Abstract
Microalgae are photosynthetic organisms with the ability to produce a variety of high-value compounds such as polyunsaturated fatty acids (PUFAs), proteins, pigments, and lipids. The high cost of microalgae production is one of the biggest obstacles for their commercialization. Plant growth regulators might be an ideal choice since they could potentially induce microalgae to produce lipids and other high-value secondary metabolites thereby reducing production cost. This study investigated the effects of eight plant growth regulators (PGRs), namely, salicylic acid (SA); 1-naphthaleneacetic acid (NAA); gibberellin (GA3); 6-benzylaminopurine (6-BA); 2,4-epibrassinolide (EBR); abscisic acid (ABA); ethephon (ETH); and spermidine (SPD) on the induction of lipids, proteins, carotenoids, and unsaturated fatty acids (UFAs) in Chlorella vulgaris. Moreover, the expression profiles of seven fatty acid biosynthethis genes were studied in the PGR-treated biomass. All PGRs used in the study caused significant increases in total lipid contents in non-dose-dependent manners when compared to control. However, lipid productivities were increased due to four of the eight PGRs (ABA, 6-BA, NAA, and ETH). Similar to lipids, total carotenoid contents were significantly higher in all of the PGR-treated microalgal biomass except ABA. However, soluble protein contents were not affected by the PGR treatments except SA at 10 mg L−1. Furthermore, 6-BA, NAA, ABA, and ETH treatments resulted in significant increases in UFAs especially DHA, linolenic acid, arachidonic acid, and EPA which were confirmed by the upregulation of fatty acid biosynthesis genes including stearoyl-ACP-desaturase, ω-3 fatty acid desaturase, biotin carboxylase, and acyl-acyl carrier protein. Our findings, therefore, indicate that the treatment with PGR used in this study could be a useful tool to produce biodiesel and other high-value metabolites from microalgal biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are a diverse group of eukaryotic microorganisms that have been exploited for the production of high-value compounds such as lipids (including omega-3 fatty acids), enzymes, polymers, toxins, antioxidants, and pigments (carotenoids) which can be used directly for numerous industrial purposes including food, feed, nutrition, cosmetics, and pharmaceuticals (Borowitzka 2013). Moreover, microalgal biomass can be a source of different types of bioenergy components including biodiesel, bioethanol, methane, alkanes, and hydrogen (Rosenberg et al. 2008). The increasing demand for an alternative source of renewable energy to replace fossil fuels has attracted considerable interest among scientists and policy makers in microalgae. However, the high cost of producing algal oil has led to the concept of “microalga biorefinery” where high-value compounds could be produced as well as biofuels to make the business economically viable (Ahmed et al. 2013).
Due to high metabolic plasticity, microalgae can be triggered to produce targeted compounds through manipulation of culture conditions or genetic engineering approaches (Catarina et al. 2011). Cultivation conditions play key roles for the ultimate use of microalgal biomass in downstream processing for the production of biodiesel and high-value products including carotenoids, proteins, and polyunsaturated fatty acids (PUFAs). Several factors, such as nitrogen deficiency, temperature, salinity, and level of irradiance, have been reported to affect the lipid contents of microalgae (Converti et al. 2009; Cha et al. 2011; Kamalanathan et al. 2016; Negi et al. 2016; Lari et al. 2016). Lei et al. (2012) reported that high-value omega-3 fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) was increased by 4.40 and 48% (dry weight of cell), respectively, through nitrogen starvation and Fe treatment in Haematococcus pluvialis.
Several plant growth regulators (PGRs), such as salicylic acid (SA), 1-naphthaleneacetic acid (NAA), gibberellin A3 (GA3), and jasmonic acid (JA), had already been investigated for their effects on the growth of microalgae in the last decade (Hunt et al. 2010; Piotrowska-Niczyporuk et al. 2012; Piotrowska-Niczyporuk and Bajguz 2014; Gao et al. 2016). The accumulation of lipids, carotenoids, monosaccharides, proteins, and chlorophyll in response to the treatment with these PGRs was also investigated in Chlorella vulgaris and H. pluvialis (Czerpak et al. 2002; Falkowska et al. 2011; Raman and Ravi 2011; Gao et al. 2013a, b). PGRs including gibberellic acids (e.g., GA3), abscisic acid (ABA), brassinosteroids (BRs), SA, JA, ethylene (ETH), auxins (e.g., NAA), cytokinins (e.g., benzylaminopurine, 6-BA), and polyamines (e.g., spermidine, SPD) are well-documented as contributing to plant growth and development. Some of the described functionalities of these PGRs include promotion of cell division, induction of bud formation, promotion of growth and inhibition of chlorophyll, and nucleic acid and protein degradation, and some PGR can maintain higher antioxidant enzyme activities and plant adaptation; accelerate ripening, senescence, and abscission; stimulate xylem differentiation and cell elongation in the hypocotyls; promote transcription of DNA and RNA in plant cells and combine proteins or phospholipids in the membrane; maintain membrane stability; and induce plant disease resistance, drought resistance, salt resistance, temperature resistance, and UV resistance (Imin et al. 2005; Hunt et al. 2010; Xu et al. 2013; Hu and Yu 2014; Liu et al. 2017a, b). However, the mechanism of action and the metabolism of these PGR in the cells remain to be fully explored (Raman and Ravi 2011).
