Abstract
Mass cultivation of the chlorophyte Chaetomorpha crassa has the potential to serve as a biological filter for the reduction of eutrophication in summertime Japanese waters. In order to clarify the suitability of C. crassa for this purpose, seasonal changes in its photosynthesis, growth, NO3–N uptake, nitrogen content, and salinity tolerance were investigated trimonthly from May 2011 to February 2012, with samples collected in Nagatsuraura Lagoon, northern Japan. Significant effects of seawater temperature on photosynthesis, growth, and nitrogen accumulation were also detected in all four seasons, and all parameters at summer temperatures (24–28 °C) were significantly greater than those at the temperatures of other seasons (8–20 °C). Moreover, compared to the other three seasons, C. crassa showed significantly higher growth rates at 16–4 psu and higher survival percentages at 8–2 psu during the summer. In conclusion, due to its high capacity for growth and nitrogen accumulation, and greater physiological tolerance of low salinity during the elevated temperature period, large-scale cultivation of C. crassa could play a significant role in the bioremediation of both saline and brackish waters during summer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last several decades coastal waters worldwide have become increasingly eutrophic as a result of runoff from land-based agriculture, huge fish aquaculture grounds, and other anthropogenic activities (Naylor et al. 2000; Read and Femandes 2003). The high nutritional status alters the characteristics of the ecosystem and causes a series of ecological events, including red tides, green tides and other disasters (Nagasoe et al. 2010; Glibert et al. 2011; Schumacher et al. 2014). Among the different methods for reducing eutrophication, seaweed cultivation is receiving more attention in recent years because of its simplification and lower cost. To date, a number of studies have investigated the bioremediation potential of different seaweed species, particularly Ulva spp. (Msuya et al. 2006; Yokoyama and Ishihi 2010; Nielsen et al. 2012), Gracilaria spp. (Hernández et al. 2006; Yang et al. 2015), Porphyra/Pyropia spp. (Kraemer et al. 2004; Carmona et al. 2006), and kelps (Reid et al. 2013; Marinho et al. 2015; Augyte et al. 2017). These previous studies found that mass cultivation of seaweeds can assimilate large amounts of nutrients and may alleviate eutrophication problem in saline waters. However, very few studies have focused on the application of seaweeds for the bioremediation of brackish waters. More information on basic ecology is essential in order to select suitable species to serve as nutrient scrubbers and reduce eutrophication in hyposaline conditions.
The filamentous green macroalga Chaetomorpha crassa (C. Agardh) Kützing is widely distributed in shallow waters throughout the world (Yoshida 1998; Lourenço et al. 2005; Bolton et al. 2007). On the coast of Japan, this species is abundant in both saline and brackish waters, sometimes forming dense mats on the sediment surface, even in aquaculture facilities (Yoshida 1998). Recently, high contents of crystalline cellulose (feedstock) have been found in the thalli of Chaetomorpha species, indicating a high potential for bioethanol production (Bastianoni et al. 2008; Wang et al. 2011). Due to its great potential value for bioenergy industry, a better understanding of the basic biology of C. crassa is essential. To date, however, except for the work of authors of this paper, no other studies have focused on this species.
In a previous paper (Gao et al. 2017b), we studied the physiological differences of three Japanese Chaetomorpha species (C. crassa, C. moniligera, and C. spiralis). We observed that C. crassa had higher growth rates and tolerance for heat stress than the other species. As a result, C. crassa was confirmed as an ideal choice for mass cultivation aimed at producing bioethanol and reducing summertime coastal eutrophication. However, in order to more fully determine the suitability of C. crassa as a biofilter, it is necessary to examine seasonal patterns in its growth and nutrient bioremediation abilities. In addition, to clarify the suitability of this species for use in brackish waters, its salinity tolerance should be compared among seasons. In general, growth, morphology, and carbon and nitrogen contents of many seaweeds exhibit seasonal patterns related to environmental factors, such as seawater temperature (Sjøtun et al. 1996; Brenchley et al. 1998; Skriptsova et al. 2004; Periyasamy et al. 2014). However, very few investigations have been conducted on the species-specific phenology of members of the Chaetomorpha genus (but see Zhang et al. 2015).
The objective of the present study was to examine the seasonal variations in the growth, nutrient accumulation, and salinity tolerance of C. crassa and to evaluate whether the species is a suitable candidate for reducing coastal eutrophication on the northern coast of Japan.
