Abstract
We investigated the impact of different nitrogen (N) and phosphorus (P) compounds and concentrations on the growth of Gyrodinium instriatum Freudenthal et Lee in laboratory experiments, and possible links to blooms of this species at Hakozaki Fishing Port, Fukuoka, Japan. G. instriatum utilized only inorganic N compounds as N sources for growth. In contrast, G. instriatum utilized many inorganic and organic phosphorus compounds. We used the Monod equation to describe the growth rate of G. instriatum in N- or P-limited batch cultures as a function of ambient nutrient concentrations. Kinetic growth parameters for maximum specific growth rate (μmax) and half-saturation nutrient concentration (K S) were 0.57 divisions d−1 and 14.2 μmol l−1, respectively, under N-limitation and 0.65 divisions d−1 and 1.75 μmol l−1, respectively, under P-limitation. Compared with these K S values, all in situ average dissolved inorganic nitrogen (DIN) concentrations in Hakozaki Fishing Port were higher than K S for N, but all in situ average dissolved inorganic phosphorus (DIP) concentrations were lower than K S for P, whether a red tide occurred or not bloom. Moreover, average DIP concentration in April (a month critical to red-tide genesis) of 2004 (a non-red-tide year) was less than half those in 2002 and 2003 (red-tide years). Thus, differences in DIP concentrations may be an important factor controlling blooms of G. instriatum in Hakozaki Fishing Port.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gyrodinium instriatum is a species of dinoflagellate that is distributed worldwide (Toriumi, 1980). Although G. instriatum is non-toxic, red-tide blooms of this species can have noxious effects. For example, in the Gulf of Guayaquil in Ecuador, there was mass mortality in shrimp farms caused by anoxic bottom water following the outbreak of a G. instriatum bloom (Jimenéz, 1993). Blooms of this species may be widely distributed: 50 km along the coast north of the Gulf of Guayaquil (Jimenéz, 1993), 150–200 km2 surface area in the Zhujiang River estuary, China (Wang et al., 2003), and 500 km2 surface area in Chi Bay, China (Zhu et al., 2004). These reports also indicate that blooms of G. instriatum cause serious damage to fishery products. However, there are few studies that examine the relationships between the environmental factors and the growth of G. instriatum or closely related species such as Gyrodinium fissum (Levander) Kofoid et Swezy (Kofoid & Swezy, 1921) and Gyrodinium uncatenum Hulburt (Hulburt, 1957; Campbell, 1973).
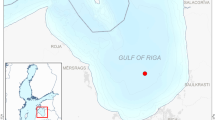
From 2002 to 2004, we investigated the species composition of phytoplankton in Hakozaki Fishing Port (lat 33°37′30″N, long 130°25′00″E), which is located inside the breakwater in the southeastern area of Hakata Bay, Japan (Fig. 1). In 2002 and 2003, G. instriatum caused intense red tides (up to over 7,000 cells ml−1 at the surface) in mid-May. In 2004, however, there was no outbreak of G. instriatum, although low densities of cells were observed. To explain the annual variability of G. instriatum in the port, our previous studies examined the effects of temperature, salinity, and irradiance on the growth of G. instriatum (Nagasoe et al., 2006a) and the inhibitory effects of Skeletonema costatum (Greville) Cleve (Nagasoe et al., 2006b). However, the effects of nutrients on the growth of G. instriatum have not yet been examined.
In environments where production is limited by a single nutrient, competitive exclusion should result in the species best able to utilize this nutrient being dominant (Tilman, 1976). A species that can utilize nutrients in various types of compounds or that requires a minimal concentration of growth-limiting nutrients has a competitive advantage. Thus, for a given red-tide species, information about the types of nutrient compounds used and the concentrations needed for growth is important for identifying the mechanisms of red-tide occurrence. To date, in the laboratory, nutritional limitation of various phytoplankton growth rates has been examined by using either the Monod (1949) or the Droop (1968) equation. The former relates the growth rate (μ) to ambient concentrations of the limiting dissolved nutrients (S); the latter relates μ to the intracellular concentrations of the limiting nutrients (cell quota). In our investigation, we analyzed ambient dissolved inorganic nutrient concentrations from 2002 to 2004. To investigate possible links between the differences in nutrient concentrations and red-tide outbreaks, we used the Monod equation to examine the effect of ambient nutrient concentrations on the growth of G. instriatum.
In this study, we used batch cultures in the laboratory to examine (1) the ability of G. instriatum to utilize various organic and inorganic nitrogen and phosphorus compounds, and (2) the effects of nutrient concentrations on the growth of G. instriatum.
