Abstract
Density functional theory (DFT) methods are employed to investigate the capability of B36 borophene nanosheets as sensors for detecting the bromoacetone (BCT) molecule. An evaluation of the structural and electronic properties of both BCT and B36 borophene is conducted. Subsequently, through computed metrics such as adsorption energy, charge density difference, and density of states, the interaction between B36 and the BCT molecule is examined via dispersion-corrected density functional theory (DFT). Employing the reduced density gradient approach for the analysis of non-covalent interactions, we further explored the nature of these interactions. The obtained results illustrate that B36 borophene nanosheets serve as effective sensors for the BCT molecule, showcasing their ability to adsorb up to five BCT molecules through an exothermic process. BCT molecules chemiadsorb onto B36 borophene by forming B‒O covalent bonds, engaging the oxygen atom of the carbonyl group in BCT with the edge boron atoms of B36 borophene. Additionally, BCT molecules physio-adsorb on both the concave and convex sides of B36 borophene, facilitated by van der Waals interactions. Ab-initio molecular dynamic simulations confirm the thermal stability of the BCT@B36 concave and convex complexes at both 300 K and 400 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Harmful and hazardous substances may infiltrate the environment through various routes, including industrial emissions, agricultural runoff, and the use of consumer products [1,2,3]. These compounds possess the potential to pose risks to human health and the environment, leading to issues such as cancer, birth defects, neurological damage, and other health concerns [4, 5]. The removal of toxic and hazardous substances from the environment involves a range of procedures and approaches aimed at reducing or eliminating pollutants that pose threats to ecosystems and human well-being.
Adsorption is one of the techniques used to remove poisonous and dangerous chemicals from various mediums like air and water [6,7,8,9,10]. This method involves the adhesion of these substances (adsorbate) to the surface of a solid material (adsorbent), which could be activated carbon [11], zeolites [12], silica gel [13], clay minerals [14,15,16], activated alumina [17], molecular sieves [18], graphene and carbon nanotubes [19], germanene [20], or other specifically designed materials. Nevertheless, owing to their distinctive characteristics at the nanoscale, 2D materials, including borophene (frequently allocated to a graphene cousin), have garnered attention for their potential applications as adsorbents. The borophene B36 nanosheet, a specific variant of 2D material comprising a single layer of boron atoms, is frequently employed as an adsorbent for toxic and hazardous substances [21,22,23,24,25,26,27,28,29]. This preference is attributed to its expansive surface area, high porosity, and exceptional chemical reactivity.
Density functional theory (DFT) computations and ab-initio molecular dynamics (MD) simulations represent computational tools employed to investigate the adsorption behavior of small compounds on adsorbent materials. DFT methods help analyze the electronic structure of both the adsorbate and adsorbent molecules, as well as their interactions (interaction between adsorbate and adsorbent) at an atomic level [29,30,31]. Meanwhile, ab-initio molecular dynamic (MD) simulations provide a comprehensive examination of the dynamic behavior of these complexes (adsorbate–adsorbent) over time and at different temperatures [32,33,34,35].
During the last decade, several researchers [21,22,23,24,25,26,27,28,29] have focused their efforts on examining and understanding the mechanisms of interaction between some extremely toxic chemicals and B36 borophene nanosheet using the density functional theory (DFT) methods. Kootenaei et al. [21] explored the reactivity and electronic sensitivity of B36 borophene toward HCOH gas. Their findings suggest that the concentration of HCOH gas notably impacts borophene conductance, indicating that an increase in the quantity of HCOH molecules leads to a corresponding elevation in conductance.
Mohsenpour and co-workers [22] employed density functional theory (DFT) calculations to explore the potential of the B36 nanosheet as a chemical sensor for the detection of pollutants such as formaldehyde, acetaldehyde, and propanal. The results show that boron atoms on the edges of the nanosheet are more reactive than those in the interior and that the number of adsorbed pollutant molecules affects both the adsorption energy and the electronic properties of the B36 nanosheet.
Li and Zhao [27] investigated the interactions between mercaptopurine and B36 using the DFT method. The observations indicated that the boron atoms positioned at the edges of B36 nanosheets exhibited higher reactivity compared to the internal boron atoms when interacting with mercaptopurine (MP). Furthermore, their conclusion underscored the pronounced tendency of MP molecules to be adsorbed, particularly via their nitrogen heads, onto the edges of B36.
Allal and all co-workers [29] conducted a computational analysis using DFT to investigate the borophene B36 nanosheet’s potential as a sensor for the harmful acrolein molecule. The results corroborated previous literature [21,22,23,24,25,26,27,28,29], confirming the borophene B36 nanosheet’s suitability as a highly promising candidate for detecting and removing acrolein molecules.
