Abstract
The marcoalga Ulva pertusa was cultured under (20 ± 2)°C, (20 ± 4)°C, (20 ± 6)°C, (20 ± 8)°C and (20 ± 10)°C circadian rhythms of fluctuating temperature conditions, and constant temperature of 20°C was used as the control. The growth rate of macroalga at (20 ± 2)°C, (20 ± 4)°C and (20 ± 6)°C were significantly higher than that at constant temperature of 20°C, while growth rate at (20 ± 8)°C and (20 ± 10)°C were significantly lower than that at constant temperature of 20°C. The growth rate of macroalga was a quadratic function of the thermal amplitude. Such a growth model can be described by G = β 0 + β 1(TA) + β 2(TA)2, where G represents the relative growth rate, TA is thermal amplitude in degree Celsius, β 0 is the intercept on the G axis, and β 1 and β 2 are the regression coefficients. The optimal thermal amplitude for the growth of thallus at mean temperature of 20°C was estimated to be ± 3.69°C. Analysis of biochemical composition at the final stages of thaulls growth revealed that diel fluctuating temperature caused various influences (P < 0.05). The content of chlorophyll, protein and total solute carbohydrate at (20 ± 2)°C and (20 ± 4)°C were slightly higher than those at constant temperature of 20°C, however no statistically significant differences were found among them (P > 0.05). While osmolytes (total solute carbohydrate and free proline) at (20 ± 10)°C were significantly higher than that at 20°C (P < 0.05). Therefore, more chlorophyll and carbohydrate production might account for the enhancement in the growth of macroalga at the diel fluctuating temperatures in the present study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organisms in the tidal zone experience major short-term and long-term or seasonal environmental variations. Aquatic organisms must be able to adapt functionally to these dynamic environmental variables (Davison & Pearson, 1996). However, most of the authors mentioned above focused on the effects of constant conditions (temperature, salinity, irradiance) or sudden environmental changes on the macroalgae. The environment of organisms in the tidal zone is variable, it fluctuates according to the tide and solar radiation cycle. Some studies on the effect of fluctuating salinity and temperature on growth were conducted in some aquatic ectotherms, they found that moderate fluctuation of environment conditions could enhance the growth of aquatic animals (Konstantinov et al., 1990; Tian et al., 2004; Tian & Dong, 2006; Mu et al., 2005; Dong et al., 2006). However, most of seaweeds are sessile organisms, in contrast to marine animals. They are unable to relocate themselves when faced with physical, chemical and biological stresses, and must tolerate the stresses, such as, changes in temperature, salinity, solar radiation, nutrients availability and grazing of herbivores. They have to acclimate (and adapt) to the prevailing conditions in their natural habitat.
At present, effects of different temperature, salinity, irradiance and nutrients on growth and metabolism of green macroalgae were studied (Taylor et al., 2001; Figueroa et al., 2003). Furthermore, the effects of oxygen production and nutrient uptake metabolism under rapid changes of temperature and salinity were also reported (Kakinuma et al., 2001, 2004; Lartigue et al., 2003; Fong et al., 1996). These authors have found the optimum growth conditions and the mechanisms of nutrient uptake metabolism of many macroalgae (Taylor et al., 2001; Liu et al., 2000; Liu & Dong, 2001a, b; Tarutani et al., 2004).
Temperature is one of the major physical factors, through its control of metabolic rates to constraints growth and is determinant for life-history patterns of organisms in the tidal zone (Davison & Pearson, 1996). In general, most of terrestrial plants grow or are cultivated at a day temperature somewhat higher than the night temperature. Some studies have investigated the effect of diel fluctuating temperature on the metabolism of some terrestrial plant species (Went, 1944; Yin et al., 1996; Bredmose & Nielsen, 2004; Shaked et al., 2004). Thermoperiodicity of growth is prevalent in terrestrial plants, and as a scientific term was firstly introduced by Went (1944). However, we have little knowledge about the adaptation mechanisms of aquatic plant to diel temperature fluctuation. The temperature change is more complex in the tidal zone than that on the terrestrial ground, because it is affected not only by solar radiation but also by the changing tide.
