Abstract
Environmental stresses such as nitrate deprivation and high light are effective at increasing lipid content in microalgae, but they can also slow down and even stop growth. In this study, the phytohormones methyl jasmonate, salicylic acid, gibberellin, abscisic acid, and ethephon were introduced to cultures of the oleaginous marine diatom Phaeodactylum tricornutum in an attempt to increase growth and lipid production. Single-factor experiments showed that the influences of some of the phytohormones were closely related to their concentrations. Methyl jasmonate, abscisic acid, and salicylic acid promoted P. tricornutum growth and lipid accumulation at certain concentrations. The differing effects of the three phytohormones on P. tricornutum may be related to the respective phytohormone’s responsive cis-regulatory elements in the upstream regions of the triacylglycerol (TAG) synthesis genes. Methyl jasmonate, abscisic acid, and salicylic acid were further studied in response surface experiments, through which a 141% increase in TAG production was attained for 10-L cultures of P. tricornutum grown under optimal conditions. This study suggests that some phytohormones can promote P. tricornutum lipid accumulation without hindering growth. It also provides another strategy for improving the production of microalgae for use as biodiesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are the optimal raw material for biodiesel, but are far from being widely used for industrial applications (Liu et al. 2017a, b). To date, there have been many studies and methods on promoting microalgae growth rate (Mohan et al. 2015) and increasing triacylglycerol (TAG) accumulation under environmental stresses (Ge et al. 2014; Peng et al. 2014; Barka et al. 2016). Yet it is difficult to simultaneously obtain rapid growth rates and high lipid production. Therefore, a new method for sustainable TAG production in microalgae has become the focus of attention.
The oleaginous Phaeodactylum tricornutum with more than 20% lipid of the dry weight (DW) is a model diatom for studying the lipid synthesis mechanism. The genome of P. tricornutum has been reported as having about 27.4 Mbp (Bowler et al. 2008). Many researchers have characterized the TAG synthesis mechanism (Yang et al. 2013; Levitan et al. 2015) and key enzymes (Guihéneuf et al. 2011; Gong et al. 2013; Niu et al. 2013; Cui et al. 2013, 2018) in P. tricornutum. The accumulation of lipids in P. tricornutum is a consequence of intermediate metabolism remodeling, especially reactions in the tricarboxylic acid and the urea cycles (Levitan et al. 2015). As with other algae, for example, Chlorella sorokiniana (Hunt et al. 2010), the synthesis and accumulation of TAG in P. tricornutum are always affected by environmental factors, especially nitrate deprivation, and biomass always declines under conditions of stress (Peng et al. 2014; Yang et al. 2014; Yu et al. 2016). Thus, the resulting two-step culture of P. tricornutum, which requires a growth phase and TAG accumulation phase, hinders its use for industrial applications because of high production costs and long culture cycles.
Several phytohormones such as salicylate and gibberellic acid have been found to affect microalgal growth and metabolism (Xu et al. 2013; Bajguz and Piotrowska-Niczyporuk 2014; Kim et al. 2016; Le Henry et al. 2017; Lin et al. 2018; Parsaeimehr et al. 2017). Naphthylacetic acid and gibberellin could promote Arthrospira platensis and Arthrospira maxima growth, boosting the growth rate of the normal culture to 150% while increasing the amount of metabolic products such as the total extracellular sugar and total intracellular protein (Chen et al. 2009). Lu et al. (2010) found that jasmonic acid methyl ester and gibberellin promoted the synthesis of the microalga Haematococcus pluvialis and accumulation of astaxanthin by regulating the key enzyme BKT in the astaxanthin synthesis pathway. Naphthylacetic acid was reported to improve the fatty acid composition of the microalga Chlorella pyrenoidosa (Liu et al. 2017a, b). Recently, Xu et al. (2017) found that 40 μM salicylic acid could stimulate TAG accumulation in P. tricornutum at the stationary phase.
