Abstract
Facile synthesis of perovskite materials for the electrochemical sensing applications are remains challenging. In the current work, a simplified electrochemical sensor system based on an electrode modifier containing LaNiO3 was developed to detect catechol (CC). The surface micrograph of LaNiO3 was examined using X-ray diffraction pattern (XRD), X-ray photoelectron spectroscopy (XPS), and High-resolution transmission electron microscopy (HRTEM). Besides, the LaNiO3 reveals a remarkable electrooxidation response for the detection of catechol by cyclic voltammetry (CV) and amperometry techniques. The suggested sensor platform shows a broad linear range for catechol detection from 5 µM to 2000 µM with LOD and sensitivity of 0.6 µM and 54 µA cm−2 mM−1, respectively, under optimised conditions. Furthermore, LaNiO3 altered GCE was fruitfully implemented for the quantification of catechol in tap water sample.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Catechol (CC, 1, 2 dihydroxybenzene) is a significant phenolic compound. It is a toxic pollutant to livestock and the environment [1]. Meanwhile, synthetic phenolic compounds are also toxic and constitute pollutants in soil, water and food. Therefore, sensing of phenolic compounds is essential for humans and the environment [2]. Catechol is widely used in the manufacture of rubber as a curing agent, skin anti-septic, antioxidant, fungicide, additives in electroplating, photographs, dyes, cosmetics, and many others [3]. It has been reported that catechol affects human health such as upper respiratory tract irritation, high blood pressure, and kidney damage due to its high toxicity and harmful effects on the environment and human health [4]. For the above reason, the determination of catechol is important in the field of environmental monitoring and industrial processes.
Numerous methods are available to determine catechol such as spectroscopic [5], chromatographic [6], chemiluminescence [7], and fluorescence [8]. The above-mentioned methods are feasible but complicated, time-consuming and costly which has limited further commercial application. In comparison to the above method, the electrochemical sensor is the best tool for detecting catechol using modified electrodes [9]. Moreover, the electrooxidation of catechol on bare glassy carbon electrode (GCE) is very poor due to its sluggish electron transfer. Instead of that, modification with nanomaterials has attained higher catalytic activity with lower oxidation potential. Previously, different types of modified electrodes were used to detect catechol like MWCNTs/SPCE [10], Tyr-AuNPs-DHP/GCE [11], AuNPs/Fe3O4-APTEs-GO/GCE [12], Au/Ni(OH)2/rGO/GCE [13], AuNPs/ZnO-Al2O3/GCE [14], and NiAl-layered/LDHs/GCE [15].
In recent years, interests have focused on nanostructured materials, particularly perovskites attained extensive use in analytical chemistry including solar cells, catalysis, biological sensors, optics, and electronics etc. More interestingly, perovskite material with ABO3 type metal oxide possesses higher electronic and ionic conductivity with enhanced catalytic activity, chemical and thermal stability by employing variation in oxygen content [16]. Specifically, LaNiO3 is an excellent material that exhibits fascinating chemical and physical characteristics and can be applied as an electrochemical sensing platform to enhance catalytic performance with higher sensitivity [17]. LaNiO3 is a well-known conducting mixed oxide material at room temperature without the use of conducting carbon additive which influences the activity [18]. There are many methods to synthesize LaNiO3 such as sol–gel, hydrothermal, and microwave techniques with diverse nanostructures such as homogeneous and anisotropic morphology, higher conductivity with abundant surface area. Compared with other methods, the simple precipitation method is very effective, low cost, and less time-consuming. Therefore, LaNiO3 is prepared by a simple precipitation method and applied for the electrochemical sensing of catechol [17, 19, 20].
In the present work, we developed LaNiO3 based sensor platform for the sensing of catechol by cyclic voltammetry and amperometry techniques. Synthesized catalyst has been characterized for material purity, elemental composition and morphological studies by XRD, XPS, and HRTEM. The as-synthesized material was used as an electrocatalyst for the detection of phenolic pollutant, catechol. Tap water provided an actual sample to demonstrate the practical application of the LaNiO3 modified electrode.