Materials and methods
Organisms and culture conditions
The clonal strain of G. instriatum used for this study was obtained by isolating a vegetative cell that germinated from bottom sediment incubated in culture flasks in the laboratory. The sediment was collected at Hakozaki Fishing Port in December 2004 and incubated at 20°C in sterilized modified SWM-3 medium (Yamasaki et al., 2007) containing GeO2 (0.1 mg l−1) under a photon flux density of 150 μmol m−2 s−1 (12:12 h light:dark [L:D]). We obtained an axenic strain of the isolate by using the method based on the phototactic ability of phytoflagellates (Imai & Yamaguchi, 1994) and by washing with capillary pipettes. A sterility test was carried out using STP (seawater with trypticase) test medium (Tatewaki & Provasoli, 1964) and the fluorochrome (4′,6-diamidino-2-phenylindole, DAPI) staining method (Porter & Feig, 1980); the test results verified that this strain was axenic. The stock culture was maintained in 100-ml flasks containing 50 ml of modified SWM-3 medium at 20°C under 150 μmol photons m−2 s−1 and 12:12 h L:D. The seawater used for the culture medium was collected from within the Tsushima Warm Current in an area around Oki Island (lat 34°24′58″N, long 130°12′20″E), in the Sea of Genkai, Japan, and aged in the laboratory for more than 1 year. And this seawater originally contained 2.5 μmol l−1 of DIN and 0.08 μmol l−1 of DIP.
Utilization of nitrogen (N) and phosphorus (P) compounds
The ability of G. instriatum to utilize various N and P compounds for growth was examined in batch culture experiments. Twenty-six nitrogen sources and 15 phosphorus sources were used in this experiment (Table 1). These are representative of natural inorganic and organic N and P sources in the environment (Sharp, 1983; van Boekel, 1991). The concentrations of N and P sources were set at 100 μmol l−1 and 25 μmol l−1, respectively. To avoid the decomposition of N and P sources during autoclave treatment (McLachlan, 1973), all compounds were added individually to the autoclaved medium (N- or P-depleted modified SWM-3) in screw-cap test tubes (18 × 180 mm), after filtration through a 0.1-μm pore size disposable syringe filter (Millipore Corporation, Bedford, MA, USA).
Gyrodinium instriatum cells for inoculation were pre-incubated for a week in N- or P-depleted media to remove excess N or P within the cells. Acclimated cells were inoculated into triplicate test tubes that contained 15 ml of media having N or P compounds. The control medium consisted of N- or P-depleted medium without any additional N or P sources. Inoculum sizes were adjusted to result in test cultures at an initial density of about 500 cells ml−1. Every two days over a 6-day period, 0.5-ml samples were taken from each test tube to determine cell densities. To determine the maximum yield, whole vegetative cells of G. instriatum in 10- to 100-μl triplicate subsamples were counted in a Sedgwick-Rafter counting chamber under an upright light microscope. Cultures were incubated under conditions of 20°C, salinity of 30, and photon flux density of 150 μmol m−2 s−1 of cool-white fluorescent illumination on a 12:12 h L:D cycle.
Effects of nitrogen (N) and phosphorus (P) concentrations on growth
The effects of nutrient concentrations on growth of G. instriatum were examined by batch culture experiments following the method of Monod (Monod, 1949). NaNO3 and NH4Cl were used as nitrogen sources and NaH2PO4 was used as a phosphorus source. Stock solutions of these nutrient sources were filtered through a 0.1-μm pore size disposable syringe filter (Millipore Corporation) into 50 ml of autoclaved N- or P-depleted modified SWM-3 medium in 100-ml flasks. The growth of G. instriatum was examined at eleven different concentrations of each nutrient (NaNO3 and NH4Cl: 0, 1, 2.5, 5, 10, 25, 50, 100, 250, 500, and 1,000 μmol l−1; NaH2PO4: 0, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50, and 100 μmol l−1). The methods for pre-incubation, inoculum sizes, cell counting and culture conditions were the same as described previously for N and P utilization experiments. The growth rate (μ; divisions d−1) for each culture determined to be in the exponential growth phase was calculated using the method of Guillard (1973). Data for measuring growth rate were collected up until day 6.
The Monod equation (1949) was used to describe the relationship between growth rate and ambient nutrient concentration:
where μ is the growth rate (divisions d−1), μmax is the maximum growth rate (divisions d−1), S is the nutrient concentration (μmol l−1), and K S is the nutrient concentration at μmax/2 (the half-saturation nutrient concentration).
Field study site and sampling method
Hakata Bay is a small and shallow bay (Fig. 1; 20 km east–west, 10 km north–south; maximum depth, 23 m; tidal range, 2 m). The southeastern section of the bay is closed off by a large breakwater, approximately 4 km long but with several openings for boat traffic. Our investigation was conducted in Hakozaki Fishing Port (Fig. 1; lat 33°37′30″N, long 130°25′00″E; water depth, 2.5–4.5 m), located inside the breakwater in the southeastern area of the bay.
Seawater sampling was conducted daily from 1 April to 30 June in 2002 to 2004 from the pier in Hakozaki Fishing Port (Fig. 1). Seawater was collected using a 1-l plastic bottle with a small lead weight attached. For sampling bottom seawater, the bottle was closed with a rubber plug attached to a string and submerged slowly so as not to resuspend bottom sediments, and then opened at depth by pulling on the string and removing the plug. Between 10:30 and 11:00 h. local time on each sampling date, seawater was collected from the surface and from the bottom layer (30 cm above the bottom). Environmental parameters (water temperature and salinity) were then immediately measured in the field and the samples were brought to the laboratory within 15 min of collection. These water samples were used for phytoplankton counts and subsequent measurement of dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphorus (DIP) concentrations. Details of these measurements are given below.