In addition to promoting accumulation of metabolites, auxins (NAA), cytokinins (6-BA), and gibberellin (GA3) have been reported to alleviate stress symptoms by inhibiting heavy metal biosorption, restoring algal growth and primary metabolite levels (Piotrowska-Niczyporuk et al. 2012). Bajguz (2000) found that 2,4-epibrassinolide (EBR) at the concentration of 10−8 M in combination with heavy metals (in the range 10−6~10−4 M) blocked metal accumulation in algal cells. So PGR can enhance the resistance of microalgae to heavy metals, reduce the accumulation of metals in microalgal cells, and improve the quality of microalgal industrial products.
Recently, the effect of these PGRs on lipid and other metabolite accumulation in microalgae is gaining attention (Bajguz and Piotrowska-Niczyporuk 2013; Salama et al. 2014; Josephine et al. 2015; Jusoh et al. 2015a, b; Lu and Xu 2015; Liu et al. 2016; Babu et al. 2017). Haematococcus pluvialis had been investigated for the expression of several genes related to lipid biosynthesis under SA and JA treatments, and these PGRs significantly affected the expression of genes (Gao et al. 2013a, b, 2016). Chlorella vulgaris is regarded as a potential candidate for microalga biodiesel production since it can accumulate high levels of lipids (Hu et al. 2008; Bajguz and Piotrowska 2009). Chlorella has also been used as a tool for studying the influences of PGR on growth and metabolite accumulation (Czerpak et al. 2006; Hu et al. 2008). Through this research, we can know the roles of PGR on the growth and metabolism of C. vulgaris which could provide a new idea for regulating the metabolism of C. vulgaris.
In this study, we investigated the effects of different concentrations of eight PGRs on the accumulation of lipids, PUFA, proteins, and carotenoids in the commercially important microalga C. vulgaris. We also studied the transcriptional expressions of fatty acid (FA) synthesis genes in order to understand the molecular mechanism of lipid bioaccumulation in C. vulgaris in response to the PGR treatments through real-time qPCR.
Materials and methods
Chemicals
All chemicals used were of analytical grade except hexane which was of chromatographic purity.
Culture of Chlorella vulgaris strain
Chlorella vulgaris FACHB-9 was from the Institute of Hydrobiology, Chinese Academy of Sciences; obtained from the Microalgae Biotechnology lab of the Shandong University of Technology, China; and maintained in BG11 growth medium at 22 ± 2 °C. The algal culture was initiated from a single colony taken from the stock agar plate and cultured in revised BG11 medium. The revised BG11 medium components are as follows (per liter): NaNO3 75 g, K2HPO4 2 g, MgSO4·7H2O 3.75 g, Na2CO3 1 g, citric acid 0.30 g, CaCl2·2H2O 1.80 g, ferric ammonium citrate 0.30 g, EDTANa2 50 mg, H3BO3 2.86 g, MnCl2·4H2O 1.86 g, ZnSO4·7H2O 0.22 g, CuSO4·5H2O 80 mg, Na2MoO4·2H2O 0.39 g, and Co(NO3)2·6H2O 50 mg. The final pH of the medium was adjusted to 7.10 with 1 M HCl. The culture medium and Erlenmeyer flasks were sterilized at 121 °C for 30 min. The microalga cultures were illuminated with cool-white fluorescent light under 36 μmol photons m−2 s−1 on a 12:12 h light/dark cycle at 24 ± 2 °C (Damiani et al. 2010). The cultures were shaken manually (approximately three to five times a day), and nutrients (two times the concentration of BG11) were added once a week.