Materials and methods
Sample collection and maintenance
Thalli of Chaetomorpha crassa were collected from natural populations in the lagoon of Nagatsuraura (38° 55′ N, 141° 46′ E), northern Japan in May (spring) 2011, August (summer) 2011, November (autumn) 2011, and February (winter) 2012. The surface seawater temperatures at the study site were also monitored monthly using a standard thermometer during April 2011 and March 2012. Samples were transported immediately to the laboratory using insulated cooler boxes filled with seawater. These thalli were rinsed several times with sterilized seawater to remove diatoms and detritus. After each sampling trip, healthy thalli were selected and cut into more than 800 fragments, each about 3 cm long, for subsequent experiments. They then were cultured in several large flasks containing 4 L of enriched 25% PESI medium (Tatewaki 1966), which was made using sterilized seawater from the coast of Ishinomaki with a salinity of 32 psu and a pH of 8.0. These fragments were maintained at the surface temperature measured during each collection trip, with an irradiance of 40 μmol photons m−2 s−1 and 12:12-h light/dark cycle for 1 day in order to reduce the negative effects of sample processing.
Photosynthesis
A differential gas volumeter called a “product-meter” (Yokohama et al. 1986) was used to measure photosynthetic rates. To take measurements, two fragments were placed in a reaction vessel containing 10 mL of sterilized seawater with 25% PESI. A compensation vessel containing only 10 mL of sterilized seawater was prepared as a control. The reaction and compensation vessels were attached to Product-meters and immersed in a thermostat-controlled water bath (Taitec CL–150F, Japan) that was maintained at a constant water temperature and shaken by means of a motor drive at 150 rpm. The vessels were illuminated from below with photo slide projector lamps (Elmo S-300, Japan) and with incandescent lamps (Philips KP-10s 100 V, 300 W; Japan). The light was reflected by mirrors placed under the water bath. Irradiance was regulated using neutral density glass filters (Toshiba TND-50, 25, 12.5, Japan) and measured with a quantum photon meter (LI-COR LI-192S, USA). The fragments were cultured for 30 min to allow them to adapt to the experimental temperature. After this equilibration period, their oxygen production was measured ten times at 3-min intervals.
To measure photosynthesis, these fragments were subjected to seven different seawater temperatures (8, 12, 16, 20, 24, 28, and 32 °C) with an irradiance of 180 μmol photons m−2 s−1. Sixteen fragments were used for each experimental treatment. The fresh weights of these fragments were measured after the experiments. The initial nutrient concentrations of the sterilized seawater were set to 0.4 mg L−1 for NO3–N and NH4–N and 0.08 mg L−1 for PO4–P.
NO3–N uptake
The product-meter was also used for measurement of uptake rates of NO3–N by comparison of the concentrations of medium (sterilized seawater with 25% PESI) between reaction and control vessels. After a 60-min shaken incubation of the fragments, the medium from reaction vessels and control vessels was collected separately, and the uptake rate of NO3–N was obtained from the differences between the concentrations analyzed by the auto-analyzer (TRAACS 800, Bran–Luebbe, Japan). To measure the uptake rates of NO3–N, these fragments were subjected to five different temperatures (16, 20, 24, 28, and 32 °C) with an irradiance of 180 μmol photons m−2 s−1. Sixteen fragments were used for each experimental treatment, and the fresh and dry weights of these fragments were measured after the experiments. The uptake rates of NO3–N were estimated using the following equation:
where VN,P = uptake rate of NO3–N (mg g−1 h−1); t = time (h); T = time interval (h); St = initial concentration at time t (mg L−1); St + T = final concentration at time t + T (mg L−1); and DW = dry weight of the fragments.
Growth and tissue nitrogen content
After each collection, a culture experiment was carried out over a period of 12 days at seven temperatures: 8, 12, 16, 20, 24, 28, and 32 °C. Each temperature treatment had four replicates. During each experiment, a 12:12-h light/dark cycle was maintained, with an irradiance of 180 μmol photons m−2 s−1 provided by 40 W cool-white fluorescent tubes. The experiments used 28 side-arm flasks, with each flask containing 500 mL of seawater enriched with 25% PESI medium amended with 1.5 mL GeO2 (3 μg mL−1) to eliminate the growth of diatoms. Ten thallus fragments were put into each flask, which was then gently aerated. The culture medium in each flask was changed every 3 days, at which times GeO2 was also added. During the culture period, these fragments were observed daily and the number of surviving fragments was recorded. When decay or discoloration extended to more than half of the fragment, it was considered to be dead. The fresh weights of all fragments prior to the experiments, and the weights of the surviving fragments at the end of the incubations, were measured after each thallus was blotted dry. The relative growth rate (RGR % day−1) of each replicate was calculated using the following equation (Bird et al. 1979; Ohno et al. 1994):
where W o is the average initial fresh weight, W t is the final fresh weight after the experiments, and t is the number of days.