On the other hand, bromoacetone CH3COCH2Br (see Fig. 1) is an exceedingly toxic chemical recognized for inducing severe irritation, burns, and tissue damage upon contact with the skin, eyes, or respiratory system [36, 37]. Its strong irritating qualities are highlighted by its historical employment as a lachrymatory weapon in chemical warfare. Because of this molecule’s hazardous nature and detrimental effects on both human health and the environment, handling and regulation of it are imperative. Exposure to this compound may cause skin irritation, eye damage, and respiratory difficulties.
In this study, we examined the electronic structures of the bromoacetone (BCT) molecule and the borophene B36 nanosheet. Our investigation focused primarily on assessing the potential of the B36 nanosheet as a chemical sensor for the BCT molecule, employing ab-initio molecular dynamic (AIMD) simulations and density functional theory (DFT) calculations.
2 Computational details
2.1 Quantum chemical calculations
All geometry optimizations in this study were carried out using ORCA program package (version 5.0.0 and 5.0.4) [38]. Firstly, the isolated electronic structures of BCT and B36 have been optimized at B3LYP-D3/def2-TZVPP [39, 40] of level theory, and then after, several configurations of interactions between BCT and B36 were investigated and analyzed. Different functionals of the Head-Gordon group [41] were also performed such as B97M, ωB97X, and ωB97M [42] in combination with the D4 dispersion correction, and using the modified triple-ζ basis sets def2-mTZVP [43]. Further, in order to predict and compare behavior of interaction between BCT and B36, we include the recent r2SCAN-3c composite method [44], based on the r2SCAN [45, 46] meta-GGA combined with the D4 dispersion correction [47] and geometrical counter poise-correction [48].
The molecular electrostatic potential (MEP) [49] was performed by ORCA package and visualized using the UCSF Chimera program (version 1.10.2) [50]. Reduced density gradient (RDG) and density of state (DOS) analyses were adopted by Multiwfn program [51]. However, Fukui function isosurface plots (electrophilic (\(f^{ - }\)) and nucleophilic (\(f^{ + }\))) were visualized by VMD program [52] based on the cube files generated by Multiwfn software.
The conceptual DFT-based global and local reactivity descriptors were estimated to predict the reactivity and reactive sites on the BCT and B36 molecules. The global reactivity descriptors such as highest occupied molecular orbital (EHOMO) and the lowest unoccupied molecular orbital (ELUMO), energy gap (\({\mathbf{\Delta E}}_{{{\mathbf{gap}}}}\)), global hardness (\({\varvec{\eta}}\)), global softness (\({\varvec{\sigma}}\)), global electrophilicity (\({\varvec{\omega}}\)), electro-donating power (\({\varvec{\omega}}^{ - }\)), electro-accepting power (\(\omega^{ + }\)), net electrophilicity (\({{\varvec{\Delta}}}\omega^{ \pm } )\), and back-donation (\({\mathbf{\Delta E}}_{{{\mathbf{back}} - {\mathbf{d}}}}\)) were calculated according to the following equation:
Furthermore, the local reactivity for both BCT and B36 molecules was examined through the Fukui indices according to the following equations [53,54,55]:
where \({\varvec{q}}_{{\varvec{K}}}\) is the gross charge of atom k at the molecule and N is the number of electrons. Therefore \({\varvec{q}}_{{\varvec{K}}} \left( {\varvec{N}} \right)\), \({\varvec{q}}_{{\varvec{K}}} \left( {{\varvec{N}} + 1} \right)\), and \({\varvec{q}}_{{\varvec{K}}} \left( {{\varvec{N}} - 1} \right)\) are charge values of atom k for neutral, cation, and anion, respectively. Local electrophilicity (\(\omega_{k}^{ + } ,\omega_{k}^{ - }\)) and local softness (\(\delta_{k}^{ + } ,\delta_{k}^{ - }\)) indexes are calculated by the following equations:
The expression used to calculate the adsorption energy is as follows [27,28,29]:
\({\text{E}}_{{\left[ {{\text{B}}_{36} @\left( {{\text{BCT}}} \right)_{{\text{n}}} } \right]}} { }\) represents the total energy of the B36@(BCT)n complex, \({\text{E}}_{{{\text{B}}_{36} }}\) denotes the energy of the isolated B36 nanosheet, and \({\text{E}}_{{{\text{BCT}}}}\) stands for the energy of the isolated single BCT molecule. The thermal stability of BCT@B36 complex is also examined by conducting the ab-initio molecular dynamic (AIMD) simulations at 300 K and 400 K.
3 Results and discussion
3.1 Structure proprieties of B36 and BCT
It has been reported in the literature [56,57,58,59,60,61,62] that the interaction between two molecular systems, specifically between an adsorbate and adsorbent surface, is highly dependent on the respective donor and acceptor abilities of the molecules involved. Molecules with high EHOMO values tend to donate electrons to a low ELUMO of suitable acceptor molecule. Charge transfer in molecular systems depends on the energy gap between EHOMO and ELUMO, a larger ΔEgap is indicative of a lower reactivity, while a smaller ΔEgap values is associated with higher reactivity of a chemical species.