2 Experimental methods
2.1 Materials
Lanthanum nitrate, nickel nitrate sodium hydroxide, NaH2PO4, Na2HPO4, sodium dodecyl sulfate (SDS), dimethyl formamide (DMF), hydroxylamine, sodium nitrite (NaNO2), sodium nitrate (NaNO3), and sodium sulphite (Na2SO3) were sourced from Sigma-Aldrich. Catechol, dopamine and uric acid were obtained from Sigma Aldrich. All chemicals have been used without additional purification. A double distillation of de-ionized water from the Milli-Q system was used for the preparation of all solutions.
2.2 Synthesis of LaNiO3
LaNiO3 nanoparticle was synthesized by a simple precipitation method. A stoichiometric amount of lanthanum nitrate (0.01 M) and nickel nitrate (0.01 M) were dissolved in 500 mL distilled water at constant stirring. Then, 0.05 M of glycine was added dropwise into the stirred solution at 100 °C. 0.01 M SDS was injected drop by drop into the hot solution and kept for 12 h at constant stirring. After 12 h, the mixture color was transferred to a dark green precipitate. The precipitate was treated to cool down to room temperature and washed many times with ethanol and water followed by dried in an oven at 85 °C for 12 h and calcinated at 600 °C for 5 h with a heating rate of 5 °C per minute. The final black color material was used as an electrocatalyst for the detection of catechol.
2.3 Fabrication of LaNiO3 modified electrode
Primarily, mirror-like surface of GCE was cleaned using 0.05 micron alumina powder on a polishing pad. Further, the electrode was washed with water and sonicated to remove alumina particles. The catalyst slurry was prepared by mixing 5 mg of LaNiO3 in 1 mL of DMF solvent and sonicated for 30 min. After sonication, 3 µL catalyst was dropped over precleaned GCE and allowed to dry. Finally, the obtained LaNiO3 modified GCE was used to detect CC in the present study as shown in Scheme 1.
3 Result and discussion
3.1 Physical characterization of LaNiO3
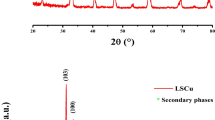
The XRD pattern of LaNiO3 is shown in Fig. 1. LaNiO3 displays the perovskite phase with crystal planes of (101), (110), (021), (003), (202), (211), (122), (220), (312), and (214) corresponding 2θ values at 23°, 32°, 40°, 41°, 47°, 53°, 58°, 68°, 78°, and 79°, respectively. The distinctive diffraction peaks of LaNiO3 exactly match with the ICDD number 00-034-1028 and a hexagonal structure [21]. We can conclude from the XRD results that LaNiO3 exists in a well-crystalline, hexagonal form with no impurities being visible. As a result, the synthesized LaNiO3 is of a high purity, proving that the synthesis approach can be applied for large-scale preparation.
HRTEM is mainly used for the detection of higher-resolution surface morphology and SAED pattern. Figure 2 shows the surface morphology and SAED pattern of LaNiO3. Several magnifications such as 200 nm, 100 nm, and 50 nm are shown in Fig. 2A, B, and C, respectively. The selected area electron diffraction pattern (SAED) of LaNiO3 is shown in Fig. 2D. Figure 2D confirms that our synthesized LaNiO3 was perfectly crystalline in nature. The d spacing values were estimated from the SAED pattern and the value are well matched with the XRD results. Based on the HRTEM micrographs, the synthesized LaNiO3 had well-crystalline nature and nanostructured material.
The XPS survey spectrum displays multiple peaks that are related to Ni 2p, La 3d, and O 1s which is shown in Fig. 3A. Figure 3B shows peaks for La 3d5/2 and La 3d3/2 at 836 eV and 854 eV, respectively, which is comparable with the results of lanthanum in a La (III) oxidation state that have been reported [22]. The higher-resolution XPS studies of Ni 2p1/2 and Ni 2p3/2 peaks and corresponding satellite peaks of LaNiO3 are shown in Fig. 3C. Figure 3C clearly indicates that Ni 2p1/2 and Ni 2p3/2 peaks were exhibited at 872 eV and 854 eV, respectively [23]. As exhibited in Fig. 3D, the binding energy (BE) of O 1s peaks observed at 529 eV and 531 eV [24, 25].