For all temperature treatments, eight surviving fragments after incubation were selected and placed into screw-top bottles and dried in a convection oven at 60 °C for 12 h. After their dry weights were measured, these fragments were crushed and analyzed for tissue nitrogen contents using an Elemental Analyzer (Eager 200; Fisons Instruments/Thermo Fisher Scientific, USA).
Salinity experiments
To compare the salinity tolerance of C. crassa among different seasons, a series of culture experiments were conducted for 12 days at five salinities (32, 16, 8, 4, and 2 psu). Each salinity treatment had four replicates. During these experiments, a temperature of 28 °C, a neutral day light cycle (12:12 h), and an irradiance of 180 μmol photons m−2 s−1 were maintained. A total of 40 side-arm flasks (500 mL) were used, with each flask containing 500 mL of seawater enriched with 25% PESI medium and 1.5 mL GeO2. Ten fragments were placed into each flask, which was then gently aerated. The culture medium in each flask was changed every 3 days. At the end of this experiment, the proportion of surviving fragments and RGR was calculated.
Data analysis
A two-way analysis of variance (ANOVA) was used to analyze the effects of temperature and season on photosynthesis, RGR, NO3–N uptake, and tissue nitrogen content of C. crassa. In addition, a separate two-way ANOVA was used to analyze the significance of effects of salinity and season on RGR of C. crassa at 28 °C. Prior to ANOVA tests, all data were assessed for normality (Shapiro-Wilk test) and homogeneity of variance (Levene test). When a significant difference was identified by ANOVA, Tukey’s multiple comparisons test was used to determine which levels of each factor produced significant differences (p < 0.05).
Results
Seawater temperature
The surface seawater temperature at the study site showed marked seasonal variation, with the maximum and minimum in August 2011 and January 2012, respectively (Fig. 1). The average temperature values during the four collection times were 17.6 °C (May), 29.4 °C (August), 16.7 °C (November), and 5.3 °C (February).
Photosynthesis
Results of two-way ANOVA showed that the photosynthetic rates of C. crassa were significantly affected by both temperature and season (temperature effect, df = 6, F = 18.204, p < 0.001; seasonal effect, df = 3, F = 7.991, p < 0.01; Fig. 2). A significant interaction between temperature and season on the photosynthetic rates was not detected (two-way ANOVA, temperature × season, df = 18, F = 2.188, p = 0.079). The photosynthetic rates at summer temperatures of 24 and 28 °C were significantly greater than those at the temperatures of other seasons (8–20 °C). The photosynthetic rate did not significantly differ among the four seasons at 8–16 and 32 °C. However, at 20–28 °C, summertime photosynthetic rates were significantly greater than those of the other three seasons. In addition, at 28 °C, the photosynthetic rates measured during spring and autumn were significantly greater than those measured during winter.
Photosynthesis–temperature curves at various temperatures per unit dry weight of thalli of C. crassa in spring, summer, autumn, and winter. Different lowercase letters indicate statistical differences among seasons at each temperature. N = 16 individuals for each treatment. Bars indicate standard errors
NO3–N uptake
The uptake rates of NO3–N of C. crassa differed significantly among the four seasons (two-way ANOVA, seasonal effect, df = 3, F = 11.667, p < 0.001) and were also significantly affected by temperature (two-way ANOVA, temperature effect, df = 4, F = 14.170, p < 0.001; Fig. 3). A significant interaction between temperature and season on the uptake rates of NO3–N was not detected (two-way ANOVA, temperature × season, df = 12, F = 1.773, p = 0.218). The uptake rates of NO3–N at summer temperatures of 24 and 28 °C were significantly greater than those at the temperatures of other seasons (16 and 20 °C). The uptake rates of NO3–N did not significantly differ among the four seasons at 16 and 32 °C. However, at 20 and 28 °C, the uptake rates of NO3–N in summer were significantly greater than those in the other three seasons. At 24 °C, the uptake rates of NO3–N in summer were significantly greater than those in winter.
NO3–N uptake–temperature curves at various temperatures per unit dry weight of thalli of C. crassa in spring, summer, autumn, and winter. Different lowercase letters indicate statistical differences among seasons at each temperature. N = 16 individuals for each treatment. Bars indicate standard errors
Growth
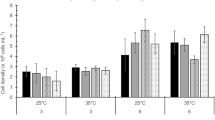
The RGRs differed significantly among four seasons (two-way ANOVA, seasonal effect, df = 3, F = 17.492, p < 0.001), and were significantly affected by temperature (two-way ANOVA, temperature effect, df = 6, F = 21.405, p < 0.001; Fig. 4). A significant interaction between temperature and season on the RGRs was not detected (two-way ANOVA, temperature × season, df = 18, F = 2.631, p = 0.061). The RGRs at summer temperatures of 24 and 28 °C were significantly greater than those at the temperatures of other seasons (8–20 °C). The RGRs did not significantly differ among four seasons at 8–16 and 32 °C. However, at 24 and 28 °C, the RGRs in summer were significantly greater than those in the other three seasons. In addition, at 24 and 28 °C, the RGRs in spring and autumn were significantly greater than those in winter. At 20 °C, the RGRs in summer were significantly greater than those in winter. During these experiments at different seasons, survival percentages of 100% were observed at all temperature levels.