Based on previous researches, it can be hypothesized that phytohormones may simultaneously increase the growth rate and TAG production of P. tricornutum and, as a result, save on both the time and costs involved in its production for biodiesel. Thus, single-factor and Box-Behnken tests were conducted to detect the influence of single phytohormones on P. tricornutum and the optimal proportions needed for the highest lipid yield. Furthermore, the expression patterns of key enzymes in the lipid synthesis pathway were analyzed to investigate the mechanism by which the phytohormones influence P. tricornutum.
Materials and methods
Culture of P. tricornutum
The P. tricornutum strain was kindly donated by Prof. Mingyan Yin of the Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences. P. tricornutum was cultured in f/2 medium (Guillard and Ryther 1962) at 23 ± 2 °C and illuminated with cool-white fluorescent light at 35 μmol photons m−2 s−1 on a 12-h:12-h light:dark cycle. The artificial seawater for the f/2 was made using Reef Salt™ (Seachem Laboratories, USA) diluted in distilled water to a concentration of 34 g L−1, and the pH level was adjusted to 7.2 with 10% hydrochloric acid.
Single-factor experiments with phytohormones
The phytohormones methyl jasmonate, salicylic acid, gibberellin, abscisic acid, and ethephon (Sigma, USA) were dissolved in ethyl alcohol before being added separately to P. tricornutum cultures at the logarithmic phase of growth. The algae were then cultured under the same conditions described above for 6 days. The final concentration gradients of methyl jasmonate, salicylic acid, and abscisic acid in the 200-mL P. tricornutum cultures were 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1. The concentrations of gibberellin were 0, 0.15, 1.50, 2.00, and 20.0 mg L−1, while those of ethephon were 0, 0.06, 0.60, 2.00, and 20.0 mg L−1. Absorption was measured at 730 nm every 24 h.
A Water-PAM fluorometer (Walz GmbH, Germany) was used to monitor Fv/Fm in P. tricornutum every 72 h. Phaeodactylum tricornutum cultures were first dark adapted for 5 min, then exposed to a saturating pulse (0.8 s; 5640 μmol photons m−2 s−1) and a set of actinic irradiances at 322 μmol photons m−2 s−1 for 0.8 s every 20 s over a 5-min interval. There was a 40-s delay between the saturating pulse and the actinic light. The experiments were performed in triplicate.
Box-Behnken experiment and response surface methodology
The Box-Behnken test design in the MyDesign-Design-Expert 8.0.6 software (www.statease.com/soft_ftp.html) was used to find the optimal amounts of additives, with the TAG content as the response value (Kansedo and Keatteong 2013). Based on the results of the single-factor experiments, methyl jasmonate at concentrations of 10 to 14 mg L−1 and salicylic acid and abscisic acid at ranges of 2 to 6 mg L−1 were studied further. Ten-liter P. tricornutum cultures were exposed to the phytohormones at these concentrations for 6 days, and the results were analyzed using analysis of variance (ANOVA). The growing conditions for the 10-L cultures were as described above with continuous aeration at 1.3 m3 min−1.
Lipid extraction and analysis
The P. tricornutum cultures from the single-factor experiments and the cultures exposed to multiple phytohormones were harvested after 6 days by centrifugation at 1500×g at 18 °C and then freeze-dried for 48 h. The resulting powders were broken down and digested with 4 mol L−1 hydrochloric acid at room temperature for 1 h, and the total lipids were extracted from the powder using the chloroform-methanol method (Yoon et al. 2012). The weight and concentration of the total lipids were measured with an electronic balance. The TAG was separated by thin-layer chromatography (silica gel plate, HSGF254, Yellow Sea, China) with a mixture of normal hexane/diethyl ether/acetic acid (70:30:1 by volume) as the mobile phase. The lipid and TAG quantification was performed by gas chromatography-mass spectrometry (GC-MS), as previously described (Yoon et al. 2012).