The calculated global reactivity descriptors such as highest occupied molecular orbital energy (EHOMO), lowest unoccupied molecular orbital energy (ELUMO), energy gap (ΔEgap), global hardness (η), global softness (σ), global electrophilicity (ω), and back-donations of electrons (ΔEback-d) are listed in Table 1. Based on the calculated reactivity descriptors, the B36 borophene exhibits high reactivity associated with low energy gap (1.881 eV) and high softness values (1.063 eV). DFT calculations revealed also that the B36 borophene has the high electrophilicity index (ω) compared to BCT molecule, which means a good acceptor behavior to interact with BCT.
The frontier molecular orbitals HOMO and LUMO were used to explain and to understand the chemical reactivity of the BCT and B36 structures. Optimized BCT and B36 electronic structures at B3LYP-D3/def2-TZVPP level of theory associated with their frontier molecular orbitals (HOMO and LUMO) are shown in Fig. 2.
One observation that can be made is that in the BTC structure, the lowest unoccupied molecular orbital (LUMO) was primarily found to be localized on the carbonyl bond (C═O), while the highest occupied molecular orbital (HOMO) is spread out over the entire molecule but has a particularly high concentration on the oxygen atom in the carbonyl group and on the bromo atom. This indicates that these atoms may be able to interact effectively with the B36 surface by providing electrons. In contrast, in the B36 structure, the electron densities of both the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are mainly spread across the surface of the B36 molecule, with a significant contribution from the boron atoms on the sides. This suggests that the most reactive sites for both electrophilic and nucleophilic attacks are located in these regions. Furthermore, the molecular electrostatic potential (MEP) analysis corroborates with the results of frontier molecular orbitals suggesting that the most reactive site on the B36 borophene are the lateral atoms of B36 structure indicated by negative charge (red surface color), although the most reactive site of the BCT molecule is the oxygen atom into the carbonyl group, as shown in Fig. 3.
In addition, the Fukui function was used to analyze the local reactivity of B36 and BCT molecules, in order to provide information on the preferential sites for electrophilic and nucleophilic attacks. The calculated condensed Fukui function, local electrophilicity, and local softness of B36 and BCT using Hirshfeld atomic charges are summarized in supplementary data Tables S1 and S2 respectively. However, the isosurface Fukui maps of the electrophilic and nucleophilic attacks are shown in Fig. 4, associated with the most high values of calculated Fukui indices \(f_{k}^{ + }\) (red) and \(f_{k}^{ - }\) (blue). Upon examining the isosurface Fukui maps presented in Fig. 4, it becomes apparent that in the case of BCT, the oxygen and bromo atoms serve as favorable sites for both nucleophilic and electrophilic attacks. Concerning the B36 surface, the boron atoms B21, B24, B27, B30, B33, and B36 are identified as preferred sites for nucleophilic attacks, while B27, B28, B29, B30, B33, and B36 atoms are deemed suitable for electrophilic attacks, as illustrated in Fig. 4.
3.2 Adsorption of single BCT molecule on the B36 nanosheet
Based on the results of DFT calculations, we have considered and compared three most stable adsorption configurations, as summarized and shown in Fig. 5a–c, the corresponding frontier molecular orbitals HOMO and LUMO are illustrated in Fig. 6.
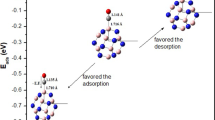
The BCT molecule can adsorb on the B36 borophene nanosheet with the formation of B‒O bond at the edge side of the B36 borophene associated with an adsorption energy of − 95.08 kj/mol. The B‒O bond established between the BCT molecule and the B36 nanosheet, with a length of 1.48 Å is slightly shorter than the sum of O−B covalent radii (\(r_{O}^{{{\text{cov}}}}\) + \(r_{B}^{{{\text{cov}}}}\) = 0.66 + 0.84 = 1.50 Å) [63], and considerably shorter than the van der Waals sum (\(r_{O}^{{{\text{van}}}}\) + \(r_{B}^{{{\text{van}}}}\) = 1.40 + 1.74 = 3.14 Å) [64], which implies the potential implication of a chemisorption process as a feasible mechanism for inhibition.
On the other hand, the BCT molecule can also interact with B36 borophene without formation of any covalent bonds between them. This interaction can occur through two distinct stable complexes: one situated at the concave side (Fig. 5b) with an interaction energy of approximately − 78.16 kj/mol and the other at the convex side with an energy of approximately − 50.07 kj/mol.
The computed distances between the atoms of BCT molecule (C, H, O, and Br atoms) and the closest boron atoms of B36 borophene in BCT@B36 concave and BCT@B36 convex complexes fall within the range of 3.06–3.10 Å. These interactions exhibit lengths that are notably surpassing the sum of the covalent radii of B−O (1.50 Å), B − H (1.15 Å), B−C (1.60 Å), and B−Br (2.04 Å) [63]. Furthermore, these lengths are comparatively longer than the van der Waals sum of the selected atoms B−O (3.14 Å), B − H (2.94 Å), B−C (3.44 Å), and B−Br (3.69 Å) [64]. Consequently, these interactions involve weak attractive forces between the BCT molecule and the B36 nanosheet.