Green macroalgae, such as Ulva, Enteromorpha, Cladophora and Chaetomorpha, are now ubiquitous in eutrophic coasts throughout the world (Raffaelli et al., 1998). When conditions are advantageous, the opportunistic genus Ulva can maintain fast-grow in the tidal zone and become the first colonizer on open substrata (Littler, 1980). U. pertusa is commonly distributed in the coastal waters of China and Japan. Some researchers have demonstrated that U. pertusa can reduce eutrophication in mariculture waters, and promote the productivity, survival rate and feeding coefficient of the culture species, such as prawn by means of polyculture (Danakusumah et al., 1991; Tan et al., 1999; Wang et al., 2001). Papers have reported that the growth of some harmful algal bloom species, such as Heterosigma akashiwo, Alexandrium tamarense and Prorocentrum micans can be strongly inhibited by U. pertusa (Jin & Dong, 2003; Jin et al., 2005). Moreover, U. pertusa can efficaciously take up nutrients from mariculture waters and improve water quality (Neori et al., 1996; Liu & Dong, 2001a, b). In present study, U. pertusa was studied under the circadian rhythms of fluctuating temperatures, aiming to investigate how the macroalga adapts to the temperature variations in the tidal zone.
Materials and methods
Plant material
Ulva pertusa, used in this study was the sterile mutant, which was kindly provided by Prof. Akira Taniguchi, Tohuku University, Japan, and was cultured aseptically in f/2 medium (Guillard & Ryther, 1962) at 20°C (constant temperature) and an irradiance of 100 μmol/(m2 s) (12:12 h light–dark cycle) in illuminating incubators. Natural seawater was subject to cotton filtration and boiling to minimize the effects of bacteria, the pH and salinity of the seawater were adjusted to 8.4 ± 0.1 and 30.0 ± 0.1, respectively.
Experimental treatment and culture condition
Discs (Ø1.05 cm, thus, 0.87 cm2) were cut from the marginal region of the thallus with a single genetic individual and were distributed to glass flasks containing 500 ml f/2 medium, and were allowed at least 24 h recuperation period at constant temperature 20°C before the experiment. A total of 36 rearing units, (glass flasks containing 500 ml f/2 medium, each unit containing 3 discs), were subjected to a constant temperature treatment (20 ± 0°C) or one of five fluctuating temperature treatments (20 ± 2°C, 20 ± 4°C, 20 ± 6°C, 20 ± 8°C and 20 ± 10°C). Six rearing units were allocated to each treatment and grown under 100 μmol/(m2 s) with a 12:12 h light–dark cycle. Growth was then followed for 12 days. The medium was renewed every day. The same photoperiod, light intensity and frequency of medium renewal was maintained throughout the experiment.
Diel temperature fluctuating mode
The temperatures were controlled by illuminating incubators. The fluctuating temperature treatments were programmed to follow a circadian rhythm (see Fig. 1) with different amplitude (A = ±2°C, ±4°C, ±6°C, ±8°C and ±10°C, respectively) around the daily mean temperature (T = 20°C). The temperature regimes were selected to match the natural temperature regime of Qingdao in a given time of a day in one flood at noon and another at midnight. A decrease (or increase) of 10°C in the medium needed about 70 min.
Growth
The initial individual fresh weight measurements were taken immediately on removal from seawater, and external water was removed from the thalli by drying them on filter paper. The mean initial weight of the thalli was 0.0218 ± 0.0007 g (Mean ± SD), and there were no differences in initial weights among treatments (P > 0.05). At 3 days, 6 days, 9 days and the end of the 12 days experiment, all thalli were weighted. At 12 days one half of disc was dried at 60°C for 24 h.