Phytoplankton counts
Cells of phytoplankton (G. instriatum and diatoms), and zooplankton (copepods + ciliates + Myrionecta rubra (Lohmann) Jankowski) were counted in the seawater sampled from Hakozaki Fishing Port; in a precise sense, copepods should be treated as individuals because each organism is multicellular, but we treated copepods as unicellular for descriptive purposes. The sample bottle was gently inverted five times before removing a subsample for cell counting. Diatoms, vegetative G. instriatum cells and zooplankton were counted in 1 ml of the sample using an upright light microscope and a counting slide with engraved lines (Rigosha Co., Ltd. Saitama, Japan). Cell densities of the organisms counted on each sampling date were determined as the average of the cell densities in surface and bottom samples. If no vegetative G. instriatum (sp.) cells could be detected in either the surface or bottom water sample, we tried to find this organism from concentrated samples. To concentrate the plankton in the samples, the 1-l surface and bottom samples were combined (2 l total) and concentrated to about 5 ml by gravity filtration through a membrane filter with a 5.0-μm pore size (Millipore Corporation) on a 47-mm polysulphone filter holder (Advantec Toyo Kaisha, Ltd., Tokyo, Japan). Vegetative cells of G. instriatum were counted in 100 μl of the concentrated sample using an upright light microscope and a counting slide with engraved lines (Rigosha). Seawater samples were not fixed before counting phytoplankton because counting occurred within 1 h of sample collection.
Analysis of in situ nutrient concentrations
Seawater samples for nutrient analysis (50 ml) were filtered through a syringe filter with a 0.22-μm pore size (Millipore Corporation) and then frozen (−30°C) until they were analyzed for DIN and DIP concentrations with an autoanalyzer (TRAACS 800; Bran + Luebbe Co., Hamburg, Germany) using the method of Strickland & Parsons (1972). In addition, during bloom events, we replaced several syringe filters in order to filtrate a seawater sample for nutrient analysis.
Results
Utilization of nitrogen (N) and phosphorus (P) compounds
Of the 26 nitrogen compounds provided as sources of N, G. instriatum grew only in the media to which inorganic nitrogen sources (NaNO3, NaNO2, and NH4Cl) were added (Fig. 2). However, the maximum yield (cells ml−1) in media with NH4Cl was lower than in that with NaNO3 or NaNO2 (about 49.8% of the maximum yield on NaNO3) (one-way ANOVA; P < 0.05). The maximum yields on all of the organic nitrogen compounds tested (urea, uric acid, 20 amino acids, and taurine) were not significantly different than that in the control (N-depleted media) (one-way ANOVA; P > 0.05). Thus, G. instriatum is apparently unable to utilize organic nitrogen for growth.
Utilization of nitrogen compounds by G. instriatum. The control had no added N source. Values are the mean and error bars are the SD (n = 3). See Table 1 for an explanation of compound abbreviations
In contrast, G. instriatum utilized a variety of inorganic and organic phosphorus compounds for growth (Fig. 3). The maximum yields on all 15 P sources tested were at least 10 times as high as those of the control (P-depleted media).
Utilization of phosphorus compounds by G. instriatum. The control had no added P source. Values are the mean and error bars are the SD (n = 3). See Table 1 for an explanation of compound abbreviations
Effects of nitrogen and phosphorus concentration on growth
We compared the growth curves of G. instriatum at the various concentrations of NaNO3, NH4Cl, and NaH2PO4 (Fig. 4). Growth of G. instriatum was observed at NaNO3 concentrations of 25 μmol l−1 and higher; the maximum yield at 25 μmol l−1 NaNO3 was twice the density at inoculation. The maximum yields increased with increasing concentration of NaNO3 to 250 μmol l−1, and became saturated at 500 μmol l−1; the cell densities at 500 and 1,000 μmol l−1 were both about 20,000 cells ml−1 (Fig. 4A). G. instriatum in 10–250 μmol l−1 NH4Cl grew to cell densities significantly greater than in the controls (one-way ANOVA; P < 0.01). Growth in 1–5 μmol l−1 NH4Cl did not differ significantly from the controls (Fig. 4B) (one-way ANOVA; P > 0.05). The highest maximum yield in medium containing NH4Cl (about 4,400 cells ml−1) was at 250 μmol l−1; this was markedly lower than the highest maximum yield with NaNO3 (about 20,000 cells ml−1 at 500 and 1,000 μmol l−1). When G. instriatum was inoculated into the medium with 500 and 1,000 μmol l−1 NH4Cl, the algal cells burst immediately.
In media containing NaH2PO4, growth of G. instriatum was observed at concentrations >1 μmol l−1, with maximum yields increasing with increases in NaH2PO4 concentrations from 1 to 100 μmol l−1 (Fig. 4C).