The inspection of the frontier molecular orbital distributions including the highest occupied molecular orbital energy (HOMO) and lowest unoccupied molecular orbital energy (LUMO) for the concave and convex BCT@B36 complexes shows that the HOMO and LUMO orbitals are entirely localized on the B36 borophene. This is in contrast to the edge configuration where the LUMO distribution is mainly localized on the BCT molecule, while the HOMO orbital is localized across the entire BCT@B36 structure.
Furthermore, the non-covalent interaction analysis based on the reduced density gradient (RDG-NCI) analysis was also carried out to examine and identify the nature of interactions responsible for its exceptional stability in the studied systems, including the intermolecular interactions in the BCT@B36 concave and BCT@B36 convex complexes. Figure 7 displays the RDG-NCI isosurfaces for the three most stable complexes, namely the edge, concave, and convex configurations (see Fig. 7a–c). The color spectrum varies from blue to red, representing values falling within the range of − 0.05 a.u. < ρ*sign(λ2) < 0.05 a.u.
Larger green isosurfaces were noticed between the BCT molecule and the B36 nanosheet in the case of the BCT@B36 concave and convex complexes (configurations b and c, respectively). These isosurfaces were distinctly characterized by the combination of light green and dark coloration.
The light green area indicates strong interactions being dominated by van der Waals forces. These contacts were observed not only between the oxygen atom of the carbonyl group and the B36 surface but also between the bromo atom and the B36, as well as between the bottommost hydrogen atoms of the methyl groups and the B36 surface. The 2D RDG scatter plots further confirm the existence of these weak interactions within the region of λ2(ρ) from − 0.002 to − 0.012 atomic units (a.u.), as illustrated in Fig. 7b and c. We can also observed a dark green area in the isosurface of BCT@B36 concave and convex complexes between carbon atom of BCT molecule and B36 surface, corresponding to the ranges between 0.00 and 0.01 a.u., as shown in the 2D scatter plot (Fig. 7b and c), due to the week interaction of van der Waals force.
On the other hand, in the most stable configuration BCT@B36 edge (configuration a), we find that not only the formation of covalent B‒O bond which stabilized the complex formed, but also a van der Waals type interaction was formed between bromo atom of BCT molecule and the side boron B atom of B36 surface. This observation is effectively depicted in Fig. 7a, where a green isosurface is visible between the bromo atom of the BCT molecule and the boron B atom of B36. Additionally, in the 2D RDG graph, this interaction is evident at approximately 0.005 and 0.015 u.a., further affirming the presence of van der Waals forces.
Additionally, we explored the charge redistribution after the interaction of the BCT molecule with the B36 nanosheet via the charge density difference (CDD) calculations. Figure 8 displays the charge density difference (CDD) plots for the most stable configurations of the interaction between the BCT molecule and B36 nanosheet. These plots utilize an isosurface value of 0.002 e/Å3, where the red cardinal regions indicate charge depletion (electron loss), while the yellow regions show charge buildup (electron enrichment). As can be visually observed in Fig. 8, the formation of direct B‒O bond is clearly shown by the most significant charge redistributions. Furthermore, it has been observed that less significant charge redistributions occur between the bromo atom of the BCT molecule and the boron B atom of the B36 nanosheet, leading to an increase in binding energy.
The analysis of charge density difference (CDD) for BCT@B36 concave and convex configurations (refer to Fig. 8b and c) showed a similar behavior and indicates the presence of an electrostatic interaction between the oxygen atom of the carbonyl group in the BCT molecule and the B36 nanosheet. This is evident from the observed charge redistribution around the C=O group and the smaller yellow regions indicating charge accumulation on the borophene surface, all without the formation of covalent bonds.
Based on the calculated molecular descriptors (Table 2), the interaction between the BCT molecule and the B36 nanosheet leads to a shift in the HOMO energy level toward higher energies. Specifically, for the BCT@B36 edge complex, it shifts to − 5.17 eV, for the BCT@B36 concave complex, it shifts to − 5.61 eV, and for the BCT@B36 convex complex, it shifts to − 5.64 eV. However, in contrast to both the BCT@B36 concave and BCT@B36 convex complexes, where the energy of the LUMO shifts to higher energy levels, the LUMO orbitals in the BCT@B36 edge complex exhibit a shift toward lower energy levels, leading to a reduction in the energy gap (ΔEgap).
Analysis of the DOS spectral results reveals also that the BCT@B36 edge configuration is more affected after interaction between BCT and B36 compared to its two analogs (BCT@B36 concave and BCT@B36 convex complexes), as shown in Fig. 9. The reduction in the HOMO–LUMO energy gap follows a descending order: BCT@B36 convex (1.89 eV) > BCT@B36 concave (1.83 eV) > BCT@B36 edge (1.17 eV). This substantial decrease in the energy gap implies a significant alteration in the electronic conductivity of the B36 nanosheet upon interaction with the BCT molecule. These beneficial changes have the potential to be applied in the development of molecular sensors.