Plant growth regulator treatments
During the exponential phase, the microalga cultures (separately grown cultures; n = 3) were treated with six different concentrations of EBR, GA3, ETH, SPD, 6-BA, NAA, SA, and ABA. All of the PGRs were dissolved in water, made into 1 g L−1 stock solutions, and stored at 4 °C. The concentrations used for treating the cultures are described in Table 1. Cultures grown in media only without PGRs were used as controls. Medium was added regularly (two times the concentration of BG11) to avoid any effect of nutrient starvation in the cultures. Biomass was collected after 15 days of cultivation and harvested by centrifugation at 7100×g for 10 min. The supernatants were discarded, and the pellets were dried at − 40 °C in a freeze-dryer and then stored at − 20 °C until use for various analyses.
Determination of total lipid content
Measurement of total lipid contents in the dried biomass was conducted following the method by Bligh and Dyer (1959) with minor modifications. In brief, 50-mg dry biomass was added to 5 mL chloroform-methanol (2:1) extraction solvent and placed in an ultrasonic crusher (VC605, Sonics, USA) in an ice bath (effective power of 240 W, cycle 2 min, work 15 s, interval 5 s). The mixture was centrifuged at 11100×g for 10 min at 4 °C, and then the supernatants were added to chloroform. An equal volume of water was added to the mixture and left for a few minutes to allow separation of organic layers. The clear layers on the top were dried at 61 °C. The total lipid contents were calculated using the following equation:
where C is the weight of the lipid layer (mg) and m is the biomass (g DW).
Lipid productivities of the hormone-treated and control cultures were calculated as follows:
where C is the weight of lipid layer (mg), V is the volume (L) of the culture medium used to culture the biomass, and D is the cultivation time (days).
Analysis of polyunsaturated fatty acids
Fatty acid methyl ester (FAME) analysis was conducted following the method described by Cha et al. (2011) with some modifications. Previously dried algae (20 mg) were used for FA analysis, and lipids present in the algal pellets were hydrolyzed and methyl-esterified by 3 mL 0.4 mol L−1 KOH/methanol solution for 1 h at 60 °C; 20 μL methyl nonadecanoate (Sigma, USA) was added as internal standard to the pellets prior to the reaction. A total of 1 mL saturated NaCl and 1 mL hexane were then added and mixed for 20 s. To separate the phase, anhydrous Na2SO4 was added to the samples and the mixture was then centrifuged at 16,000×g for 3 min. A total of 1 μL of the hexane layer was injected into a GC-2010 gas chromatograph (Shimadzu Corporation, Japan) fitted with a HP-FFAP capillary column (0.25 mm inner diameter × 30 cm length) and a flame ionization detector. Nitrogen was used as carrier gas at a constant flow rate of 30 mL min−1. Split injections at 1:18 were performed at 300 °C, and the oven temperatures applied were 120 °C for 1 min, then increased to 210 °C at 6 °C min−1, and held for 10 min. Identification of FAs was accomplished by comparing the peaks and retention times of the FAMEs to those of the reference standard Supelco 37 Component FAME Mix (Sigma Aldrich, China).
Measurement of crude protein content
Freeze-dried biomass (50-mg DW from separately grown cultures; n = 3) was dissolved in 20 mL 0.20 M PBS (8 g NaCl, 0.20 g KCl, 1.44 g Na2HPO4, and 0.24 g KH2PO4, dissolved in 900 mL double-distilled water; hydrochloric acid to adjust the pH value to 7.40; add water to the volume of 1 L; room temperature preservation) with ultrasonic fragmentation (VC605, Sonics, USA). The following conditions are used for ultrasonic disruption: effective power of 240 W, work 15 s, interval 5 s, cycle 2 min. Supernatants were harvested by centrifugation at 7100×g for 10 min and were used for measurement of protein contents. The protein contents in the hormone treatments and control were determined according to the Bradford method (Bradford 1976).
The protein contents were calculated using the following equation:
where C is the weight of protein (mg) and m is the biomass (g DW).
Measurement of total carotenoids
Microalgal biomass (50-mg DW) was transferred to 10 mL 90% acetone and kept in the dark at 4 °C for 24 h. Total carotenoids in the biomass were calculated following the method described by Lichtenthaler (1987).
RNA isolation and gene expression profiling
Fresh microalgal biomass was frozen in liquid nitrogen and then ground into a fine powder. The total RNA was subsequently extracted using Trizol reagent according to the manufacturer’s instructions and dissolved in diethypyrocarbonate-treated water. In this protocol, RNA was digested with DNaseI. The cDNA used for real-time qPCR was synthesized from total RNA using SuperScript III First-Strand Synthesis Kit (Invitrogen, USA).