Expression pattern of TAG synthesis enzymes in P. tricornutum
To investigate the influences of the three phytohormones methyl jasmonate, salicylic acid, and abscisic acid on the lipid synthesis pathway, the expression patterns of the key enzymes in P. tricornutum for TAG synthesis were examined by quantitative PCR (Q-PCR). The key enzymes included acetyl-CoA carboxylase (ACC, NC_011686.1 and NC_011698.1), long-chain acyl-coenzyme A synthetases (LACS, KF359938.1, KF359939.1, KF359940.1, KF359941.1, and KF359942.1), lysophosphatidic acid acyltransferase (LPAAT, JQ837824.1), and diacylglycerol acyltransferase (DGAT, HQ589265.1, JQ837823.1, JX469837.1, XP_002184474.1). The Q-PCR primers for these enzymes are listed in Table 1. The cis-regulatory elements in the 5′ upstream region of the key enzymes’ genes were analyzed through the PLANTCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Phaeodactylum tricornutum induced by the three phytohormones for 0, 3, and 6 days were harvested and frozen in liquid N2. RNA was isolated from algal samples using RNAiso extraction reagent (TAKARA, China) according to the manufacturer’s instructions. cDNA synthesis and relative quantitative real-time PCR were performed as described by Cui et al. (2018) using the Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, USA) with SYBR Green PCR Master Mix (TAKARA, China). The mRNA expression level was normalized using the 18S cDNA gene as the internal control.
Statistical analysis
All experiments were performed using biological triplicates to ensure reproducibility. Values are presented as means ± SD. Statistical analyses were performed using the SPSS statistical package (SPSS Inc., USA). Paired sample t tests were applied. Differences were considered statistically significant when p values < 0.05.
Results
Effects of phytohormones on growth and TAG accumulation in P. tricornutum
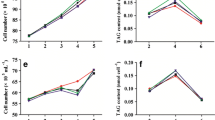
In the single-factor experiments, methyl jasmonate, salicylic acid, and abscisic acid were found to stimulate the growth rate of P. tricornutum to 0.7 to 0.9 mg L−1 day−1 at several concentrations. These include methyl jasmonate at 10 and 14 mg L−1; salicylic acid at 2, 6, and 30 mg L−1; and abscisic acid at 2 mg L−1. Meanwhile, gibberellin and ethephon did not promote the growth of P. tricornutum at any of the tested concentrations (Fig. 1).
Growth curves of P. tricornutum cultures containing the phytohormones. aP. tricornutum containing methyl jasmonate. The numbers in the figure are the concentrations of methyl jasmonate: 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1. bP. tricornutum containing salicylic acid. The numbers in the figure are the concentrations of salicylic acid: 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1. cP. tricornutum containing abscisic acid. The numbers in the figure are the concentrations of abscisic acid: 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1. dP. tricornutum containing gibberellin with. The numbers in the figure are the concentrations of gibberellin: 0, 0.15, 1.50, 2.00, and 20.0 mg L−1. eP. tricornutum containing ethephon. The numbers in the figure mean the concentrations of ethephon: 0, 0.06, 0.60, 2.00, and 20.0 mg L−1. (Data are mean ± SD, n = 3)
The DW, TAG productivity, and TAG content (TAG/DW%) of P. tricornutum following 6 days of induction with the phytohormones are shown in Table 2. The TAG productivity of P. tricornutum cultures induced by methyl jasmonate, salicylic acid, and abscisic acid at some concentrations was higher than that of the control. For methyl jasmonate, the highest biomass and TAG productivity of P. tricornutum were achieved at 10 mg L−1, which is 1.51-fold the control value. Meanwhile, for abscisic acid, the highest TAG production was 1.97-fold of the control at 10 mg L−1. In contrast, gibberellin and ethephon caused the TAG productivity of P. tricornutum to decline by a large margin.
The TAG content in P. tricornutum induced by these phytohormones in most tested concentrations—except 10 mg L−1 methyl jasmonate, 0.15 and 1.5 mg L−1 gibberellin, and 0.06 mg L−1 ethephon—was lower than that of the control. This suggests that salicylic acid and abscisic acid could promote the growth of P. tricornutum to achieve high TAG production, but not strengthen TAG synthesis. The phytohormones gibberellin and ethephon may promote TAG synthesis at several concentrations, but inhibit growth at the same time.
The Fv/Fm of P. tricornutum showed a slight decline during the late logarithmic phase of growth (Fig. 2). Methyl jasmonate, salicylic acid, and abscisic acid did not influence photosynthetic activity (p > 0.05, two-way repeated measures ANOVA), while gibberellin and ethephon limited photosynthetic activity after 3 days of induction.