3.3 Ab-initio molecular dynamic (AIMD) simulations of adsorption
The ab-initio molecular dynamic (AIMD) simulations were executed using the ORCA software (version 5.0.4) [38, 65]. For all systems, a time step of 1.0 fs was employed, and the temperature was set at T = 300 K.
We explore the thermal stability and predict the behavior of both concave and convex BCT@B36 complexes using AIMD simulations at temperatures of 300 K and 400 K. The computational results from AIMD simulations for both types of complexes at the 300 K and 400 K temperatures are depicted in Fig. 10.
As illustrated in Fig. 10, the root mean square deviation (RMSD) curves exhibit consistent periodic fluctuations for both concave and convex BCT@B36 complexes at 300 K and 400 K. The comparison between the two RMSD of BCT@B36 concave and BCT@B36 convex clearly indicates that BCT@B36 convex is the more thermally stable configuration at 300 K. Upon comparing the two RMSD curves at 300 K and 400 K for BCT@B36 complexes, it becomes evident that the higher the simulation temperature, the more significant the geometric fluctuation attributed to thermal motion.
3.4 Adsorption of multiple BCT molecules on the B36 nanosheet
We have investigated the impact of concentration by examining the adsorption of one to six bromoacetone (BCT) molecules on borophene B36 nanosheet. With this aim in mind, we chose the most stable complex featuring a singly edge-adsorbed BCT. Subsequently, additional BCT molecules were systematically introduced, progressing incrementally at the edge, concave, and convex sides, with a primary emphasis on edge-side adsorption.
We conducted a reoptimization procedure for each configuration using the B3LYP-D3BJ-SMD/Def2-SVP level of theory, with RIJCOSX approximation. Subsequently, for each newly optimized complex, an exploration of various possible configurations was compared.
Therefore, Figs. 11 and 12 depict the most stable configurations, while Table 3 provides detailed information on the adsorption energies of multiple BCT molecules on the B36 nanosheet.
Based on DFT calculation results (Table 3, Figs. 11 and 12), B36 borophene nanosheet has the capacity to absorb more than a single BCT molecule. It is worth noting that B36 has the capability to capture up to five BCT molecules (an exothermic process), facilitated by spontaneous attachment corroborated by the negative adsorption energy, as illustrated in Table 3.
Contrarily, in the case of the adsorption of six BCT molecules on B36 borophene, the calculation results yield a positive value for the adsorption energy (an endothermic process), except for the configuration 4-AC-EDGE + 1AC-CONCAVE + 1AC-CONVEX, which exhibits a negative adsorption energy, suggesting a strong interaction between the BCT and B36 borophene molecules. The calculated adsorption energies at the B97-D3(BJ)/def2-SVP level (Table 3) reveal that the most stable complexes (2-EDGE, 3-EDGE, 4-EDGE, and 5-EDGE) feature the adsorption of BCT molecules at the edge sites of the B36 nanosheet.
We also note that in configurations involving four, five, and six molecules of BCT adsorbed on B36 borophene, certain BCT molecules underwent dissociation reactions into acetonyl radicals and bromine atom fragments during the adsorption process on B36 borophene, as illustrated in Fig. 12 (4-EDGE (a), 5-EDGE (a–d), and 6-EDGE (b and c)).
Nevertheless, in the 5-EDGE + 1-CONCAVE configuration, the BCT molecule formed a covalent B‒O bond with B36 borophene, leading to a bond length of 1.43 Å. This measured distance is marginally less than the combined covalent radii of O−B (\(r_{O}^{{{\text{cov}}}}\) + \(r_{B}^{{{\text{cov}}}}\) = 0.66 + 0.84 = 1.50 Å) [63], suggesting the plausibility of a chemisorption process as the mechanism for adsorption.
4 Conclusion
In summary, a computational study has been performed on the B36 borophene nanosheets as sensors for detecting bromoacetone (BCT) molecule. The main conclusions that can be drawn from this study are the following:
-
The B36 borophene nanosheets effectively senses the BCT molecule, adsorbing up to five BCT molecules via an exothermic process.
-
DFT calculations revealed that the most stable configurations among the complexes are BCT@B36 edge, BCT@B36 concave, and BCT@B36 convex.
-
The BCT molecule can adsorb through the formation of B‒O bond, involving the oxygen atom of the carbonyl group in BCT and the edge boron atoms of B36 borophene, indicating a chemisorption process.
-
The BCT molecule can also physio-adsorb on both the concave and convex sides of B36 borophene nanosheets, facilitated by van der Waals interactions.
-
The results of ab-initio molecular dynamics (AIMDs) validate the thermal stability of both the BCT@B36 concave and BCT@B36 convex complexes at temperatures of 300 K and 400 K.
Data availability
Not applicable.