Using gene sequences retrieved from NCBI database (HM560033, HM560034, HM560035, HM560036, and HM560037), primers were designed according to Lei et al. (2012) (Table 2). Primers were synthesized using Nanjing Genscript (China). Seven known FA synthesis genes, biotin carboxylase (BC), 3-ketoacyl carrier protein synthase gene (KAS), acyl-acyl carrier protein (ACP), acyl carrier protein thioesterase (FATA), ω-3 fatty acid desaturase (FAD), malonyl-CoA:ACP transacylase (MCTK), and stearoyl-ACP-desaturase (SAD), were investigated in this study (Fig. 1).
Fatty acid biosynthesis pathway in green microalgae (cited from Lei et al. 2012)
Real-time qPCR analysis was conducted on an ABI-7900HT System (Applied Biosystems, USA) using SYBR green fluorescence (Applied Biosystems), using actin gene as the internal control. Each PCR reaction consisted of 10 μL SYBR green, 0.5 μL dNTP, 1 μL Taq DNA polymerase, 7.5 μL diH2O, 1 μL of primer mixture (forward and reverse, 0.5 μL each), and 2 μL of cDNA (2 μg μL−1). The thermal cycles were set as follows: stage 1, 95 °C for 10 min; stage 2, 45 cycles of 95 °C for 15 s and 60 °C for 1 min; and stage 3, 1 cycle of 95 °C for 2 min, 60 °C for 15 s, and 95 °C for 15 s. The 2−△△CT method was used to analyze quantitative real-time qPCR data (Gao et al. 2012a, b).
Statistical analysis
All experiments were performed in triplicates. The effects of various PGR on the accumulation of lipids, FAMEs, proteins, and carotenoids and the expressions of FA biosynthesis-relevant genes in C. vulgaris strain were analyzed by one-way analysis of variance (ANOVA) and LSD multiple-comparison tests and were used to detect differences among the groups of different trials. p values of less than 0.05 were considered to be statistically significant. Results were analyzed by Origin 9.0 and SPSS 17.0, and data expressed as mean ± standard error (SE).
Results
Total lipid and lipid productivity
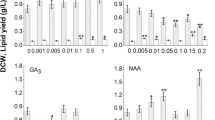
Higher lipid contents compared to controls (135.97 ± 22.58 mg g−1 DW) were found for all of the eight PGR treatments of C. vulgaris cells after 15 days of cultivation. The most significant effects were observed in the 20 mg L−1 ABA and 0.2 mg L−1 ETH treatments where the total lipid contents were 409.63 ± 10.35 and 367.26 ± 8.056 mg g−1 DW, respectively (Fig. 2). The 6-BA, EBR, NAA, SA, and SPD treatments showed increased lipid production at only one or two concentrations in a non-dose-dependent manner (Fig. 2).
We also calculated lipid productivities of C. vulgaris biomass in response to PGR treatments after 15 days of cultivation. Increases of lipid productivities were observed due to ABA (0.50 mg L−1, 6.91 ± 0.42 mg L−1 day−1; 1 mg L−1, 6.97 ± 0.24 mg L−1 day−1; 20 mg L−1, 6.58 ± 0.17 mg L−1 day−1; 50 mg L−1, 9.56 ± 0.26 mg L−1 day−1), 6-BA (1 mg L−1, 7.61 ± 0.63 mg L−1 day−1), ETH (0.01 mg L−1, 7.58 ± 0.29 mg L−1 day−1; 0.15 mg L−1, 6.89 ± 0.31 mg L−1 day−1), and NAA (50 mg L−1, 7.08 ± 0.38 mg L−1 day−1) treatments when compared to controls (4.79 ± 0.80 mg L−1 d−1; Fig. 3). However, no significant increase in lipid productivity could be found for EBR, GA3, SA, and SPD (Supplementary Fig. 1).
Lipid productivity (mg L−1 day−1) in Chlorella vulgaris biomass at different concentrations of abscisic acid (ABA), benzylaminopurine (6-BA), ethylene (ETH), and 1-naphthaleneacetic acid (NAA) after 15 days of cultivation. Shown are mean values and SEs from three separately grown cultures. The asterisks indicate statistically significant differences compared to controls
In the FAME analysis, some changes in the contents of PUFAs (EPA, DHA, linolenic acid (LNA), and arachidonic acid (ARA)) due to treatments with 6-BA, NAA, ABA, and ETH were found (Supplementary Fig. 4), although these FAs were at undetectable levels in the controls (Table 3). The percentages of LNA varied from 14.38 ± 0.34 to 53.09 ± 3.25% of total FAs in all treatments except the lower concentrations of BA (0.01 to 0.10 mg L−1) which had undetectable levels similar to the controls. Some detectable levels of EPA, DHA, and ARA were also found in the treated microalgal biomass with some exceptions (Table 3). Significant increases in the percentages of C15:0 and C18:0 were observed in all treated samples when compared to controls (Table 3).