The Fv/Fm of P. tricornutum cultures with phytohormones. a Cultures with methyl jasmonate. The 8 different concentrations of methyl jasmonate were 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1. b Cultures with salicylic acid. The 8 different concentrations of salicylic acid were 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1. c Cultures with abscisic acid. The 8 different concentrations of abscisic acid were 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1. d Cultures with gibberellin. The 5 different concentrations of gibberellin were 0, 0.15, 1.50, 2.00, and 20.0 mg L−1. e Cultures with ethephon. The 5 different concentrations of ethephon were 0, 0.15, 1.50, 2.00, and 20.0 mg·L−1. (Data are mean ± SD, n = 3)
Based on these results, the phytohormones methyl jasmonate, salicylic acid, and abscisic acid could improve the TAG production as well as the growth of P. tricornutum; thus, they were further analyzed for optimization. Gibberellin and ethephon did not increase growth or TAG production, and thus were not evaluated further.
Effects of methyl jasmonate, salicylic acid, and abscisic acid on the TAG synthesis pathway
The TAG content (TAG product per 1 mg DW) in P. tricornutum induced by methyl jasmonate, salicylic acid, and abscisic acid did not rise obviously or even declined in most cases as listed in Table 2, which may indicate the TAG synthesis pathway in P. tricornutum was not strengthened by these phytohormones. To survey the influences of methyl jasmonate, salicylic acid, and abscisic acid on the TAG synthetic pathway, the key enzymes related to ACC1, ACC2, LAPPT, and so on were further monitored by Q-PCR every 72 h (Fig. S1). The R2 values for all the primer pairs were all higher than 0.90. The results showed that these key enzymes’ expression were related to the phytohormone concentrations and the inducing time (Figs. 3, 4, and 5), and were suppressed in most sets except 6-day inducing of 10 mg L−1 methyl jasmonate (Fig. 3).
The expression pattern of the key enzyme genes in TAG synthetic pathway in P. tricornutum with methyl jasmonate induction. The P. tricornutum cultures were conducted with Q-PCR for every 3 days and each sample has three biological replicates, while each biological triplicate sample has three analytical triplicates in Q-PCR. The numbers on the X-axis are the concentrations of methyl jasmonate: 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1
The expression pattern of the key enzyme genes in TAG synthetic pathway in P. tricornutum with salicylic acid induction. The P. tricornutum cultures were conducted with Q-PCR for every 3 days and each sample has three biological replicates, while each biological triplicate sample has three analytical triplicates in Q-PCR. The numbers on the X-axis are the concentrations of salicylic acid: 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1
The expression pattern of the key enzyme genes in TAG synthetic pathway in P. tricornutum with abscisic acid induction. The P. tricornutum cultures were conducted with Q-PCR for every 3 days and each sample has three biological replicates, while each biological triplicate sample has three analytical triplicates in Q-PCR. The numbers on the X-axis ae the concentrations of abscisic acid: 0, 1, 2, 6, 10, 14, 18, and 30 mg L−1
The cis-regulatory elements responsive to the three phytohormones in these gene upstream regions were also analyzed and are listed in Table 3. The methyl jasmonate-responsive elements (CGTCA-motif and TGACG-motif) were found in the upstream regions of most genes except DGAT1, while the abscisic acid- and salicylic acid-responsive elements were present in just some of the gene upstream regions. Considering the Q-PCR results (Fig. 3) and the TAG content measurements (Table 2), methyl jasmonate appears to influence TAG content through the expression of the key enzymes in the TAG synthesis pathway. Its most effective concentration range for increasing TAG synthesis in P. tricornutum was 10 to 14 mg L−1 for 6 days. Salicylic acid and abscisic acid suppressed genes’ expression at all the concentrations, including DGAT2A which contained the regulatory elements responsive to the phytohormones, and the TAG content of P. tricornutum induced by the two phytohormones decreased. Based on these results, salicylic acid and abscisic acid were predicted to increase TAG production through biomass but not the TAG content.