We determined the relationship between growth rates of G. instriatum and the concentrations of NaNO3 and NaH2PO4 (Fig. 5), but not for NaH4Cl, because of the low number of concentrations of NH4Cl concentrations that resulted in growth. Based on the approximation of parameters using the non-linear least-squares method, the values of μmax and K S for NaNO3 were 0.57 divisions d−1 and 14.2 μmol l−1, respectively, and for NaH2PO4 are 0.65 divisions d−1 and 1.75 μmol l−1, respectively. Using these values, Equations 2 and 3 describe the growth rate of G. instriatum as a function of each inorganic nutrient concentration (S):
Growth rates (μ) of G. instriatum as a function of ambient N or P concentration (S), as determined from the Monod equation (1949). Symbols are the experimental mean values and error bars are the SD (n = 3). Solid black lines show simulated values
Field investigations of G. instriatum abundance
Gyrodinium instriatum cell densities in Hakozaki Fishing Port were calculated daily from April to June in 2002–2004 (Fig. 6). In 2002 and 2003, G. instriatum increased in abundance from the end of April and formed intensive red tides in mid-May (average of surface and bottom cell density; 3,721 cells ml−1 in 2002 and 2,382 cells ml−1 in 2003). Subsequently, cell densities gradually decreased, and G. instriatum cells were not observed after mid-June in 2002 or 2003. In contrast, there was no outbreak of G. instriatum in April–June 2004. Whether a red tide occurred or not, low cell densities of G. instriatum were observed in early April. However, cell density of this organism did not increase during April 2004.
In situ concentrations of DIN and DIP from 2002 to 2004
Concentrations of DIN and DIP were monitored daily from April to June from 2002 to 2004 at Hakozaki Fishing Port (Fig. 7A, B). The concentrations of dissolved inorganic macronutrients are of particular interest during April because this period is important for the initial proliferation of G. instriatum, which has subsequent outbreaks in mid-May of bloom years at Hakozaki Fishing Port (Fig. 6).
Daily values for A dissolved inorganic nitrogen (DIN), B dissolved inorganic phosphorus (DIP), and C G. instriatum cell density at Hakozaki Fishing Port during the study period (April–June of 2002–2004). The values in boxes are average concentrations (μmol l−1) of each nutrient during the periods indicated by arrows
Nitrate accounted for more than 80% of DIN concentrations. Therefore, we considered fluctuations in DIN concentrations in the port as changes in nitrate levels. Both DIN and DIP concentrations fluctuated widely in the red-tide years 2002 and 2003; we observed many pulses of higher nutrient concentrations. In contrast, in 2004 (without a red tide), and particularly during April, there were only occasional pulses of higher nutrient levels; the concentrations of nutrients and the magnitude of changes remained low during April. The average concentrations during April (and the average concentration from April to June) in each year were 35.8 μmol l−1 (39.5 μmol l−1) in 2002, 51.2 μmol l−1 (46.1 μmol l−1) in 2003, and 29.8 μmol l−1 (39.3 μmol l−1) in 2004 for DIN (Fig. 7A), and 0.7 μmol l−1 (0.5 μmol l−1) in 2002, 0.5 μmol l−1 (0.5 μmol l−1) in 2003, and 0.2 μmol l−1 (0.5 μmol l−1) in 2004 for DIP (Fig. 7B). The average concentrations of DIN and DIP during April 2004 (non-red-tide year) were lower than those in April 2002 and 2003 (red-tide years) (Fig. 7). These differences were statistically significant for DIN only between 2003 and 2004 (one-way ANOVA; P < 0.05), but for DIP the differences were significant between both red-tide years (2002 and 2003) and the non-red-tide year (2004) (one-way ANOVA; P < 0.05). In particular, the average DIP concentration during April in 2004 was less than half that in 2002 and 2003 (Fig. 7B).
Following the G. instriatum red tides (more than 2,000 cells ml−1) in 2002 and 2003, the blooms gradually declined and disappeared almost entirely by early to mid-June in 2002 and 2003 (Fig. 7C). During these periods of declining G. instriatum blooms, the concentrations of DIP and the magnitude of the variations remained at low levels; the average DIP concentrations during the period of decline were 0.2 μmol l−1 in 2002 and 2003 (Fig. 7B).
Daily fluctuations in physical and biological parameters in Hakozaki Fishing Port
Daily water temperatures and salinities in Hkozaki Fishing Port are shown in Fig. 8. The temperature on 1 April was 15.1°C in 2002, 13.0°C in 2003, and 13.3°C in 2004, and increased steadily thereafter (Fig. 8A). Linear regression analysis yielded the same slope (rate of temperature increase) of 0.11°C d−1 during the study period in each year (r = 0.97, 0.97, and 0.98 in 2002, 2003, and 2004, respectively). Salinities from April to June ranged from 22.2 to 33.0 in 2002, 21.6 to 34.1 in 2003, and 20.2 to 33.4 in 2004 (Fig. 8B).
Zooplankton densities showed inconsistent patterns during the April–June period in the 3 years of observations (Table 2). The average monthly densities were: April, 0.4 cells ml−1 (2002), 1.3 cells ml−1 (2003), and 0.1 cells ml−1 (2004); May, 1.6 cells ml−1 (2002), 0.3 cells ml−1 (2003), and 0 cells ml−1 (2004); and June, 2.9 cells ml−1 (2002), 2.6 cells ml−1 (2003) and 3.2 cells ml−1 (2004) (Table 2). Zooplankton densities were lower in April and May than in June in each year.