Tissue nitrogen contents
Results of two-way ANOVA showed that the tissue nitrogen contents differed significantly among the four seasons (seasonal effect, df = 3, F = 8.005, p < 0.01; Fig. 5). However, there were no significant effects of temperature (temperature effect, df = 6, F = 1.929, p = 0.159) and no significant interaction (temperature × season, df = 18, F = 1.332, p = 0.301). The tissue nitrogen contents in summer were greater than those in the other three seasons at all temperature levels. Especially at 20–28 °C, significantly greater values were found in summer.
Salinity tolerance
At 32 and 16 psu, C. crassa thalli fragments had survival percentages of 100%. At 8 and 4 psu, the survival percentages of C. crassa in summer were 82.5 and 60%, respectively (Fig. 6). These survival percentages are higher than those in the other three seasons. At a lower salinity of 2 psu, all samples in spring, autumn, and winter had died by the end of the experiments. In contrast, at this low salinity, survival percentages of 22.5% were found in summer.
The RGRs differed significantly among four seasons (two-way ANOVA, seasonal effect, df = 3, F = 16.701, p < 0.001) and were significantly affected by salinity (two-way ANOVA, salinity effect, df = 4, F = 26.832, p < 0.001; Fig. 7). A significant interaction between salinity and season on the RGRs was not detected (two-way ANOVA, salinity × season, df = 12, F = 2.089, p = 0.107). Although there were no significant differences in RGRs between 32 and 16 psu, the RGRs decreased significantly with decreasing salinities thereafter. At 32–4 psu, the RGRs in summer were significantly greater than those in the other three seasons. Moreover, the RGRs in spring and autumn were significantly greater than those in winter at 32 and 16 psu.
Discussion
In the present study, the RGR of C. crassa at 28 °C was significantly greater than those of other temperature levels. Similar response has been found in Chaetomorpha linum, with optimal growth temperatures of 25 and 28 °C (Xu and Lin 2008). Deng et al. (2012) also reported 25–29 °C and 21–29 °C as suitable temperatures for the growth of gametophyte and sporophyte of Chaetomorpha valida, with optimal temperatures of 25 and 29 °C at which maximum growth rate occurred. On the other hand, there were little changes in the growth of C. crassa at 8 °C. This may indicate that this species lack the capacity to adapt to low temperature conditions in winter. This hypothesis is supported by a previous finding that low temperatures up to 10 °C retarded normal growth of Chaetomorpha melagonium (Patel 1971). Compared to the upper lethal temperatures of 26–30 °C for other Chaetomorpha species (Bischoff and Wiencke 1993; Gao et al. 2017b), C. crassa showed a higher value at 33–35 °C (Gao et al. 2017b; this study), indicating a greater heat tolerance and higher potential for cultivating in summer. Furthermore, in this study, the photosynthesis and growth of C. crassa exhibited significant seasonal variations, with maximum values in summer. Deng et al. (2012) also reported that C. valida often dominates aquaculture ponds and grows more luxuriantly in summer than in winter. In Japan, the cultivation of most seaweeds are limited to winter and spring, and very few seaweed crops are suitable for cultivation in the summer. These characteristics are likely to qualify C. crassa for the contribution of water quality improvements in summer by mass cultivation.
Seasonal variations of ambient temperature may have compelled seaweeds to develop different strategies for growth and survival (Lüning 1990). One of these strategies is a differential temperature response according to seasonal acclimation, a set of adaptations detected in many representatives of both perennial seaweeds and those with an isomorphic life cycle. For instance, as temperature acclimation occurs, some seaweeds change their temperature requirements for growth (Bischoff and Wiencke 1993). Evidence of this has been observed in several kelp species, which exhibited different optimal growth temperatures at different developmental stages and thus synchronized their growth periods to ambient temperatures (Komazawa et al. 2015; Gao et al. 2017a). In contrast, this study found that the optimum temperature for growth of C. crassa remain stable at 28 °C between different seasons. This finding concurs with studies of some green and red seaweeds that showed little adaptation to temperature fluctuations (Yarish et al. 1987; Bischoff and Wiencke 1993). Like these species, Chaetomorpha may also have no mechanism to adaptively adjust their metabolisms to changing temperatures.