Combination of methyl jasmonate, abscisic acid, and salicylic acid for inducing TAG accumulation in P. tricornutum
Based on the single-factor experiments, the effects of methyl jasmonate, abscisic acid, and salicylic acid were further examined using the response surface test. Ten-liter cultures of P. tricornutum with three phytohormones inducing were established according to the design of the software, and the resulting TAG products (Table 4) were used as the response values to find the optimal conditions for growth. These results were then analyzed using ANOVA. The resulting parameters calculated based on TAG yield as the response are presented in Table 5 and the response surface model was established with p < 0.05. The combination of methyl jasmonate and salicylic acid (group AB in Table 5) and of methyl jasmonate and abscisic acid (group AC in Table 5) may increase TAG production in P. tricornutum cultures, much more than just one of these phytohormones will do (Table 5). And salicylic acid (group C in Table 5) may be more effective to increase TAG production in P. tricornutum than the other two phytohormones (group A and group B) during inter-group analysis. Finally, according to the model, 6.83 mg L−1 day−1 TAG product, which was 2.41-fold the control value, was obtained from 10-L cultures of P. tricornutum with 14 mg L−1 methyl jasmonate, 6 mg L−1 abscisic acid, and 2 mg L−1 salicylic acid. The combined influence of the three phytohormones was stronger than just one of them for increasing TAG accumulation in P. tricornutum.
Discussion
In the photoautotrophic culture of P. tricornutum, some environmental conditions such as nitrate deprivation and high light could increase TAG content 2- to 3-fold (Peng et al. 2014; Yang et al. 2014; Yu et al. 2016). But these environmental conditions, especially nitrate deprivation, inhibit algal growth when P. tricornutum is exposed to a nitrate-deprived medium at the logarithmic growth phase. In this study, the effects of five commonly used phytohormones—methyl jasmonate, salicylic acid, gibberellin, abscisic acid, and ethephon—on TAG accumulation in this diatom were investigated. All of these five phytohormones play important roles in the growth and development of the higher plants (Hara et al. 2012; Vankova 2012; Muhammad et al. 2013; Ozturk et al. 2018; Thongkum et al. 2018). They also participate in the signaling of biotic and abiotic stress responses in the higher plants (Hara et al. 2012; Vankova 2012; Jiang et al. 2018). Salicylic acid could increase the heat stress tolerance of bread wheat seeds (Kousar et al. 2018) and hinder the biotrophic pathogen, for example, Pseudomonas syringae (Vlot et al. 2009). The phytohormones were also used to increase the secondary metabolites in higher plants and algae. The methyl jasmonate promoted astaxanthin synthesis in H. pluvialis (Lu et al. 2010) and TAG synthesis in P. tricornutum (Table 2).
In this study, salicylic acid increased TAG productivity in P. tricornutum by stimulating algal growth but not the TAG content per DW, but interestingly, it was found to stimulate TAG synthesis of P. tricornutum at the stationary phase (Xu et al. 2017). The effects of salicylic acid application depend on numerous factors such as the species and the developmental stage of the plant (Hara et al. 2012). The extent of salicylic acid’s various influences on P. tricornutum may be related to the growth phases: the stationary phase in the research by Xu et al. (2017) and the logarithmic phase in the present study.
Fv/Fm is the maximum photochemical efficiency of open reaction centers in photosystem II, and has been used as a character for reflecting the PSII efficiency of a light-dependent process. Environmental factors and endogenous diel patterns can both impact the Fv/Fm value (Cosgrove and Borowitzka 2011). In the present study, environmental factors such as light, temperature, and nutrient status were set as the parallel parameters during all the experiments, and the added phytohormones in the cultures were the only extracellular factor to influence Fv/Fm. Gibberellin and ethephon limited algae growth and decreased Fv/Fm, whereas methyl jasmonate, salicylic acid, and abscisic acid did not influence Fv/Fm though they stimulated algal growth and promoted TAG synthesis (Table 2 and Figs. 2, 3, 4, and 5) in P. tricornutum. These results may be related to the cellular metabolisms influenced by the respective phytohormones and further studies including the enzymatic reaction of PSII, the transcriptomes, and metabolome analysis will be needed.