We compared the abundance of G. instriatum and diatoms from April to June in the 2002–2004 period at Hakozaki Fishing Port (Fig. 9). During May in 2002 and 2003, there was a relatively long period when diatoms remained at low cell densities, and G. instriatum became abundant during this period. In contrast, in 2004, diatoms remained at high cell densities throughout the study period; especially during April and May, decreases in diatom cell density were followed immediately by a return to high cell densities. April is the most important month for the initial proliferation of G. instriatum, and the average diatom cell density during April was 458.7 cells ml−1 in 2002, 101.9 cells ml−1 in 2003, and 593.3 cells ml−1 in 2004.
Discussion
The ability to use the various nutrient components available for growth differs among phytoplankton species, and differences in the ability to utilize organic nutrients in particular is one of the factors that accounts for in situ species succession (Bonin & Maestrini, 1981). In natural aquatic environments, urea represents the majority of the organic nitrogen, and is important for phytoplankton as a nitrogen source (Sharp, 1983). Dissolved amino acids are also utilized by several phytoplankton species (Iwasaki, 1979; Flynn & Butler, 1986). In this study, however, G. instriatum grew only in culture media containing inorganic nitrogen (nitrate, nitrite, and ammonium), and not in any of the media with organic nitrogen (Fig. 2). Thus, a continuous supply of inorganic nitrogen would be essential for G. instriatum blooms.
Among the P compounds tested, p-nitrophenylphosphate (NPP), which is not a natural component of dissolved organic phosphorus, has been used as an artificial substrate to test for alkaline phosphatase activity (APA) (van Boekel, 1991). Our results indicate that G. instriatum has APA. There have been several reports that glycerophosphate (GYP), one of the organic phosphorus species that we tested, is utilized by many red-tide phytoplankton species (Mahoney & McLaughlin, 1977; Iwasaki, 1979). However, the raphidophytes Chattonella antiqua (Hada) Ono (Nakamura & Watanabe, 1983; Yamaguchi, 1996), Chattonella marina (Subrahmanyan) Hara et Chihara (Yamaguchi, 1996), and Heterosigma akashiwo (Hada) Hada (Watanabe et al., 1982), and the diatom Skeletonema costatum (Yamaguchi, 1996) cannot use GYP for growth. Therefore, compared to these and similar species, G. instriatum would have an advantage in forming red-tides in terms of utilizing organic phosphorus.
Our results also indicate that G. instriatum cannot achieve high cell density when ammonium is at very high concentrations. High concentrations of ammonium are known to inhibit the growth of many phytoplankton species, although the degree of inhibition varies with the species (Norris & Chew, 1975; Yamaguchi, 1994). For example, the ammonium concentrations that inhibit growth are ≥50 μmol l−1 for H. akashiwo (Takahashi & Fukazawa, 1982) and Cochlodinium polykrikoides Margareff (Lee, 2008), and ≥150 μmol l−1 for C. antiqua (Nakamura & Watanabe, 1983). However, the growth of Gymnodinium nagasakiense Takayama et Adachi (=Karenia mikimotoi (Miyake et Kominami ex Oda) Hansen et Moestrup) (Yamaguchi, 1994) and Heterocapsa circularisquama Horiguchi (Yamaguchi et al., 2001) was not inhibited at 250 μmol l−1 ammonium. In the present study, we observed growth of G. instriatum at 250 μmol l−1, but substantial inhibition of growth at ammonium concentrations of 500 μmol l−1 and higher. Thus, G. instriatum has a higher ammonium tolerance than H. akashiwo, C. polykrikoides and C. antiqua. However, our field observations indicate that G. instriatum never encountered such high concentrations because the ammonium concentrations in Hakozaki Fishing Port were less than 10–20 μmol l−1.
The half-saturation nutrient concentration (K S) have been reported for the growth of several harmful flagellates. These values for N and P were, respectively, 0.8 and 0.14 μmol l−1 for G. nagasakiense (= K. mikimotoi) (Yamaguchi, 1994), 2.16 and 1.39 μmol l−1 for C. polykrikoides (Kim, 2003), 0.3 and 0.2 μmol l−1 for H. akashiwo (Zhang et al., 2006), and 9.0 and 0.8 μmol l−1 for Chattonella subsalsa (Zhang et al., 2006). In comparison with these species, G. instriatum (14.2 μmol l−1 for K NS and 1.75 μmol l−1 for K PS: in this study) has a high nutrient demand for growth.
Gyrodinium instriatum has a resting cyst stage in its life cycle (Uchida et al., 1996; Shikata et al., 2008). Therefore, studies of the developmental mechanisms of G. instriatum red tides should analyze environmental factors during the specific period when vegetative cells are in the water column. Shikata et al. (2008) found that excystment of G. instriatum occurred at low temperatures (10 and 15°C). We found vegetative G. instriatum cells in the water column at Hakozaki Fishing Port on the first day of April, when water temperatures were already 10–15°C (Fig. 8A), regardless of subsequent red-tide occurrence (Fig. 6). During April, numbers of G. instriatum vegetative cells increased progressively in the red-tide years (2002 and 2003), but failed to increase in the non-red-tide year (2004) (Fig. 6). Thus, April appears to be the most important period for the initial proliferation of germinated G. instriatum, which has outbreaks in mid-May of bloom years at Hakozaki Fishing Port.