Effect on crude protein contents
The PGRs did not affect crude protein contents in C. vulgaris cells (Supplementary Fig. 2) except for SA which caused increases in protein at 10 mg L−1 when compared to controls (39.76 ± 5.56 vs 24.42 ± 3.46 mg g−1 DW in controls; Fig. 4).
Effect on total carotenoid contents
Similar to lipids, total carotenoids in C. vulgaris cells increased in the PGR treatments used in our study; however, the increases were not dose-dependent (Fig. 5). In the case of 6-BA treatments, significant increases in total carotenoid contents were found for all concentrations when compared to controls (treatments 2.22 ± 0.35 to 4.12 ± 0.42 mg g−1 DW; controls 1.54 ± 0.18 mg g−1 DW; Fig. 5). Significant increases in total carotenoid contents were also observed due to 0.50 mg L−1 EBR, 0.20 mg L−1 ETH, 15 mg L−1 GA3, 15 mg L−1 NAA, 20 mg L−1 SA, and 15 mg L−1 SPD treatments. However, all concentrations of ABA reduced total carotenoid content in C. vulgaris cells (Fig. 5).
Expression of fatty acid synthesis-related genes
The expressions of FA synthesis genes were studied using quantitative real-time qPCR. Among the seven genes investigated in our study, all except SAD were upregulated in C. vulgaris in response to ABA and ETH treatments (Fig. 6). A similar pattern was observed for the treatments with SA and SPD where two genes (KAS, FATA) were upregulated. NAA treatment also upregulated two genes, namely, BC and SAD; however, they were different from SA and SPD treatments. Only one of the seven genes was found to be upregulated in response to treatments with 6-BA (SAD), EBR (FAD), and GA3 (BC) (Fig. 6).
Relative expression levels of fatty acid biosynthesis genes (BC, ACP, MCTK, KAS, FATA, FAD, and SAD) in Chlorella vulgaris in response to treatments with eight PGRs after 15 days of cultivation. Shown are mean values and SEs from three separately grown cultures. The asterisks indicate statistically significant differences compared to controls
Discussion
PGRs play a critical role in plant growth and development (Cooke et al. 2002). Ethylene is considered to play major roles in regulating plant defense responses against abiotic stresses (Bari et al. 2009). In the present study, PGR treatments increased lipid content significantly compared with the controls. The best stimulatory effect on lipid was 20 mg L−1 ABA treatments with 3.01-fold increase compared to controls then 0.20 mg L−1 ETH with 2.70-fold of controls, and other PGR treatments also had different levels of increased lipid accumulation. Converti et al. (2009) reported that lipid content of C. vulgaris under low nitrogen increased from 5.90 to 16.41%, a 2.70-fold increase compared to controls. Jusoh et al. (2015a) reported that 100 μM indole-3-acetic acid (IAA) induced 390.00 mg g−1 DW of lipids in C. vulgaris resulting in 39% increase in total lipid contents compared to controls. They also reported 54% increase in lipid accumulation in C. vulgaris in response to 45 μM JA treatment (Jusoh et al. 2015b). The present study observed higher levels of lipid induction in C. vulgaris due to PGR treatments than these authors.
Babu et al. (2017) reported 49 and 84% increases in lipid productivity in Chlorella sorokiniana due to 10 μM IAA and 10−3 μM diethyl aminoethyl hexanoate (DA-6) treatments. Similarly, up to fourfold increase in lipid productivity was reported in Chlorella pyrenoidosa and Scenedesmus quadricauda when treated with 60 mg L−1 indole-3-propionic acid (IPA) (Liu et al. 2016). However, decreased lipid productivity was reported in S. quadricauda after treatment with 1 and 2 μM ABA (Sulochana and Arumugam 2016). Our results are in disagreement with Sulochana and Arumugam (2016) as we observed increased lipid productivity due to ABA indicating the effect of PGR might be species-specific. However, four of the eight PGRs used in this study did cause increase in lipid productivity indicating that not all PGRs are effective in causing improved lipid productivity in C. vulgaris. In the present study, increased accumulation of PUFAs such as ARA (4.18 ± 0.16%), LNA (63.17 ± 1.12%), EPA (0.28 ± 0.05%), and DHA (0.53 ± 0.04%) was observed due to 6-BA, NAA, ABA, and ETH treatments in C. vulgaris. Moreover, higher proportions of unsaturated fatty acids were detected in the PGR-treated biomass than in the controls. Our results are in agreement with the findings of Park et al. (2013) and Liu et al. (2017a, b) who reported increased FAME yield in Chlamydomonas reinhardtii due to ABA treatment and increased unsaturated fatty acid accumulation in C. vulgaris cells due to NAA treatment, respectively. Therefore, our results indicate that addition of PGR can be a useful tool to induce high-value PUFA biosynthesis in C. vulgaris cells.