The relative elements responsive to these phytohormones were found in the 5′ upstream region of the TAG synthesis-related genes in this study. The gene expression patterns in P. tricornutum induced by phytohormones were consistent with the TAG content changes compared with those of the control. Methyl jasmonate promoted TAG synthesis and upregulated the genes related to TAG synthesis, while salicylic acid and abscisic acid declined TAG synthesis and downregulated these genes. Methyl jasmonate at the concentration of 10 mg L−1 could promote the TAG synthesis-related gene expression (such as ACC1, LACS1, LACS2, LACS5, LAPPT, DGAT2A in Fig. 3) and increase the TAG content to 3.92% compared with 3.85% TAG content in the control. Meanwhile, salicylic acid and abscisic acid downregulated the TAG synthesis-related gene expression (ACC1, ACC21, LACS1, LACS2, DGAT2A, DGAT2D,WS/DGAT) at the concentration of 2 mg L−1 (Figs. 4 and 5) and decline the TAG content to 3.58 and 3.84% respectively (Table 2). TAG synthesis and accumulation in P. tricornutum are closely related to endogenous intermediate metabolism (Levitan et al. 2015) and are influenced by many factors such as light and nutrients. The phytohormones methyl jasmonate, salicylic acid, and abscisic acid may act as signals for regulating internal mechanisms such as the tricarboxylic acid cycle and starch synthesis, but not directly induce TAG synthesis. The mechanisms of the phytohormones influencing TAG synthesis and accumulation will be explored in future studies.
The three phytohormones—methyl jasmonate, salicylic acid, and abscisic acid—were found to increase TAG production in P. tricornutum, while salicylic acid and abscisic acid just stimulated the growth rate. The combination of these three phytohormones could increase TAG productivity to 6.00 mg L−1 day−1, which is higher than that of P. tricornutum with N deprivation (5.40 mg L−1 day−1; Yang et al. 2013; Cui et al. 2018). The TAG content of P. tricornutum induced by phytohormones was 1.7-fold than that of the control, indicating that TAG accumulation is strengthened by the combination of the three phytohormones. These results suggest that phytohormone induction is a promising method for improving TAG accumulation in P. tricornutum for industrial use.
References
Bajguz A, Piotrowska-Niczyporuk A (2014) Interactive effect of brassinosteroids and cytokinins on growth, chlorophyll, monosaccharide and protein content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem 80:176–183
Barka F, Angstenberger M, Ahrendt T, Lorenzen W, Bode HB, Büchel C (2016) Identification of a triacylglycerol lipase in the diatom Phaeodactylum tricornutum. Biochim Biophys Acta 1861:239–248
Bowler C, Allen AE, Badger JH, Grimwood J, Jabbari K, Kuo A, Maheswari U, Martens C, Maumus F, Otillar RP, Rayko E, Salamov A, Vandepoele K, Beszteri B, Gruber A, Heijde M, Katinka M, Mock T, Valentin K, Verret F, Berges JA, Brownlee C, Cadoret JP, Chiovitti A, Choi CJ, Coesel S, de Martino A, Detter JC, Durkin C, Falciatore A, Fournet J, Haruta M, Huysman MJJ, Jenkins BD, Jiroutova K, Jorgensen RE, Joubert Y, Kaplan A, Kröger N, Kroth PG, la Roche J, Lindquist E, Lommer M, Martin–Jézéquel V, Lopez PJ, Lucas S, Mangogna M, McGinnis K, Medlin LK, Montsant A, Secq MPO–L, Napoli C, Obornik M, Parker MS, Petit JL, Porcel BM, Poulsen N, Robison M, Rychlewski L, Rynearson TA, Schmutz J, Shapiro H, Siaut M, Stanley M, Sussman MR, Taylor AR, Vardi A, von Dassow P, Vyverman W, Willis A, Wyrwicz LS, Rokhsar DS, Weissenbach J, Armbrust EV, Green BR, van de Peer Y, Grigoriev IV (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456:239–244