The relative growth rate (RGR) in terms of the fresh weight was calculated as the following:
RGR (% day−1) = 100 × (lnW t-lnW 0)/T
where W t and W 0 are the final and initial fresh weight of the thallus, respectively; T is the duration of the experiment.
Biochemical composition analysis
At the end of the experiment all discs were cut in two parts, one half of disc determined chlorophyll a and chlorophyll b, and other three half a discs determined protein, total soluble carbohydrate and proline, respectively. Surplus discs were kept at −70°C. Chlorophyll a and chlorophyll b were determined as described by Jeffrey & Humphrey (1975). Protein was determined as described by Bradford (1976) with bovine serum albumin as the standard. Total soluble carbohydrate was measured by the anthrone reaction with glucose as the standard (Yemm & Willis, 1954). Free proline was estimated by the method of Bates et al. (1973) with L-proline as the standard.
Data treatments and statistical analysis
Data set was analysed using one-way ANOVA, fluctuation amplitude treatment being the factor, by SPSS for Windows (Version 11.0). When differences were detected, means were compared using Duncan multicomparative analysis. Differences were considered significant if P < 0.05. In some cases, percentage and ratio data were arcsine transformed in order to meet normality and homoscedasticity.
Results
Growth
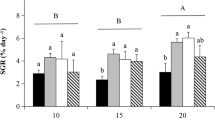
The change in the RGR of test thalli under different treatments was shown in Fig. 2. It can be seen that different diel fluctuating temperatures had various influences on the growth of U. pertusa. RGRs of the thalli at (20 ± 2)°C, (20 ± 4)°C and (20 ± 6)°C were significantly greater than that at constant temperature of 20°C (P < 0.05), while no significant difference was found between (20 ± 8)°C and constant temperature of 20°C (P > 0.05). The lowest growth of the thalli occurred at (20 ± 10)°C, and its growth rate was significantly lower than that at constant temperature of 20°C (P < 0.05).
The relative growth rate (RGR) of U. pertusa at different thermal regimes. C20, thalli grown at constant temperature; F2, F4, F6, F8 and F10, thalli grown at different fluctuating temperatures with fluctuation amplitude of ± 2, ± 4, ± 6, ± 8 and ± 10. Means with different letters in the same day in culture are significantly different (P < 0.05). Error bars represent 1 SE
The RGR was a quadratic polynomial function of the studied thermal amplitude, and can be described by following equation,
where G represents the relative growth rate on a 12-day basis, TA is thermal amplitude in degree Celsius, β 0 is the intercept on the G axis, and β 1 and β 2 are the regression coefficients, respectively. Their detail data is listed in Table 1.
Based on the equation, the optimal thermal amplitude for the growth of thalli at mean temperature of 20°C was estimated to be ±3.69°C.
Biochemical composition
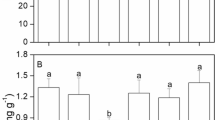
Some differences in biochemical composition of discs, related to the temperature fluctuation, were observed (see Table 2). The final content of chlorophyll a (Chl-a) and chlorophyll b (Chl-b) was slightly higher at (20 ± 2)°C and (20 ± 4)°C than those at constant temperature of 20°C, however no statistically significant differences were found among them (P > 0.05). The lowest levels of Chl-a and Chl-b occurred at (20 ± 10)°C, and their contents were significantly lower than those at 20°C (P < 0.05). The content of free proline at (20 ± 10)°C was significantly higher than at 20°C (P < 0.05). The P/C was slightly higher at constant temperature of 20°C than that at the temperature fluctuation, no statistically significant differences were found among constant temperature of 20°C and (20 ± 2)°C, (20 ± 4)°C, (20 ± 6)°C, and (20 ± 10)°C, while the P/C at (20 ± 8)°C was significantly lower than that at constant temperature of 20°C.