Chaetomorpha crassa also exhibited higher nutrient uptake rates and tissue nitrogen contents during the summer than during the other seasons. In natural populations of some seaweeds, the pool of nitrogen that is maintained as a storage reserve can vary on a seasonal basis in response to changes in the external availability of nitrogen (Asare and Harlin 1983; Fujita 1985). Similarly, in Chaetomorpha linum, the size of the internal nitrogen pools changed nearly fourfold when grown under N-saturating or N-limiting conditions (McGlathery et al. 1996). In the present study, all C. crassa thalli were cultured under identical nitrogen enrichment; therefore, the difference in nitrogen reserves is unlikely to be due to different nutrient concentrations in the ambient environment. Rather, our results indicate that under N-saturated conditions, the nitrogen pool of C. crassa may be affected by seawater temperature. The nitrogen pool of this species may become larger in the high summertime seawater temperatures, resulting in improved capacity for nutrient uptake and assimilation.
In the present study, the lower lethal salinity of C. crassa occurred at 16–8 psu, which is generally consistent with the < 15 psu for C. linum reported by Xu and Lin (2008). These authors also reported that the highest growth rate of C. linum occurred at 30 psu, significantly higher than those of the other lower salinity levels. However, there were no significant differences in growth rates of C. crassa between 32 and 16 psu. Moreover, C. crassa thalli had survival percentages of 100% at 16 psu. At lower salinities of 8 and 4 psu, the survival percentage ranges were still 72.5–82.5% and 45–60%, respectively. It appears that this species is not particularly sensitive to salinity fluctuations and has a great capacity to thrive in coastal waters where heavy runoff and freshwater influxes always occur. This hypothesis may be supported by our field observations that C. crassa is the dominant primary producer in Nagatsuraura lagoon. These indicate that it is practicable to cultivate this species in hyposaline conditions.
A two-factor experiment in southern California revealed that nitrogen enrichment could ameliorate the negative effect of reduced salinity on the growth and condition of Ulva (Enteromorpha) intestinalis. Increased tissue nitrogen contents may have improved the healthy state of the algae and thus increased its tolerance to low salinity (Kamer and Fong 2001). Adequate nutrients can contribute directly to osmoregulation, providing a possible mechanism by which U. intestinalis adapted to reduced salinity (Cohen and Fong 2004). Supporting this interpretation, Fong et al. (1996) showed that under N-sufficient conditions, U. intestinalis was more tolerant of low salinity than its competitors. In our study, C. crassa exhibited greater tolerance to low salinity during summer than during the other seasons. Coincidentally, the nutrient uptake rate and nitrogen content were also highest at summertime temperatures. Therefore, we suggest that its greater tolerance to low salinity in summer may be partially associated with its higher capacity for nitrogen accumulation during the same period.
One important standard in selecting seaweed species for bioremediation in an integrated aquaculture system is their resistance to large fluctuations in environmental conditions, including temperature and salinity (de Paula Silva et al. 2008; Kang et al. 2013). The results of this study showed that C. crassa is a eurythermic and euryhaline species, indicating an excellent candidate for co-culturing with aquatic animals. Actually, successful trials have been conducted that integrated aquaculture of tiger prawns with species from the genus Chaetomorpha has resulted in increased growth rate of tiger prawns (Tsutsui et al. 2010, 2015). However, species with promise need to be assessed in situ in operational cultivation systems, as the transfer from controlled conditions to field can provide contrasting outcomes (Paul and de Nys 2008). de Paula Silva et al. (2008) reported that Chaetomorpha indica, as a targeted species for bioremdiation, had excellent growth under controlled conditions but failed to grow (essentially died) under short-term in situ trials. Therefore, further research to identify the applicability of C. crassa in integrated aquaculture systems is warranted.
Recently, it has been demonstrated that different algal species have a differential capacity to absorb nitrate and ammonium from seawater, implying that diverse species assemblages of seaweeds are more effective at obtaining nutrients (Bracken and Stachowicz 2006; Kang et al. 2011). Therefore, we suggest that polycultures, rather than monocultures, may be a more effective way to improve water quality. In addition to C. crassa, other seaweeds also exhibit a wide range of tolerance to fluctuating environmental conditions, including members of genera Ulva (Taylor et al. 2001; Wang et al. 2007; Mantri et al. 2010) and Gracilaria (Choi et al. 2006; Thomsen and McGlathery 2007). Together with C. crassa, representatives of these groups would be suitable choices for mixed bioremediation polycultures. Similarly, Buschmann et al. (2008) also proposed that Macrocystis pyrifera and Gracilaria chilensis should be introduced simultaneously at different depths to increase the nutrient removal effectiveness from the environment. Therefore, further research should be conducted to identify potential differences in the assimilation characteristics of nutrients between different algal species and appropriate combinations.