References
Deng, J., Guo, J., Zhou, X., Zhou, P., Fu, X., Zhang, W., Lin, K.: Hazardous substances in indoor dust emitted from waste TV recycling facility. Environ. Sci. Pollut. Res. 21(12), 7656–7667 (2014)
Deng, J., Guo, J., Zhou, X., Zhou, P., Fu, X., Zhang, W., Lin, K.: Hazardous substances in indoor dust emitted from waste TV recycling facility. Environ. Sci. Pollut. Res. 21, 7656–7667 (2014)
McCunney, R.J.: Health effects of work at waste water treatment plants: a review of the literature with guidelines for medical surveillance. Am. J. Ind. Med. 9, 271–279 (1986)
Chandiramouli, R., Nagarajan, V.: Transport studies of CeO2 molecular device and adsorption behavior of CO on CeO2 device: a first-principles investigation. J. Comput. Electron. 16, 316–324 (2017)
Golding, J.: Unnatural constituents of breast milk-medication, lifestyle, pollutants, viruses. Early. hum. dev. 49, S29–S43 (1997)
DeCoste, J.B., Peterson, G.W.: Metal-organic frameworks for air purification of toxic chemicals. Chem. Rev. 114, 5695–5727 (2014)
Gopakumar, D.A., Pasquini, D., Henrique, M.A., de Morais, L.C., Grohens, Y., Thomas, S.: Meldrum’s acid modified cellulose nanofiber-based polyvinylidene fluoride microfiltration membrane for dye water treatment and nanoparticle removal. ACS. Sustain. Chem. Eng. 5, 2026–2033 (2017)
Lyu, Y., Brusseau, M.L., Chen, W., Yan, N., Fu, X., Lin, X.: Adsorption of PFOA at the air-water interface during transport in unsaturated porous media. Environ. Sci. Technol. 52, 7745–7753 (2018)
Saravanan, A., Senthil, K.P., Jeevanantham, S., Karishma, S., Tajsabreen, B., Yaashikaa, P.R., Reshma, B.: Effective water/wastewater treatment methodologies for toxic pollutants removal: processes and applications towards sustainable development. Chemosphere 280, 130595 (2021)
Bhuvaneswari, R., Nagarajan, V., Chandiramouli, R.: First-principles analysis of the detection of amine vapors using an antimonene electroresistive molecular device. J. Comput. Electron. 18, 779–790 (2019)
Srivastava, V.C., Mall, I.D., Mishra, I.M.: Adsorption of toxic metal ions onto activated carbon. Chem. Eng. Process. 47, 1269–1280 (2008)
Tang, Z.K., Nozue, Y., Goto, T.: Quantum size effect on the excited state of HgI2, PbI2 and BiI3 clusters and molecules in zeolite LTA. J. Phys. Soc. Jpn. 61, 2943 (1992)
Sigot, L., Ducom, G., Germain, P.: Adsorption of octamethylcyclotetrasiloxane (D4) on silica gel (SG): retention mechanism. Microporous Mesoporous Mater. 213, 118–124 (2015)
Besghaier, S., Cecilia, J.A., Chouikhi, N., Vilarrasa-García, E., Rodríguez-Castellón, E., Chlendi, M., Bagane, M.: Glyphosate adsorption onto porous clay heterostructure (PCH): kinetic and thermodynamic studies. Braz. J. Chem. Eng. 39, 903–917 (2022)
Vinuth, M., Naik, H.S.B., Mahadevaswamy, M.M., Prabhakara, M.C.: Environmentally benign Fe(III)–montmorillonite for rapid adsorption of methylene blue dye in aqueous medium under ambient conditions. Fash. Text. 4, 8 (2017)
Scholtzová, E., Jankovič, L., Tunega, D.: Montmorillonite as an anti-tuberculosis rifampicin drug carrier: DFT and experimental study. Clays Clay Miner. 71, 229–241 (2023)
Jin-feng, P., Wu, R., Chen, X.: Preparation of benzene adsorption materials using waste activated alumina. J. Shanghai Jiaotong Univ. Sci. 17, 373–376 (2012)
Manjare, S.D., Ghoshal, A.K.: Comparison of adsorption of ethyl acetate on activated carbon and molecular sieves 5A and 13X. J. Chem. Eng. Data 51, 1185–1189 (2006)
Bardi, N., Giannakopoulou, T., Vavouliotis, A., Trapalis, C.: Electrodeposited films of graphene, carbon nanotubes, and their mixtures for supercapacitor applications. ACS. Appl. Nano. Mater. 3, 10003–10013 (2020)
Snehha, P., Nagarajan, V., Chandiramouli, R.: Germanene nanotube electroresistive molecular device for detection of NO2 and SO2 gas molecules: a first-principles investigation. J. Comput. Electron. 18, 308–318 (2019)
Kootenaei, S.A., Ansari, G.: B36 borophene as an electronic sensor for formaldehyde: quantum chemical analysis. Phys. Lett. A 380, 2664–2668 (2016)