3.2 Electrochemical characterization studies of LaNiO3 modified electrode
3.2.1 Cyclic voltammetry
Synthesized LaNiO3 was utilized for the electrochemical oxidation of catechol. LaNiO3 altered GCE was employed for the electrochemical oxidation of catechol in 0.1 M PBS using CV technique (pH = 7). Figure 4 shows CVs of bare GCE and LaNiO3/GCE in the presence and absence of catechol. Curve ‘a’ and ‘b’ indicate that bare GCE and LaNiO3 altered GCE in the absence of catechol. There was no current response in pure PBS electrolyte. Curve ‘c’ and ‘d’ indicate the bare GCE and modified GCE with the existence of 1 mM catechol at 10 mV s−1 scan rate. However, compared to bare GCE, LaNiO3 modified GCE shows higher current density response towards the sensing of catechol. The observed higher current response of LaNiO3/GCE to the electrooxidation of catechol is attributed to the electrocatalytic activity of LaNiO3.
The pH effect on electrochemical sensing of catechol at LaNiO3/GCE was investigated using CV studies with 1 mM catechol at a sweeping rate of 10 mV s−1. Figure 5A exhibits CVs of catechol at different pH such as 6.0, 6.5, 7.0, 7.5, and 8.0. Figure 5B illustrates the increment in peak current when the pH increases from pH 6 to pH 7, then it starts to diminish when pH increases further. Therefore, the optimized PBS (pH = 7) was chosen for further electrochemical studies. From the results, the probable reaction mechanism of catechol at LaNiO3 altered GCE is displayed in Fig. 5C.
Electrocatalytic behavior of LaNiO3/GCE at various concentrations of catechol has been investigated using CV in 0.1 M PBS in pH = 7 (10 mV s−1). The CV responses for electrocatalytic determination of catechol at LaNiO3/GCE upon each addition of catechol are disclosed in Fig. 6A. Anodic peak current augmented with the increment of catechol concentration from 0.05 to 10 mM. Figure 6B displayed that anodic peak current varies linearly with catechol concentration in the range from 0.05 to 7 mM. From the calibration curves in Fig. 6B, the obtained sensitivity value is 95 µA cm−2 mM−1 with its corresponding linear regression equation Ip.a. = 95x + 39 × (C) mM (R2 = 0.989).
A Cyclic voltammograms of different concentrations of catechol at LaNiO3/GCE. Concentration of catechol 0.05–10 mM. Scan rate = 10 mV s−1. B Calibration plot. C Cyclic voltammograms of 1 mM catechol at LaNiO3/GCE with an increment of scanning rate starts from 10 to 150 mV s−1 and D Dependence of J νs (scan rate)1/2
To understand kinetics, CVs of LaNiO3/GCE in 0.1 M PBS with the existence of 1 mM catechol were recorded at various scan rates. Figure 6C shows the oxidative peak current amplified steadily with an increment of scan rate starting from 10 to 150 mV s−1 and further positive shift was observed in the peak potential. The peak current was correlated with scan rate as displayed in Fig. 6D (R2 = 0. 992). Linear response was observed for the current density with square root of scan rate, which signifies the electron transfer process was under mass-transfer control.
3.2.2 Amperometric study
Amperometric measurement was carried out at an optimized applied potential of 0.3 V using LaNiO3/GCE by successive addition of different concentrations of catechol in 0.1 M PBS (pH 7). Figure 7A exhibits the response of modified electrode at different concentrations of catechol from 5 µM to 8 mM. Figure 7B displays the current response of LaNiO3 for the successive addition of catechol. By increasing the catechol concentration from 5 to 2000 µM, current response increased gradually. From the calibration plot in Fig. 7B, sensitivity and detection limit (LOD) were calculated as 54 µA cm−2 mM−1 and 0.6 µM, respectively, with Ip.a. = 54 x + 4.9 × (C) mM linear regression equation (R2 = 0.994). A comparison of analytical parameters of our proposed sensor with other modified electrodes for catechol sensing is displayed in Table 1. It can be seen that our proposed electrode shows wide concentration range compared to other electrodes.