In conclusion, C. crassa exhibited the greatest growth rates, nutrient uptake rates and low salinity tolerance at the high temperature of 28 °C during summer. This finding indicates that C. crassa would be a suitable candidate for bioremediation of both saline and brackish waters during summer.
References
Asare SO, Harlin MM (1983) Seasonal fluctuation in tissue nitrogen for five species of perennial macroalgae in Rhode Island sound. J Phycol 19:254–257
Augyte S, Yarish C, Redmond S, Kim JK (2017) Cultivation of a morphologically distinct strain of the sugar kelp, Saccharina latissima forma angustissima, from coastal Maine, USA, with implications for ecosystem services. J Appl Phycol 29:1967–1976
Bastianoni S, Coppola F, Tiezzi E, Colacevich A, Borghini F, Focardi S (2008) Biofuel potential production from the Orbetello lagoon macroalgae: a comparison with sunflower feedstock. Biomass Bioenergy 32:619–628
Bird NL, Chen LCM, McLachlan J (1979) Effects of temperature, light and salinity on growth in culture of Chondrus crispus, Furcellaria lumbricalis, Gracilaria tikvahiae (Gigartinales, Rhodophyta) and Fucus serratus (Fucales, Phaeophyta). Bot Mar 22:521–527
Bischoff B, Wiencke C (1993) Temperature requirements for growth and survival of macroalgae from Disko Island (Greenland). Helgol Meeresunters 47:167–191
Bolton JJ, Oyieke HA, Gwada P (2007) The seaweeds of Kenya: checklist, history of seaweed study, coastal environment, and analysis of seaweed diversity and biogeography. S Afr J Bot 73:76–88
Bracken MES, Stachowicz JJ (2006) Seaweed diversity enhances nitrogen uptake via complementary use of nitrate and ammonium. Ecology 87:2397–2403
Brenchley J, Raven J, Johnston A (1998) Carbon and nitrogen allocation patterns in two intertidal fucoids: Fucus serratus and Himanthalia elongata (Phaeophyta). Eur J Phycol 33:307–313
Buschmann AH, Varela DA, Hernández-González MC, Huovinen P (2008) Opportunities and challenges for the development of an integrated seaweed-based aquaculture activity in Chile: determining the physiological capabilities of Macrocystis and Gracilaria as biofilters. J Appl Phycol 20:571–577
Carmona R, Kraemer GP, Yarish C (2006) Exploring northeast American and Asian species of Porphyra for use in an integrated finfish–algal aquaculture system. Aquaculture 252:54–65
Choi HG, Kim YS, Kim JH, Lee SJ, Park EJ, Ryu J, Nam KW (2006) Effects of temperature and salinity on the growth of Gracilaria verrucosa and G. chorda, with the potential for mariculture in Korea. J Appl Phycol 18:269–277
Cohen RA, Fong P (2004) Physiological responses of a bloom-forming green macroalga to short-term change in salinity, nutrients, and light help explain its ecological success. Estuaries 27:209–216
Deng YY, Tang XR, Huang BX, Ding LP (2012) Effect of temperature and irradiance on the growth and reproduction of the green macroalga, Chaetomorpha valida (Cladophoraceae, Chlorophyta). J Appl Phycol 24:927–933
Fong P, Boyer KE, Desmond JS, Zedler JB (1996) Salinity stress, nitrogen competition, and facilitation: what controls seasonal succession of two opportunistic green macroalgae? J Exp Mar Biol Ecol 206:273–221
Fujita RM (1985) The role of nitrogen status in regulating transient ammonium uptake and nitrogen storage by macroalgae. J Exp Mar Biol Ecol 92:283–301
Gao X, Endo H, Nagaki M, Agatsuma Y (2017a) Interactive effects of nutrient availability and temperature on growth and survival of different size classes of Saccharina japonica (Laminariales, Phaeophyceae). Phycologia 56:253–260
Gao X, Endo H, Agatsuma Y (2017b) Comparative study on the physiological differences between three Chaetomorpha species from Japan in preparation for cultivation (Laminariales, Phaeophyceae). J Appl Phycol. https://doi.org/10.1007/s10811-017-1306-0
Glibert PM, Burkholder JM (2011) Harmful algal blooms and eutrophication: “strategies” for nutrient uptake and growth outside the Redfield comfort zone. Chin J Oceanol Limnol 29:724–738
Hermández I, Pérez-Pastor A, Vergara JJ, Martínez-Aragón JF, Fernández-Engo MÁ, Pérez-Lloréns JL (2006) Studies on the biofiltration capacity of Gracilariopsis longissima: from microscale to macroscale. Aquaculture 252:43–53