Mohsenpour, Z., Shakerzadeh, E., Zare, M.: Quantum chemical description of formaldehyde (HCHO), acetaldehyde (CH3CHO) and propanal (CH3CH2CHO) pollutants adsorption behaviors onto the bowl-shaped B36 nanosheet. Adsorption 23, 1041–1053 (2017)
Valadbeigi, Y., Farrokhpour, H., Tabrizchi, M.: Adsorption of small gas molecules on B36 nanocluster. J. Chem. Sci. 127, 2029–2038 (2015)
Rostami, Z., Soleymanabadi, H.: N–H bond cleavage of ammonia on graphene-like B36 borophene: DFT studies. J. Mol. Model. 22, 70 (2016)
Omidvar, A.: Borophene: a novel boron sheet with a hexagonal vacancy offering high sensitivity for hydrogen cyanide detection. Comput. Theor. Chem. 1115, 179–184 (2017)
Rastgou, A., Soleymanabadi, H., Bodaghi, A.: DNA sequencing by borophene nanosheet via an electronic response: a theoretical study. Microelectron. Eng. 169, 9–15 (2017)
Li, W., Zhao, T.: Borophene as an carrier for mercaptopurine drug: electronic study via density-functional theory computations. J. Mol. Model. 29, 325 (2023)
Arabieh, M., Azar, Y.T., Sepehrian, H., Fasihi, J.: DFT exploration of adsorptive performances of borophene to small sulfur-containing gases. J. Mol. Model. 28, 146 (2022)
Allal, H., Belhocine, Y., Rahali, S., Damous, M., Ammouchi, N.: Structural, electronic, and energetic investigations of acrolein adsorption on B36 borophene nanosheet: a dispersion-corrected DFT insight. J. Mol. Model. 26, 128 (2020)
Damous, M., Allal, H., Belhocine, Y., Maza, S., Merazig, H.: Quantum chemical exploration on the inhibition performance of indole and some of its derivatives against copper corrosion. J. Mol. Liq. 340, 117136 (2021)
Bousba, S., Allal, H., Damous, M., Maza, S.: Computational DFT analysis and molecular modeling on imidazole derivatives used as corrosion inhibitors for aluminum in acidic media. Comput. Theor. Chem. 1225, 114168 (2023)
Petrushenko, I.K., Ivanov, N.A., Petrushenko, K.B.: Theoretical investigation of carbon dioxide adsorption on Li+-decorated nanoflakes. Molecules 26, 7688 (2021)
Liu, J., Xu, X., Anantram, M.P.: Role of inelastic electron–phonon scattering in electron transport through ultra-scaled amorphous phase change material nanostructures. J. Comput. Electron. 13, 620–626 (2014)
Böselt, L., Thürlemann, M., Riniker, S.: Machine learning in QM/MM molecular dynamics simulations of condensed-phase systems. J. Chem. Theory Comput. 17, 2641–2658 (2021)
Meng, S., Fu, X., Jiang, L., Shi, L., Wang, X., Liu, X., Wang, J.: Theoretical calculations and experiments on the thermal properties of fluorinated graphene and its effects on the thermal decomposition of nitrate esters. Nanomaterials 12, 621 (2022)
Szinicz, L.: History of chemical and biological warfare agents. Toxicology 214, 167–181 (2005)
Heyl, M., McGuire, R.: Analytical chemistry associated with the destruction of chemical weapons. Springer, Dordrecht (1997)
Neese, F.: The ORCA program system. WIREs Comput. Mol. Sci. 2, 73–78 (2012)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Weigend, F., Ahlrichs, R.: Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297 (2005)
Head-Gordon, M., Rico, R.J., Oumi, M., Lee, T.J.: A doubles correction to electronic excited states from configuration interaction in the space of single substitutions. Chem. Phys. Lett. 219, 21–29 (1994)
Najibi, A., Goerigk, L.: The nonlocal kernel in van der Waals density functionals as an additive correction: an extensive analysis with special emphasis on the B97M-V and ωB97M-V approaches. J. Chem. Theory Comput. 14, 5725–5738 (2018)
Chan, B., Dawson, W., Nakajima, T.: Searching for a reliable density functional for molecule–environment interactions, found B97M-V/def2-mTZVP. J. Phys. Chem. A 126, 2397–2406 (2022)
Ehlert, S., Huniar, U., Ning, J., Furness, J.W., Sun, J., Kaplan, A.D., Perdew, J.P., Brandenburg, J.G.: r2SCAN-D4: dispersion corrected meta-generalized gradient approximation for general chemical applications. J. Chem. Phys. 154, 061101 (2021)
Furness, J.W., Kaplan, A.D., Ning, J., Perdew, J.P., Sun, J.: Accurate and numerically efficient r 2 SCAN meta-generalized gradient approximation. J. Phys. Chem. Lett. 11, 8208–8215 (2020)
Palm, W.U., Schmidt, N., Stahn, M., Grimme, S.: A kinetic study of the photolysis of sulfamethoxazole with special emphasis on the photoisomer. Photochem. Photobiol. Sci. 22, 615–630 (2022)
