-7