Kamer K, Fong P (2001) Nitrogen enrichment ameliorates the negative effects of reduced salinity on the green macroalga Enteromorpha intestinalis. Mar Ecol Prog Ser 218:87–93
Kang YH, Park SR, Chung IK (2011) Biofiltration efficiency and biochemical composition of three seaweed species cultivated in a fish-seaweed integrated culture. Algae 26:97–108
Kang YH, Hwang JR, Chung IK, Park SR (2013) Development of a seaweed species-selection index for successful culture in a seaweed-based integrated aquaculture system. J Ocean Univ China 12(1):125–133
Komazawa I, Sakanishi Y, Tanaka J (2015) Temperature requirements for growth and maturation of the warm temperate kelp Eckloniopsis radicosa (Laminariales, Phaeophyta). Phycol Res 63:64–71
Kraemer GP, Carmona R, Chopin T, Neefus C, Tang XR, Yarish C (2004) Evaluation of the bioremediatory potential of several species of the red alga Porphyra using short-term measurements of nitrogen uptake as a rapid bioassay. J Appl Phycol 16:489–497
Lourenco SO, Barbarino E, Nascimento A, Paranhos R (2005) Seasonal variations in tissue nitrogen and phosphorus of eight macroalgae from a tropical hypersaline coastal environment. Cryptogam Algol 26:355–371
Lüning K (1990) Seaweed biogeography and ecophysiology. Wiley, New York, p 527
Mantri VA, Singh RP, Bijo AJ, Kumari P, Reddy CRK, Bhavanath J (2010) Differential response of varying salinity and temperature on zoospore induction, regeneration and daily growth rate in Ulva fasciata (Chlorophyta, Ulvales). J Appl Phycol 23:243–250
Marinho GS, Holdt SL, Birkeland MJ, Angelidaki I (2015) Commercial cultivation and bioremediation potential of sugar kelp, Saccharina latissima, in Danish waters. J Appl Phycol 27:1963–1973
McGlathery KJ, Pedersen MF, Borum J (1996) Changes in intracellular nitrogen pools and feedback controls on nitrogen uptake in Chaetomorpha linum (Chlorophyta). J Phycol 32:393–401
Msuya FE, Kyewalyanga MS, Salum D (2006) The performance of the seaweed Ulva reticulata as a biofilter in a low-tech, low-cost, gravity generated water flow regime in Zanzibar, Tanzania. Aquaculture 254:284–292
Nagasoe S, Shikata T, Yamasaki Y, Matsubara T, Shimasaki Y, Oshima Y, Honjo T (2010) Effects of nutrients on growth of the red-tide dinoflagellate Gyrodinium instriatum Freudenthal et Lee and a possible link to blooms of this species. Hydrobiologia 651:225–238
Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MC, Clay J, Folke C, Lubchenco J, Mooney H, Troell M (2000) Effect of aquaculture on world fish supplies. Nature 405:1017–1024
Nielsen MM, Bruhn A, Rasmussen MB, Olesen B, Larsen MM, Møller HB (2012) Cultivation of Ulva lactuca with manure for simultaneous bioremediation and biomass production. J Appl Phycol 24:449–458
Ohno M, Largo DB, Ikumoto T (1994) Growth rate, carrageenan yield and gel properties of cultured kappa-carrageenan producing red alga Kappaphycus alvarezii (Doty) Doty in the subtropical waters of Shikoku, Japan. J Appl Phycol 6:1–5
Patel R (1971) Growth of members of Cladophorales in experimental culture. Phykos 10:40–53
Paul NA, de Nys R (2008) Promise and pitfalls of locally abundant seaweeds as biofilters for integrated aquaculture. Aquaculture 281:49–55
de Paula Silva PH, McBride S, de Nys R, Paul NA (2008) Integrating filamentous ‘green tide’ algae into tropical pond-based aquaculture. Aquaculture 284:74–80
Periyasamy C, Anantharaman P, Balasubramanian T, Subba Rao PV (2014) Seasonal variation in growth and carrageenan yield in cultivated Kappaphycus alvarezii (Doty) Doty on the coastal waters of Ramanathapuram district, Tamil Nadu. J Appl Phycol 26:803–810
Read P, Fernandes T (2003) Management of environmental impacts of marine aquaculture in Europe. Aquaculture 226:139–163
Reid GK, Chopin T, Robinson SMC, Azevedo P, Quinton M, Belyea E (2013) Weight ratios of the kelps, Alaria esculenta and Saccharina latissima, required to sequester dissolved inorganic nutrients and supply oxygen for Atlantic salmon, Salmo salar, in integrated multi-trophic aquaculture systems. Aquaculture 408–409:34–46