We consider whether some factor other than nutrient concentrations (i.e., temperature, salinity, grazer populations or interspecific relationships with diatoms) determined the presence or absence of G. instriatum red tides from 2002 to 2004 at Hakozaki Fishing Port. Water temperatures during the study period each year were almost the same (Fig. 8A). Moreover, G. instriatum is a euryhaline species, with an optimum salinity range for growth from 10 to 35 (Nagasoe et al., 2006a). The salinity in Hakozaki Fishing Port during the study period ranged from 20.2 to 34.1 over the 3-year study (2002–2004; Fig. 8B). Thus, the salinity was always within the optimum salinity range for growth of G. instriatum, even in the non-red-tide year (2004).
There were no consistent differences in the time of appearance or the numbers of zooplankton from April to June between red-tide and non-red-tide years. Even though the grazing pressure in 2004 was the lowest among the 3 years, there was little growth of G. instriatum during April of this year (Table 2). Thus, it is unlikely that direct grazing pressure by zooplankton inhibited the progress of G. instriatum blooms.
Phytoplankton species such as the diatom S. costatum have been shown to have an allelopathic effect on G. instriatum. The growth rates and maximum cell densities of G. instriatum decreased in cultures containing high densities of S. costatum cells (Nagasoe et al. 2006b). Our data for G. instriatum and diatom abundances from April to June in the 2002–2004 period at Hakozaki Fishing Port show long periods in May 2002 and 2003 when diatoms remained at low cell densities and G. instriatum grew quickly to form dense blooms (Fig. 9). The absence of a similar period of low diatom cell density in 2004 suggests that the allelopathic effect of diatoms may influence outbreaks of G. instriatum red tides. However, April is the most important month for the initial proliferation of G. instriatum, and the average diatom cell density during April 2002 (red-tide year: 458.7 cells ml−1) was almost as high as in 2004 (non-red-tide year: 593.3 cells ml−1). However, it was only in April 2002 and not in 2004 that G. instriatum grew steadily, leading to the rapid increase in densities during the period of low diatom cell density in May (Figs. 6, 9). Thus, some factor other than allelopathic interactions with diatoms must influence the initial proliferation of G. instriatum during April.
According to our field measurements of nutrients indicated average DIN and DIP concentrations during April, the values of the non-red-tide year (2004) were lower than those in the red-tide years (2002 and 2003). In comparison with the K S values that we determined in the laboratory, all in situ average DIN concentrations were higher than K NS, but all in situ average DIP concentrations were lower than K PS, whether a red tide occurred or not. This suggests that G. instriatum growth was consistently limited by DIP concentrations at Hakozaki Fishing Port. The average DIP concentration in April 2004 was less than half that in 2003 and less than one-third that in 2002. In addition, DIP concentrations decreased and remained low during the periods of bloom decline in 2002 (average, 0.2 μmol l−1) and 2003 (0.2 μmol l−1) (Fig. 7B). These DIP concentrations are of the same order as the average DIP concentration in April 2004. This combined evidence suggests that low DIP concentrations affected the initial proliferation of G. instriatum during April 2004. If true, this suggests that the DIP concentrations that restrict G. instriatum growth in the natural environment are lower than the K PS estimated in this study. Future studies should examine minimum cell quotas for G. instriatum under semi-continuous N- or P-limitation in chemostat culture.
Our culture experiments indicate that G. instriatum can utilize various dissolved organic phosphorus (DOP) compounds for growth (Fig. 3). This implies that G. instriatum populations at Hakozaki Fishing Port could utilize any available DOP to grow to red-tide densities, even if the DIP concentration was below K PS. Unfortunately, we only measured DOP at Hakozaki Fishing Port in 2004, so we cannot compare DOP concentrations between red-tide and non-red-tide years. Our 2004 results indicate very low DOP concentrations (range, 0–0.25 μmol l−1). Yanagi (1999) and Yanagi & Onitsuka (2000) reported seasonal variations of nutrients in Hakata Bay from April 1993 to March 1994. They found little DOP loading from the surrounding land or the bay bottom through the year, and the DOP concentrations were high from July to October due to large phytoplankton populations. In other words, phytoplankton are likely to be the major source of DOP in Hakata Bay. In our study, diatom cell densities were slightly lower during April of 2002 and 2003 than in April 2004 (Fig. 9), so there would not be much difference in the April DOP concentrations from 2002 to 2004. Thus, we consider DIP depletion as one of the major factors that prevented a G. instriatum red tide in 2004. Future studies should investigate the role of DOP in the occurrence of G. instriatum red tides.
Conclusion
Our results indicate: (1) the nitrate-rich environment of Hakozaki Fishing Port should be favorable for growth of G. instriatum, which readily utilizes DIN but cannot utilize organic nitrogen for growth. (2) The concentration of DIP at Hakozaki Fishing Port in April 2004 (non-red-tide year) was the lowest during the 3-year period of this study (2002–2004). (3) Although G. instriatum can use DOP for growth, there was little detectable DOP at Hakozaki Fishing Port in 2004. We conclude that phosphorus depletion may be an important factor controlling the blooms of G. instriatum at Hakozaki Fishing Port.