In the present study, no effect on protein biosynthesis was observed in C. vulgaris cells due to PGR treatments with the exception of 10 mg L−1 SA (1.63-fold of control). Our results are in agreement with Czerpak et al. (2002) who also reported increased protein accumulation in C. vulgaris due to SA treatment. Additionally, increased protein bioaccumulation was reported in C. vulgaris in response to IAA, IPA, and indole-3-butyric acid (IBA) treatments (Bajguz and Piotrowska-Niczyporuk 2013) and also in Scenedesmus obliquus due to IAA and diethyl aminoethyl hexanoate (DAH) supplementation (Salama et al. 2014). The lack of effect on protein biosynthesis in response to other PGRs in our study is consistent with the findings of Hunt et al. (2010) who reported that SPD, NAA, and GA3 had no significant effect on protein biosynthesis in Chlorella sorokiniana.
In the present study, we observed all of the PGRs except ABA induced C. vulgaris cells to synthesize carotenoids. Our results are in agreement with Ahmed et al. (2014) who reported increased carotenoid accumulation in Dunaliella salina and Tetraselmis suecica due to 10 μM methyl jasmonate and 70 μM salicylic acid, respectively. Increased carotenoid was also reported in S. quadricauda in response to 2,4-epibrassinolide (EBL) and IAA treatments (Kozlova et al. 2017). Furthermore, higher astaxanthin biosynthesis was reported in H. pluvialis as a result of ABA, SA, JA, EBL, and GA3 treatments (Gao et al. 2012a, b, 2013a, b). Higher biosynthesis of carotenoids is usually related to inhibition of chloroplast damage to cells exposed to environmental stresses (Gao et al. 2012a, b). The higher biosynthesis of carotenoids observed in the present study is thus an indication that PGR triggered the protection mechanism of C. vulgaris cells.
In the present study, six fatty acid synthesis genes were upregulated in response to ABA and ETH treatments. This was consistent with our findings that ABA and ETH induced accumulation of lipids in C. vulgaris cells. Lei et al. (2012) reported that all the selected genes coding for fatty acid synthesis were upregulated coupled with an increase in lipid contents in H. pluvialis under stress conditions (including nitrogen depletion, 450 μM FeSO4, 45 mM NaAC, high temperature, and low temperature). Thus, our results indicate that the presence of ABA and ETH in the media caused stress to C. vulgaris cells triggering significant expression of fatty acid synthesis genes which leads to higher accumulation of lipids in these cells.
The expression of SAD gene was significantly upregulated by 6-BA and NAA treatments in the present study. SAD is a rate-limiting enzyme in the biosynthesis of monounsaturated fatty acids from their saturated fatty acid precursors (Uto 2016). Lei et al. (2012) reported that SAD gene expression had a negative correlation with C18:0 but a positive correlation with C18:1 level. The synthesis of PUFAs in microalgae begins with the synthesis of C18:1 from C18:0 (Salas and Ohlrogge 2002). The high SAD upregulation, therefore, indicates that 6-BA and NAA can induce higher accumulation of PUFAs in C. vulgaris cells which can be corroborated by the high PUFA contents in 6-BA- and NAA-treated biomass (Table 3). Similarly, FAD gene expression has a positive correlation with PUFA biosynthesis (Jusoh et al. 2015a, b). The high upregulation of FAD gene as well as the higher biosynthesis of PUFA in ABA- and ETH-treated microalgal biomass in our study confirmed the role this gene plays in the fatty acid biosynthesis pathway. Our results also indicate that ABA, ETH, 6-BA, and NAA could be very useful tools to induce PUFA (i.e., LNA and DHA) in green microalgae through upregulation of key biosynthesis genes.
Conclusions
The use of PGR was a useful tool to induce C. vulgaris cells to accumulate lipids, soluble proteins, carotenoids, and PUFA in the present study. ABA was found to be the most effective for lipid production as it could increase total lipid contents and lipid productivities by up to 3.01-fold and 2-fold when compared to controls. 6-BA, NAA, ABA, and ETH treatments induced C. vulgaris cells to accumulate PUFA especially LNA, DHA, ARA, and EPA. Similar to lipids, accumulation of carotenoids was also induced in C. vulgaris cells by all PGRs used in this study except ABA. On the contrary, only SA was effective in inducing protein accumulation. Overall, treatment with PGR used in this study could be a useful tool to produce biodiesel and other high-value metabolites from microalgal biomass.