Chen Y, Zhao P, Wang XQ, Pang GC (2009) Effect on biomass and the content of active components of Spirulina sp. by three phytohormones. Mar Sci 33:11–16 (In Chinese)
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 1–17
Cui YL, Zheng GT, Li XQ, Lin HZ, Jiang P, Qin S (2013) Cloning and characterization of a novel diacylglycerol acyltransferase from the diatom Phaeodactylum tricornutum. J Appl Phycol 25:1509–1512
Cui YL, Zhao JL, Wang YC, Qin S, Lu YD (2018) Characterization and engineering of a dual-function diacylglycerol acyltransferase in theoleaginous marine diatom Phaeodactylum tricornutum. Biotechnol Biofuels 11:32
Ge F, Huang W, Chen Z, Zhang C, Xiong Q, Bowler C (2014) Methylcrotonyl-coA carboxylase regulates triacylglycerol accumulation in the model diatom Phaeodactylum tricornutum. Plant Cell 26:1681–1697
Gong YM, Zhang J, Guo X, Wan X, Liang Z, Hu CJ, Jiang M (2013) Identification and characterization of PtDGAT2B, an acyltransferase of the DGAT2 acyl-coenzyme A: diacylglycerol acyltransferase family in the diatom Phaeodactylum tricornutum. FEBS Lett 587:481–487
Guihéneuf F, Leu S, Zarka A, Khozin-Goldberg I, Khalilov I, Boussiba S (2011) Cloning and molecular characterization of a novel acyl-CoA: diacylglycerol acyltransferase 1-like gene (PtDGAT1) from the diatom Phaeodactylum tricornutum. FEBS J 278:3651–3666
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol 8:229–239
Hara M, Furukawa J, Sato A (2012) Abiotic stress and role of salicylic acid in plants. In: Ahamd P (ed) Abiotic stress response in plants. Spinger, New York, pp 235–251
Hunt RW, Chinnasamy S, Bhatnagar A, Das KC (2010) Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana. Appl Biochem Biotechnol 162:2400–2414
Jiang XJ, Lin HT, Lin MS, Chen YH, Wang H, Lin YX, Shi J, Lin YF (2018) A novel chitosan formulation treatment induces disease resistance of harvested litchi fruit to Peronophythora litchii in association with ROS metabolism. Food Chem 266:299–308
Kansedo J, Keatteong L (2013) Process optimization and kinetic study for biodiesel production from non-edible sea mango (Cerbera odollam) oil using response surface methodology. Chem Eng J 214:157–164
Kim S-H, Lim SR, Hong S-J, Cho B-K, Lee H, Lee C-G, Choi H-K (2016) Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J Agric Food Chem 64:4807–4816
Kousar R, Qureshi R, Jalal-Ud-Din MM, Shabbir G (2018) Salicylic acid mediated heat stress tolerance in selected bread wheat genotypes of Pakistan. Pak J Bot 50:2141–2146
Le Henry M, Charton M, Alignan M, Maury P, Luniov A, Pelletier I, Pontalier P-Y, Binder BM, Vaca-Garcia C, Chervin C (2017) Ethylene stimulates growth and affects fatty acid content of Synechocystis sp. PCC 6803. Algal Res 26:234–239
Levitan O, Dinamarca J, Zelzion E, Lun DS, Guerra LT, Kim MK, Kim J, Van Mooy BA, Bhattacharya D, Falkowski PG (2015) Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutum under nitrogen stress. Proc Nat Acad Sci 112:412–417
Lin B, Ahmed F, Du H, Li Z, Yan Y, Huang Y, Cui M, Yin Y, Li B, Wang M, Meng C, Gao Z (2018) Plant growth regulators promote lipid and carotenoid accumulation in Chlorella vulgaris. J Appl Phycol 30:1549–1561
Liu JY, Song Y, Qiu W (2017a) Oleaginous microalgae Nannochloropsis as a new model for biofuel production: review & analysis. Renew Sust Energ Rev 72:154–162
Liu F, Wang C, Wang ZY, Liu TT, Li YQ (2017b) Effect of induction with phytohormones analogs on biomass and lipid accumulation in Chlorella vulgaris cells. Chin J Biol 30:390–394 (In Chinese)
Lu YD, Jiang P, Liu SF, Gan QH, Cui HL, Qin S (2010) Methyl jasmonate- or gibberellins A3-induced astaxanthin accumulation is associated with up-regulation of transcription of b-carotene ketolase genes (bkts) in microalga Haematococcus pluvialis. Bioresour Technol 101:6468–6474