References
Bonin, D. J. & S. Y. Maestrini, 1981. Importance of organic nutrients for phytoplankton growth in natural environments: implications for algal species succession. Canadian Bulletin of Fisheries and Aquatic Sciences 210: 279–291.
Campbell, P. H., 1973. Studies on brackish water phytoplankton. Sea Grant Publication UNC-SG-73-07, University of North Carolina. Chapel Hill, North Carolina.
Droop, M. R., 1968. Vitamin B12 and marine ecology. IV. The kinetics of uptake growth and inhibition in Monochrysis lutheri. Journal of the Marine Biological Association of the United Kingdom 48: 689–733.
Flynn, K. J. & I. Butler, 1986. Nitrogen sources for the growth of marine microalgae: role of dissolved free amino acids. Marine Ecology Progress Series 34: 281–304.
Guillard, R. R. L., 1973. Division rate. In Stein, J. R. (ed.), Handbook of Phycological Methods: Culture Methods and Growth Measurements. Cambridge University Press, Cambridge: 289–311.
Hulburt, E. M., 1957. The taxonomy of unarmored Dinophyceae of shallow embayments on Cape-Cod, Massachusetts. The Biological Bulletin 112: 196–219.
Imai, I. & M. Yamaguchi, 1994. A simple technique for establishing axenic cultures of phytoflagellates. Bulletin of the Japanese Society of Microbial Ecology 9: 15–17.
Iwasaki, H., 1979. Physiological ecology of red tide flagellates. In Levandovsky, M. & S. H. Hunter (eds), Biochemistry and Physiology of Protozoa, Vol. 1, 2nd ed. Academic Press, New York: 357–393.
Jimenéz, R., 1993. Ecological factors related to Gyrodinium instriatum bloom in the inner estuary of the Gulf of Guayaquil. In Smayda, T. J. & Y. Shimizu (Eds), Toxic Phytoplankton Blooms in the Sea. Proceedings of the 5th International Conference on Toxic Marine Phytoplankton, Newport, Rhode Island, U.S.A., 28 October–1 November 1991. Elsevier, Amsterdam: 257–262.
Kim, D. I., 2003. Physiological and ecological studies on harmful red tide dinoflagellate Cochlodinium polykrikoides (Margalef). Ph.D. dissertation, Kyushu University, Fukuoka (in Japanese).
Kofoid, C. A. & O. Swezy, 1921. The free-living unarmored Dinoflagellata. Memoirs University of California, Vol. 5. California Press, Berkeley.
Lee, Y. S., 2008. Utilization of various nitrogen, phosphorus, and selenium compounds by Cochlodinium polykrikoides. Journal of Environmental Biology 29: 799–804.
Mahoney, J. B. & J. J. A. McLaughlin, 1977. Association of phytoflagellate blooms in Lower New York Bay with hypertrophication. Journal of Experimental Marine Biology and Ecology 28: 53–65.
McLachlan, J., 1973. Growth media-marine. In Stein, J. R. (ed.), Handbook of Phycological Methods. Culture Methods and Growth Mesurements. Cambridge University Press, Cambridge: 25–51.
Monod, J., 1949. The growth of bacterial cultures. Annual Review of Microbiology 3: 371–394.
Nagasoe, S., D. I. Kim, Y. Shimasaki, Y. Oshima, M. Yamaguchi & T. Honjo, 2006a. Effects of temperature, salinity and irradiance on the growth of the red tide dinoflagellate Gyrodinium instriatum Freudenthal et Lee. Harmful Algae 5: 20–25.
Nagasoe, S., S. Toda, Y. Shimasaki, Y. Oshima, T. Uchida & T. Honjo, 2006b. Growth inhibition of Gyrodinium instriatum (Dinophyceae) by Skeletonema costatum (Bacillariophyceae). African Journal of Marine Science 28: 325–329.
Nakamura, Y. & M. M. Watanabe, 1983. Growth characteristics of Chattonella antiqua Part 2. Effects of nutrients on growth. Journal of the Oceanographical Society of Japan 39: 151–155.
Norris, L. & K. K. Chew, 1975. Effect of environmental factors on growth of Gonyaulax catenella. In LoCicero, V. R. (ed.), Proceedings of the first international conference on Toxic Dinoflagellate Blooms, November, 1974, Boston, Massachusetts. Massachusetts Science and Technology Foundation, Massachusetts: 143–152.
Porter, K. G. & Y. S. Feig, 1980. The use of DAPI for identifying and counting aquatic microflora. Limnology and Oceanography 25: 943–948.
Sharp, J. H., 1983. The distributions of inorganic nitrogen and dissolved and particulate organic nitrogen in the sea. In Carpenter, E. J. & D. G. Capone (eds), Nitrogen in the Marine Environment. Academic Press, New York: 1–35.