References
Ahmed F, Fanning K, Schuhmann H, Netzel M, Schenk PM (2013) Microalgae: a valuable source of natural carotenoids with potential health benefits. In: Yamaguchi (ed) Carotenoids: food sources, production and health benefits. New York, pp 143–164
Ahmed F, Fanning K, Netzel M, Schenk PM (2014) Induced carotenoid accumulation in Dunaliella salina and Tetraselmis suecica by plant hormones and UV-C radiation. Appl Microbiol Biotech 99:9407–9416
Babu AG, XG W, Kabra AN, Kim DP (2017) Cultivation of an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitation. Algal Res 23:178–185
Bajguz A (2000) Blockade of heavy metals accumulation in Chlorella vulgaris cells by 2,4-epibrassinolide. Plant Physiol Biochem 38:797–801
Bajguz A, Piotrowska A (2009) Conjugates of auxin and cytokinin. Phytochemistry 70:957–969
Bajguz A, Piotrowska-Niczyporuk A (2013) Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem 71:290–297
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Molecul Biol 69:473–488
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Physiol Pharmacol 37:911–917
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Catarina GA, Amaro HM, Xavier MF (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
Cha TS, Chen JW, Goh EG, Aziz A, Loh SH (2011) Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour Technol 102:10633–10640
Converti A, Casazza AA, Ortiz EY, Perego P, Borghi MD (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Cooke TJ, Poli DB, Sztein AE, Cohen JD (2002) Evolutionary patterns in auxin action. In: Catherine PR (ed) Auxin molecular biology. Springer, Dordrecht, pp 319–338
Czerpak R, Bajguz A, Gromek M, Kozłowska G, Nowak I (2002) Activity of salicylic acid on the growth and biochemism of Chlorella vulgaris Beijerinck. Acta Physiol Plant 24:45–52
Czerpak R, Piotrowska A, Szulecka K (2006) Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris. Acta Physiol Plant 28:195–203
Damiani MC, Popovich CA, Constenla D, Leonardi PI (2010) Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour Technol 101:3801–3807
Falkowska M, Pietryczuk A, Piotrowska A, Bajguz A, Grygoruk A, Czerpak R (2011) The effect of gibberellic acid (GA(3)) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (Chlorophyceae) Beijerinck exposed to cadmium and lead stress. Polish J Env Stud 20:53–59
Gao ZQ, Meng CX, Zhang XW, Xu D, Zhao YF, Wang YT, Lv HX, Yang LM, Chen LL, Ye NH (2012a) Differential expression of carotenogenic genes, associated changes on astaxanthin production and photosynthesis features induced by JA in H. pluvialis. PLoS One 7:e42243
Gao ZQ, Meng CX, Zhang XW, Xu D, Miao XX, Wang YT, Yang LM, Lv HX, Chen LL, Ye NH (2012b) Induction of salicylic acid (SA) on transcriptional expression of eight carotenoid genes and astaxanthin accumulation in Haematococcus pluvialis. Enz Microb Tech 51:225–230
Gao ZQ, Gao HZ, Meng CX (2013a) Effects of abscisic acid (ABA) carotenogenesis expression and astaxanthin accumulation in Haematococcus pluvialis. Res. J Biotech 8:9–15
Gao ZQ, Meng CX, Gao HZ, Zhang XW, Xu D, Su YF, Wang YY, Zhao YR, Ye NH (2013b) Analysis of mRNA expression profiles of carotenogenesis and astaxanthin production of Haematococcus pluvialis under exogenous 2,4-epibrassinolide (EBR). Biol Res 46:201–206
Gao ZQ, Miao XX, Zhang XW, Wu GX, Guo YY, Wang MM, Li B, Li XB, Gao YH, Hu S, Sun JT, Cui JL, Meng CX, Li Y (2016) Comparative fatty acid transcriptomic test and iTRAQ-based proteomic analysis in Haematococ cuspluvialis upon salicylic acid (SA) and jasmonic acid (JA) inductions. Algal Res 17:277–284
Hu Y, Yu D (2014) Brassinosteroid insensitive interacts with abascisic acid insensitive 5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell 26:4394–4408
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Hunt RW, Chinnasamy S, Bhatnagar A, Das KC (2010) Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana. Appl Biochem Biotech 162:2400–2414
Imin N, Nizamidin M, Daniher D, Nolan KE, Rose RJ, Rolfe BG (2005) Proteomic analysis of somatic embryogenesis in Medicago truncatula eplant cultures grown under 6-benzylaminopurine and 1-naphthaleneacetic acid treatments. Plant Physiol 137:1250–1260