Bursch, M., Neugebauer, H., Ehlert, S., Grimme, S.: Dispersion corrected r2SCAN based global hybrid functionals: r2SCANh, r2SCAN0, and r2SCAN50. J. Chem. Phys. 156, 134105 (2022)
Kruse, H., Grimme, S.: A geometrical correction for the inter- and intra-molecular basis set superposition error in Hartree-Fock and density functional theory calculations for large systems. J. Chem. Phys. 136, 154101 (2012)
Leboeuf, M., Köster, A.M., Jug, K., Salahub, D.R.: Topological analysis of the molecular electrostatic potential. J. Chem. Phys. 111, 4893–4905 (1999)
Goddard, T.D., Huang, C.C., Ferrin, T.E.: Visualizing density maps with UCSF Chimera. J. Struct. Biol. 157, 281–287 (2007)
Lu, T., Chen, F.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012)
Hsin, J., Arkhipov, A., Yin, Y., Stone, J.E., Schulten, K.: Using VMD: an introductory tutorial. Curr. Protoc. Bioinform. 24(1), 5–7 (2008)
Korchowiec, J., Gerwens, H., Jug, K.: Relaxed Fukui function indices and their application to chemical reactivity problems. Chem. Phys. Lett. 222, 58–64 (1994)
Kinkar, R.R., Hirao, K., Pal, S.: On non-negativity of Fukui function indices II. J. Chem. Phys. 113, 1372–1379 (2000)
Allal, H., Nemdili, H., Zerizer, M.A., Zouchoune, B.: Molecular structures, chemical descriptors, and pancreatic lipase (1LPB) inhibition by natural products: a DFT investigation and molecular docking prediction. Struct. Chem. 35(1), 223–239 (2023)
Sakki, B., Said, M.E., Mezhoud, B.: Experimental and theoretical study on corrosion inhibition of pyridinium salts derivatives for API 5L Gr.B steel in acidic media. J. Adhes. Sci. Technol. 36, 2245–2268 (2022)
Bouasla, S., Sobhi, C., Allal, H., Mecibah, W., Cherifi, M., Bougdah, I.: Synthesis, antioxidant and dft study of some 4-methyl-2h- chromen-2-one derivatives. Rev. Roum. Chim. 66, 653–660 (2021)
Ammouchi, N., Allal, H., Zouaoui, E., Dob, K., Zouied, D., Bououdina, B.: Extracts of Ruta chalepensis as green corrosion inhibitor for copper CDA 110 in 3% NaCl medium: experimental and theoretical studies. Anal. Bioanal. Electrochem. 11, 830–850 (2019)
Kaur, D., Sharma, R., Aulakh, D.: The hydrogen bond donor and acceptor ability of thioformic acid. Struct. Chem. 22, 1015–1030 (2011)
Chopra, N., Kaur, D., Chopra, G.: Hydrogen bonded complexes of oxazole family: electronic structure, stability, and reactivity aspects. Struct. Chem. 29, 341–357 (2018)
Ajmal, M., Ali, U., Javed, A., Tariq, A., Arif, Z., Iqbal, J., Shoaib, M., Ahmed, T.: Designing indaceno thiophene–based three new molecules containing non-fullerene acceptors as strong electron withdrawing groups with DFT approaches. J. Mol. Model. 25, 311 (2019)
El-Shishtawy, R.M., Asiri, A.M., Aziz, S.G., Elroby, S.A.K.: Molecular design of donor-acceptor dyes for efficient dye-sensitized solar cells I: a DFT study. J. Mol. Model. 20, 2241 (2014)
Cordero, B., Gómez, V., Platero-Prats, A.E., Reves, M., Echeverría, J., Cremades, E., Barragán, F., Alvarez, S.: Covalent radii revisited. Dalton Trans. (2008). https://doi.org/10.1039/B801115J
Batsanov, S.S.: Van der Waals radii of Elements. Inorg. Mater. 37, 871–885 (2001)
Brehm, M.: ORCA 5.0 Molecular dynamics module. (2021)
Acknowledgements
The authors express their gratitude to the University of Constantine 1 for its support in the completion of this work.
Funding
The authors assert the absence of any funding sources.
Author information
Authors and Affiliations
Contributions
M.T.—Investigation and ORCA software. H.A.—Administration of projects, reviewing and editing, and validating. S.B.—Reviewed the manuscript. F.B.—Investigation, multiwfn software, and RDG analysis. S.M.—Investigation, multiwfn software, and CDD analysis. M.D.—Writing the manuscript. A. B.—Writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taier, M., Allal, H., Bousba, S. et al. A computational investigation on the adsorption behavior of bromoacetone on B36 borophene nanosheets. J Comput Electron 23, 931–944 (2024). https://doi.org/10.1007/s10825-024-02192-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-024-02192-3