Discussion
Results from this study showed that thermoperiodicity is also occurred in coastal aquatic algae, at average temperature of 20°C the temperature fluctuation of (20 ± 2)°C, (20 ± 4)°C and (20 ± 6)°C produced better growth than that at constant temperature of 20°C (P < 0.05), however, the higher fluctuation of (20 ± 8)°C and (20 ± 10)°C did not show any positive influence on the growth of U. pertusa compared with that at constant temperature. Growth rate of U. pertusa at (20 ± 10)°C was significantly lower than at constant temperature (P > 0.05) (Fig. 2).
It can be seen that diel temperature fluctuations exerted various influences on the biochemical composition of discs. The content of Chl-a and protein at (20 ± 2)°C and (20 ± 4)°C were slightly higher than those at constant temperature of 20°C, which indicates that the synthesization of some biochemical products was accelerated within the optimal temperature amplitude, consequently it accelerated the increase of growth and biomass.
Synthesis and accumulation of organic osmolytes are widespread in plants (Klotke et al., 2004; Davison & Pearson, 1996). Osmolutes, such as proline are maintained through a combination of synthesis and catabolism in response to stress (Bates et al., 1973). In our study, the content of free proline at higher temperature fluctuations (20 ± 8°C, 20 ± 10°C is higher than that at constant temperature of 20°C.
When plants are exposed to higher day temperatures and lower night temperatures plant production is improved through carbon balance between photosynthesis and respiration (Went, 1944; Mitchell et al., 1991; Grimstad & Grimanslund, 1993). Therefore, more fixed carbon may be available for growth and defence (Bradfield & Stamp, 2004). However this theory cannot explain why lower day temperatures and higher night temperatures also could improve plant growth. Fluctuating temperature is a stressor compared with constant temperature, and stress may play a dual role. In the present study, (20 ± 2)°C and (20 ± 4)°C has a positive effect for macroalga, the growth and anabolism of macroalga was accelerated, while with the increase of amplitude (>±8°C), positive effect turned into negative effect, the growth of macroalga was inhibited.
Until now, few investigations have probed into what may contribute to the enhancement in growth of plant (Sun et al., 2000). The activity of amylase and glucose-6-phosphate dehydrogenase of wheat growing in diurnal fluctuation of temperature was higher in day and lower in night, and the amylase and glucose-6-phosphate dehydrogenase of the wheat growing in constant temperature had no the characteristic (Sun et al., 2000). In future research, it is needed to find out whether the optimal amplitude of fluctuation activates more enzymes cooperative and compatible or the distress inhibits the activity of key enzymes.
References
Bates, L. S., R. P. Waldren & I. D. Teare, 1973. Rapid determination of free proline for water-stress studies. Plant and Soil 39: 205–207.
Bradfield, M. & N. Stamp, 2004. Effect of nighttime temperature on tomato plant defensive chemistry. Journal of Chemical Ecology 30: 1713–1721.
Bradford, M. M., 1976. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principles of protein-dye binding. Analytical Biochemistry 72: 248–254.
Bredmose, N. & J. Nielsen, 2004. Effects of thermoperiodicity and plant population density on stem and flower elongation, leaf development, and specific fresh weight in single stemmed rose (Rosa hybrida L.) plants. Scientia Horticulturae 100: 169–182.
Danakusumah, E., S. Kadowaki & H. Hirata, 1991. Effects of coexisting Ulva pertusa on the producton of kuruma prawn. Bulletin of the Japanese Society of Scientific Fisheries 57: 1579–1603.
Davison I. R. & G. A. Pearson, 1996. Stress tolerance in intertidal seaweeds. Journal of Phycology 32: 197–211.
Dong, Y., S. Dong, X. Tian, F. Wang & M. Zhang, 2006. Effects of diel temperature fluctuations on growth, oxygen consumption and proximate body composition in the sea cucumber Apostichopus japonicus Selenka. Aquaculture 2006 255: 514–521.