Mohan SV, Rohit MV, Chiranjeevi P, Chandra R, Navaneeth B (2015) Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: progress and perspectives. Bioresour Technol 184:169–178
Muhammad S, Zora S, Ahmad SK (2013) Time of methyl jasmonate application influences the development of ‘Cripps Pink’ apple fruit color. J Sci Food Agr 93:611–618
Niu YF, Zhang M, Li D, Yang W, Liu J, Bai W, Li HY (2013) Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum. Mar Drugs 11:4558–4569
Ozturk B, Bektas E, Aglar E, Karakaya O, Gun S (2018) Cracking and quality attributes of jujube fruits as affected by covering and pre-harvest Parka and GA(3) treatments. Sci Hortic 240:65–71
Parsaeimehr A, Mancera-Andrade EI, Robledo-Padilla F, Iqbal HMN, Parra-Saldivar R (2017) A chemical approach to manipulate the algal growth, lipid content and high-value alpha-linolenic acid for biodiesel production. Algal Res 26:312–322
Peng X, Liu S, Zhang W, Zhao Y, Chen L, Wang H (2014) Triacylglycerol accumulation of Phaeodactylum tricornutum with different supply of inorganic carbon. J Appl Phycol 26:131–139
Thongkum M, McAtee PM, Schaffer RJ, Allan AC, Ketsa S (2018) Characterization and differential expression of ethylene receptor genes during fruit development and dehiscence of durian (Durio zibethinus). Sci Hortic 240:623–630
Vankova R (2012) Abscisic acid signaling in plants. In: Ahamd P (ed) Abiotic stress response in plants. Springer, New York, pp 235–251
Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Xu F, Chen X, Yang Z, Jin P, Wang K, Shang H, Wang X, Zheng Y (2013) Maintaining quality and bioactive compounds of broccoli by combined treatment with 1-methylcyclopropene and 6-benzylaminopurine. J Sci Food Agric 93:1156–1161
Xu J, Fan X, Li X, Liu G, Zhang Z, Zhu Y, Fu Z, Qian H (2017) Effect of salicylic acid on fatty acid accumulation in Phaeodactylum tricornutum during stationary growth phase. J Appl Phycol 29:2801–2810
Yang ZK, Niu YF, Ma YH, Xue J, Zhang MH, Yang WD, Liu JS, Lu SH, Guan LF, Li HY (2013) Molecular and cellular mechanisms of neutral lipid accumulation in diatom following nitrogen deprivation. Biotechnol Biofuels 6:67
Yang ZK, Ma YH, Zheng JW, Yang WD, Liu JS, Li HY (2014) Proteomics to reveal metabolic network shifts towards lipid accumulation following nitrogen deprivation in the diatom Phaeodactylum tricornutum. J Appl Phycol 26:73–82
Yoon K, Han D, Li Y, Sommerfeld M, Hu Q (2012) Phospholipid:diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell 24:3708–3724
Yu SJ, Shen XF, Ge HQ (2016) Role of sufficient phosphorus in biodiesel production from diatom Phaeodactylum tricornutum. Appl Microbiol Biotechnol 100:6927–6934
Acknowledgements
We would like to acknowledge with apologies many excellent studies that could not be cited due to space limitations. We are grateful to the anonymous reviewers for their valuable improvement to this manuscript. We thank Natalie Kim, PhD, from Liwen Bianji, Edanz Editing, China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2016YFB0601001), the Science and Technology Service Network Initiative of the Chinese Academy of Sciences (KFJ-STS-ZDTP-023), the Project of Innovation & Development of Marine Economy (HHCL201803), the seed project of Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences(YIC755031013).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(PNG 4542 kb)
Rights and permissions
About this article
Cite this article
Chu, J., Li, Y., Cui, Y. et al. The influences of phytohormones on triacylglycerol accumulation in an oleaginous marine diatom Phaeodactylum tricornutum. J Appl Phycol 31, 1009–1019 (2019). https://doi.org/10.1007/s10811-018-1623-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1623-y