Josephine A, Niveditha C, Radhik A, Shali AB, Kumar TS, Dharani G, Kirubagaran R (2015) Analytical evaluation of different carbon sources and growth stimulators on the biomass and lipid production of Chlorella vulgaris—implications for biofuels. Biomass Bioenergy 75:170–179
Jusoh M, Loh SH, Chuah TS, Aziz A, Cha TS (2015a) Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 111:65–71
Jusoh M, Loh SH, Chuah TS, Aziz A, Cha TS (2015b) Elucidating the role of jasmonic acid in oil accumulation, fatty acid composition and gene expression in Chlorella vulgaris (Trebouxiophyceae) during early stationary growth phase. Algal Res 9:14–20
Kamalanathan M, Pierangelini M, Shearman LA, Gleadow R, Beardall J (2016) Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. J Appl Phycol 28:1509–1520
Kozlova TA, Hardy BP, David P, Levin B (2017) Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Res 27:325–334
Lari Z, Moradi-kheibari N, Ahmadzadeh H, Abrishamchi P, Moheimani NR, Murry MA (2016) Bioprocess engineering of microalgae to optimize lipid production through nutrient management. J Appl Phycol 28:3235–3250
Lei A, Chen H, Shen G, Hu Z, Chen L, Wang J (2012) Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnol Biofuels 5:18–28
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Liu JY, Qiu W, Song YM (2016) Stimulatory effect of auxins on the growth and lipid productivity of Clorella pyrenoidosa and Scenedesmus quadricauda. Algal Res 18:273–280
Liu TT, Liu F, Wang C, Wang ZY, Li YQ (2017a) The boosted biomass and lipid accumulation in Chlorella vulgaris by supplementation of synthetic phytohormone analogs. Bioresour Technol 232:44–52
Liu J, Qiu W, Xia D (2017b) Brassinosteroid improves lipid productivity and stress tolerance of Chlorella cells induced by high temperature. J Appl Phycol https://doi.org/10.1007/s10811-017-1223-2
Lu YD, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci 20:273–282
Negi S, Barry AN, Friedland N, Sudasinghe N, Subramanian S, Pieris S, Holguin FO, Dungan B, Schaub T, Sayre R (2016) Impact of nitrogen limitation on biomass, photosynthesis, and lipid accumulation in Chlorella sorokiniana. J Appl Phycol 28:803–881
Park WK, Yoo G, Moon M, Kim CW, Choi YE, Yang JW (2013) Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Appl Biochem Biotech 171:1128–1142
Piotrowska-Niczyporuk A, Bajguz A (2014) The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul 73:57–66
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Zyłkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65
Raman V, Ravi S (2011) Effect of salicylic acid and methyl jasmonate on antioxidant systems of Haematococcus pluvialis. Acta Physiol Plant 33:1043–1049
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Salama ES, Kabra AN, Ji MK, Kim JR, Min B, Jeon BH (2014) Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour Technol 172:97–03
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403:25–34
Sulochana SB, Arumugam M (2016) Influence of abscisic acid on growth, biomass and lipid yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresour Technol 213:198–203
Uto Y (2016) Recent progress in the discovery and development of stearoyl CoA desaturase inhibitors. Chem Phys Lipids 197:3–12
Xu F, Chen X, Yang Z, Jin P, Wang K, Shang H, Wang X, Zheng Y (2013) Maintaining quality and bioactive compounds of broccoli by combined treatment with 1-methylcyclopropene and 6-benzylaminopurine. J Sci Food Agric 93:1156–1161
Funding
The present study was supported by Key Research and Development Program of Shandong Province (2016GSF121030, 2017GSF21105, 2017CXGC0309), the National Natural Science Foundation of China (31170279, 41106124), the Natural Science Foundation of Shandong province (ZR2011DM006, ZR2011CQ010), the Project of Shandong Province Higher Educational Science and Technology Program (J17KA132), and the Supporting Project for Young Teachers in Shandong University of Technology (4072–114021).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Supplementary Figure 1
(JPEG 823 kb)
Supplementary Figure 2
(JPEG 1897 kb)
Supplementary Figure 4
(PDF 64.7 kb)
Rights and permissions
About this article
Cite this article
Lin, B., Ahmed, F., Du, H. et al. Plant growth regulators promote lipid and carotenoid accumulation in Chlorella vulgaris . J Appl Phycol 30, 1549–1561 (2018). https://doi.org/10.1007/s10811-017-1350-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1350-9