Figueroa, F. L., C. Nygård, N. Ekelund & I. Gómez, 2003. Photobiological characteristics and photosynthetic UV responses in two Ulva species (Chlorophyta) from southern Spain. Journal of Photochemistry and Photobiology B: Biology 72: 35–44.
Fong, P., K. E. Boyer, J. S. Desmond & J. B. Zedler, 1996. Salinity stress, nitrogen competition, and facilitation: What controls seasonal succession of two opportunistic green macroalgae? Journal of Experimental Marine Biology and Ecology 206: 203–221.
Grimstad, S. O. & E. Grimanslund, 1993. Effect of different day and night temperature regimes on greenhouse cucumber young plant production, flower bud formation and early yield. Scientia Horticulturae 53: 191–204.
Guillard, R. R. & J. H. Ryther, 1962. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervaceae (Cleve) Gran. Canadian Journal of Microbiology 8: 229–239.
Jeffrey, S. W. & G. F. Humphrey, 1975. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemistry and Physiology Pflanzen 167: 191–194.
Jin, Q. & S. Dong, 2003. Comparative studies on the allelopathic effects of two different strains of Ulva pertusa on Heterosigma akashiwo and Alexandrium tamarense. Journal of Experimental Marine Biology and Ecology 293: 41–55.
Jin, Q., S. Dong & C. Wang, 2005. Allelopathic growth inhibition of Prorocentrum micans (Dinophyta) by Ulva pertusa and Ulva linza (Chlorophyta) in laboratory cultures. European Journal of Phycology 40: 31–37.
Kakinuma, M., Y. Kuno & H. Amano, 2004. Salinity stress responses of a sterile mutant of Ulva pertusa (Ulvales, Chlorophyta). Fisheries Science 70: 1177–1179.
Kakinuma, M., N. Shibahara, H. Ikeda, M. Maegawa & H. Amano, 2001. Thermal stress responses of a sterile mutant of Ulva pertusa (Chlorophyta). Fisheries Science 67: 287–294.
Klotke, J., J. Kopka, N. Gatzke & A. G. Heyer, 2004. Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation—evidence for a role of raffinose in cold acclimation. Plant, Cell and Environment 27: 1395–1404.
Konstantinov, A. S., V. V. Zdanovich & D. G. Tikhomirov, 1990. The effect of temperature oscillation on metabolic rate and energetics of young fish. Joural of Ichthyology 29: 1019–1027.
Lartigue, J., A. Neill, B. L. Hayden, J. Pulfer & J. Cebrian, 2003. The impact of salinity fluctuations on net oxygen production and inorganic nitrogen uptake by Ulva lactuca (Chlorophyceae). Aquatic Botany 75: 339–350.
Littler, M. M., 1980. Morphological form and photosynthetic performances of marine macroalgae: Tests of a functional/form hypothesis. Botanica Marina 22: 161–165.
Liu, J. & S. Dong, 2001a. Comparative studies on utilizing nitrogen capacity between two macroalgae Gracilaria tenuistipitata Var. Liui (Rhodophyta) and Ulva pertusa (Chlorophyta): I. Nitrogen storage under nitrogen enrichment and starvation. Journal of Environmental Sciences 13: 318–322.
Liu, J. & S. Dong, 2001b. Comparative studies on utilizing nitrogen capacity between two macroalgae Gracilaria tenuistipitata Var. Liui (Rhodophyta) and Ulva pertusa (Chlorophyta):II. Feedback control of intracellular nitrogen pools on nitrogen uptake. Journal of Environmental Sciences 13: 323–327.
Liu J., S. Dong, X. Liu & S. Ma, 2000. Response of macroalgae Gracilaria tenuistipitata var. liui (Rhodophyta) to iron stress. Journal of Applied Phycology 12: 605–612.