Shikata, T., S. Nagasoe, T. Matsubara, Y. Yamasaki, Y. Shimasaki, Y. Oshima, T. Uchida, I. R. Jenkinson & T. Honjo, 2008. Encystment and excystment of Gyrodinium instriatum Freudenthal et Lee. Journal of Oceanography 64: 355–365.
Strickland, J. D. H. & T. R. Parsons, 1972. A Practical Handbook of Seawater Analysis, Vol. 167, 2nd ed. Bulletin of the Fiseries Research Board of Canada, Ottawa.
Takahashi, M. & N. Fukazawa, 1982. A mechanism of “red-tide” formation. II. Effect of selective nutrient stimulation on the growth of different phytoplankton species in natural water. Marine Biology 70: 267–273.
Tatewaki, M. & L. Provasoli, 1964. Vitamin requirements of three species of Antithamnion. Botanica Marina 6: 193–203.
Tilman, D., 1976. Ecological competition between algae—experimental confirmation of resource-based competition theory. Science 192: 463–465.
Toriumi, S., 1980. Synopsis of red tide organisms. Fish Agency, Japan Government, Tokyo (in Japanese).
Uchida, T., Y. Matsuyama, M. Yamaguchi & T. Honjo, 1996. The life cycle of Gyrodinium instriatum (Dinophyceae) in culture. Phycological Research 44: 119–123.
van Boekel, W. H. M., 1991. Ability of Phaeocystis sp. to grow on organic phosphates: direct measurement and prediction with the use of an inhibition constant. Journal of Plankton Research 13: 959–970.
Wang, H. K., L. M. Huang, X. P. Huang, X. Y. Song, H. J. Wang, N. J. Wu & C. Li, 2003. A red tide caused by Gyrodinium instriatum and its environmental characters in Zhujiang River estuary. Redai Haiyang Xuebao 22: 55–62. (in Chinese with English abstract).
Watanabe, M. M., Y. Nakamura, S. Mori & S. Yamochi, 1982. Effects of physico-chemical factors and nutrients on the growth of Heterosigma akashiwo Hada from Osaka Bay, Japan. Japanese Journal of Phycology 30: 279–288.
Yamaguchi, M., 1994. Physiological ecology of the red tide flagellate Gymnodinium nagasakiense (Dinophyceae). Mechanism of the red tide occurrence and its prediction. Bulletin of Nansei National Fisheries Research Institute 27: 251–394. (in Japanese with English abstract).
Yamaguchi, M., 1996. Keisou-rui no eiyouen-riyou-tokusei oyobi Chattonella tono eiyouen-kyougou. In Agriculture, Forestry and Fisheries Research Council, Ministry of Agriculture, Forestry and Fisheries of Japan (ed.), Yūgai-Akashio no Seitaigakuteki-Seigyo ni yoru Higai-Boujyo-Gijyutsu no Kaihatsu ni kansuru Kenkyu. Nourinkousai-kai, Tokyo: 44–59 (in Japanese).
Yamaguchi, M., S. Itakura & T. Uchida, 2001. Nutrition and growth kinetics in nitrogen- or phosphorus-limited cultures of the ‘novel red tide’ dinoflagellate Heterocapsa circularisquama (Dinophyceae). Phycologia 40: 313–318.
Yamasaki, Y., S. Nagasoe, T. Matsubara, T. Shikata, Y. Shimasaki, Y. Oshima & T. Honjo, 2007. Allelopathic interactions between the bacillariophyte Skeletonema costatum and the raphidophyte Heterosigma akashiwo. Marine Ecology Progress Series 339: 83–92.
Yanagi, T., 1999. Seasonal variations in nutrient budgets of Hakata Bay, Japan. Journal of Oceanography 55: 439–448.
Yanagi, T. & G. Onitsuka, 2000. Seasonal Variation in lower trophic level ecosystem of Hakata Bay, Japan. Journal of Oceanography 56: 233–243.
Zhang, Y., F. Fu, E. Whereat, K. J. Coyne & D. A. Hutchins, 2006. Bottom-up controls on a mixed-species HAB assemblage: A comparison of sympatric Chattonella subsalsa and Heterosigma akashiwo (Raphidophyceae) isolates from the Delaware Inland Bays, USA. Harmful Algae 5: 310–320.
Zhu, X. S., B. Yi, Y. H. Dong & L. F. Yang, 2004. A primary study on one of the “bilateral” red tide at Chi Bay of Pearl River Estuary. Haiyang Huanjing Kexue 23: 41–44. (in Chinese with English abstract).
Acknowledgements
The authors thank the staff of Fukuoka Fisheries and Marine Technology Research Center (FFMTRC), especially C. Yamamoto, for permitting us to use the autoanalyzer and other FFMTRC facilities. We also thank Dr. Y. Matsuyama for providing helpful insights on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: D. P. Hamilton
Rights and permissions
About this article
Cite this article
Nagasoe, S., Shikata, T., Yamasaki, Y. et al. Effects of nutrients on growth of the red-tide dinoflagellate Gyrodinium instriatum Freudenthal et Lee and a possible link to blooms of this species. Hydrobiologia 651, 225–238 (2010). https://doi.org/10.1007/s10750-010-0301-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0301-0