Schumacher J, Dolch T, Reise K (2014) Transitions in sandflat biota since the 1930s: effects of sea-level rise, eutrophication and biological globalization in the tidal Bay Konigshafen, northern Wadden Sea. Helgol Mar Res 68:289–298
Sjøtun K, Fredriksen S, Rueness J (1996) Seasonal growth and carbon and nitrogen in canopy and first-year plants of Laminaria hyperborea (Laminariales, Phaeophyceae). Phycologia 35:1–8
Skriptsova A, Khomenko V, Isakov V (2004) Seasonal changes in growth rate, morphology and alginate content in Undaria pinnatifida at the northern limit in the Sea of Japan (Russia). J Appl Phycol 16:17–21
Tatewaki M (1966) Formation of a crustose sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 6:62–66
Taylor R, Fletcher RL, Raven JA (2001) Preliminary studies on the growth of selected ‘green tide’ algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate. Bot Mar 44:327–336
Thomsen MS, McGlathery KJ (2007) Stress tolerance of the invasive macroalgae Codium fragile and Gracilaria vermiculophylla in a soft-bottom turbid lagoon. Biol Invas 9:499–513
Tsutsui I, Kanjanaworakul P, Srisapoome P, Aue-umneoy D, Hamano K (2010) Growth of giant tiger prawn, Penaeus monodon Fabricius, under co-culture with a discarded filamentous seaweed, Chaetomorpha ligustica (Kützing) Kützing, at an aquarium-scale. Aquacult Int 18:545–553
Tsutsui I, Songphatkaew J, Meeanan C, Aue-umneoy D, Sukchai H, Pinphoo P, Klomkling S, Ganmanee M, Sudo H, Hamano K (2015) Co-culture with Chaetomorpha sp. enhanced growth performance and reduced feed conversion ratio of the giant tiger prawn, Penaeus monodon. Int Aquat Res 7:193–199
Wang QH, Dong SL, Tian XL, Wang F (2007) Effects of circadian rhythms of fluctuating temperature on growth and biochemical composition of Ulva pertusa. Hydrobiologia 586:313–319
Wang X, Liu XH, Wang GY (2011) Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J Integr Plant Biol 53(3):246–252
Yang YF, Chai ZY, Wang Q, Chen WZ, He ZL, Jiang SJ (2015) Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res 9:236–244
Yarish C, Kirkman H, Lüning K (1987) Lethal exposure times and preconditioning to upper temperature limits of some temperate North Atlantic red algae. Helgol Meeresunters 41:323–327
Xu YJ, Lin J (2008) Effect of temperature, salinity, and light intensity on the growth of the green macroalga, Chaetomorpha linum. J World Aquacult Soc 39:847–851
Yokohama Y, Katayama N, Furuya K (1986) An improved type of “product-meter”, a differential gas-volumeter, and its application to measuring photosynthesis of seaweeds. Jpn J Phycol 34:37–42
Yokoyama H, Ishihi Y (2010) Bioindicator and biofilter function of Ulva spp. (Chlorophyta) for dissolved inorganic nitrogen discharged from a coastal fish farm–potential role in integrated multi-trophic aquaculture. Aquaculture 310:74–83
Yoshida T (1998) Marine algae of Japan. Uchida Roukakuho Publishing Co., Ltd, Tokyo, 56 pp
Zhang Y, Gong QL, Li JY (2015) Seasonal nutrient uptake by Chaetomorpha linum (O. F. Muller) Kützing under different environmental factors. Transac Oceanol Limnol 1:50–62 (in Chinese)
Acknowledgements
We sincerely thank Professor Emeritus K. Taniguchi (deceased) of Tohoku University for supporting this study. We are also grateful to the staff of the Miyagi Prefecture Fisheries Technology Center for allowing us to use their seawater for culture experiments and measurements of photosynthesis and nutrient uptake and to Prof. O. Nishimula and Dr. K. Ito for helping with the analysis of seawater nutrient concentrations and tissue nitrogen contents. We are also grateful to T. Igarashi for his support of the C. crassa collection and seawater temperature measurement in Nagatsuraura. This work was partly supported by a grant-in-aid from the Japanese Society for Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, X., Endo, H. & Agatsuma, Y. Seasonal changes in photosynthesis, growth, nitrogen accumulation, and salinity tolerance of Chaetomorpha crassa (Cladophorales, Chlorophyceae). J Appl Phycol 30, 1905–1912 (2018). https://doi.org/10.1007/s10811-017-1381-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1381-2