Mitchell, R. A. C., D. W. Lawlor & A. T. Young, 1991. Dark respiration of winter wheat crops in relation to temperature and simulated photosynthesis. Annals of Botany 67: 7–16.
Mu, Y., F. Wang, S. Dong, S. Dong & C. Zhu, 2005. Effects of salinity fluctuation in different ranges on intermolt period and growth of juvenile Fenneropenaeus chinensis. Acta Oceanologia sinica 24: 1–7.
Neori, A., M. D. Krom, S. P. Ellner, C. E. Boyd, D. Popper, R. Rabinovitch, P. J. Davison, O. Dvir, D. Zuber, M. Ucho, D. Angel & H. Gordin, 1996. Seaweed biofilters as regulators of water quality in integrated fish-seaweed culture units. Aquaculture 141: 183–199.
Raffaelli, D. G., J. A. Raven & L. J. Poole, 1998. Ecological impact of green macroalgal blooms. Oceanography and Marine Biology 36: 97–125.
Shaked, R., K. Rosenfeld & E. Pressman, 2004. The effect of low night temperatures on carbohydrates metabolism in developing pollen grains of pepper in relation to their number and functioning. Scientia Horticulturae 102: 29–36.
Sun, C., J. Mao, B. Bai & Y. Bai, 2000. Studies of enzymology on diurnal change of temperature accelerating the rate of wheat seedling growth. Journal of Jilin Agricultural University 22: 30–33 (in Chinese, with English abstract).
Tan, H., J. Li & Y. Lei, 1999. A preliminary study on purification of water contaminated by Haliotis dicus hannai lno. Tropic Oceanology 18: 20–26.
Tarutani, K., Y. Niimura & T. Uchida, 2004. Short-term uptake of dissolved organic nitrogen by an axenic strain of Ulva pertusa (Chlorophyceae) using 15N isotope measurements. Botanica Marina 47: 248–250.
Taylor, R., R. L. Fletcher & J. A. Raven, 2001. Preliminary studies on the growth of selected ‘green tide’ algae in laboratory culture: Effects of irradiance, temperature, salinity and nutrients on growth rate. Botanica Marina 44: 327–336.
Tian, X. & S. Dong, 2006. The effects of thermal amplitude on the growth of Chinese shrimp Fenneropenaeus chinensis (Osbeck, 1975). Aquaculture 251: 516–524.
Tian, X., S. Dong & F. Wang, 2004. The effects of temperature changes on the oxygen consumption of Chinese shrimp. Journal of Experimental Marine Biology and Ecology 310: 59–72.
Wang, J., C. Jin, X. Zhang & G. Liu, 2001. Polyculture of experiment Penaeus chinensis with various biomass of Ulva pertusavar. Journal of Fisheries of China 25: 32–37 (in Chinese, with English abstract).
Went, F. W., 1944. Plant growth under controlled conditions. II. Thermoperiodicity in growth and fruiting of the tomato. American Journal of Botany 31: 135–149.
Yemm, E. W. & A. J. Willis, 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal 57: 508–514.
Yin, X., M. J. Kropff & J. Goudriaan, 1996. Differential effects of day and night temperature on development to flowering in rice. Annals of Botany 77: 203–213.
Acknowledgements
The authors thank Prof. Taniguchi for his kind supply of the axenic nonsexual strain of U. pertusa, and Dr. Yunwei Dong and Xidan Zhao providing assistance in the care and maintenance of the experiment. This work was supported by National Eleventh Five-year Scientific and Technological Key Project (Grant No. 2006BAD09A01) and Hi-Tech Research and Development Program of China (Grant No. 2006AA10Z409).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. M. Thomaz
Rights and permissions
About this article
Cite this article
Wang, Q., Dong, S., Tian, X. et al. Effects of circadian rhythms of fluctuating temperature on growth and biochemical composition of Ulva pertusa . Hydrobiologia 586, 313–319 (2007). https://doi.org/10.1007/s10750-007-0700-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-0700-z