Selectivity of the proposed sensor for the sensing of catechol was further assessed by amperometric analysis at 0.3 V (0.1 M PBS). Figure 8A depicts the amperometric response of LaNiO3/GCE for the determination of catechol in the existence of common interferents with 50-fold higher concentration of resorcinol, hydroquinone, ammonia nitrate, sodium nitrite, sodium bromate, potassium chloride, calcium carbonate, sodium sulfite, ascorbic acid, glucose, and urea. The obtained amperometric response clearly indicates that our proposed sensor could selectively sense the catechol and no significant current change was observed for the addition of other interferents. Figure 8B depicts the corresponding bar diagram of the interference which are included along with catechol. The above result clearly explains that our proposed sensor has high selectivity to detect catechol even in the presence of a high concentration of common interferent exist in solution.
A Amperometric responses of LaNiO3/GCE modified electrode to the addition of 100 µL stock solution addition of catechol and addition of 50-fold high concentration of resorcinol, hydroquinone, ammonia nitrate, sodium nitrite, sodium bromate, potassium chloride, calcium carbonate, sodium sulfite, ascorbic acid, glucose, and urea at 0.3 V. B Corresponding bar diagram of the interference study
3.2.3 Practical application
Real time application of LaNiO3/GCE sensor examined with the utilization of real sample (tap water). The tap water was collected from our institute (Karaikudi, Tamil Nadu, India). To perform a real sample analysis of catechol, a known amount (10 mM) of catechol was spiked in the water sample. The amperometric measurements were carried out by adding different known concentrations of catechol via the standard spiking method. A known quantity of standard catechol solution (25 and 50 µM) was spiked into the 0.1 M PBS as illustrated in Fig. 9A. The corresponding linear calibration plot of different addition of catechol into the real sample is shown in Fig. 9B. The obtained data validate that our sensor could be effective for catechol detection in water samples with good RSD values.
3.2.4 Stability and reproducibility
Stability of the LaNiO3/GCE was determined with 1 mM of catechol by continuously recording 100 cyclic voltammograms as shown in Fig. 10. Stable current was observed with less than 5% relative standard deviation (RSD) in their current values. The CV results reveal that the LaNiO3/GCE modified electrodes exhibits appreciable stability. Reproducibility of the fabricated electrode was carried out using 5 different modified electrodes with an RSD of less than 4.5%, which confirms that LaNiO3 modified GCEs have appreciable fabrication reproducibility. Hence, LaNiO3/GCE can be employed for practical applications.
4 Conclusion
In summary, we have explored a highly sensitive catechol sensor by employing a LaNiO3 perovskite nanostructure-modified electrode. The XPS spectra revealed the various oxidation states of the as-prepared materials. The proposed sensor exhibited enhanced analytical features towards the determination of catechol with higher sensitivity (54 µA cm−2 mM−1), lower detection limit (0.6 µM), eminent selectivity, wide linear range (5 to 2000 µM), and stability. Moreover, the estimation of catechol in tap water encourages the chance for real time application towards the proposed LaNiO3 as a modifier. Hence, it is believed that the LaNiO3 nanocomposite-modified electrode can be considered as a promising material for sensor application.
Data availability
Data will be available on request.
References
Liang Y, Li J, Zhao Y (2017) Poly (sulfosalicylic acid)/ multi-walled Carbon Nanotube Modified Electrode for the Electrochemical Detection of Catechol. Int J Electrochem Sci 12:9512–9522. https://doi.org/10.20964/2017.10.20
Dorraji PS, Jalali F (2015) A Nanocomposite of Poly (melamine) and Electrochemically Reduced Graphene Oxide Decorated with Cu Nanoparticles: Application to Simultaneous Determination of Hydroquinone and Catechol. J Electrochem Soc 162:237–244. https://doi.org/10.1149/2.0951509jes
Matos F, De, Deon M, Nicolodi S et al (2018) Electrochimica acta magnetic silica / titania xerogel applied as electrochemical biosensor for catechol and catecholamines. Electrochim Acta 264:319–328. https://doi.org/10.1016/j.electacta.2018.01.127
Coros M, Pog F, Lidia M (2017) Graphene-porphyrin composite synthesis through graphite exfoliation: the electrochemical sensing of catechol. Sens Actuators B. https://doi.org/10.1016/j.snb.2017.09.205
Smith SJ, Noble ÆCJ, Palmer ÆRC et al (2008) Structural and spectroscopic studies of a model for catechol oxidase. J Biol Inorg Chem 13:499–510. https://doi.org/10.1007/s00775-007-0334-7
Lan L, Wu S, Yu S et al (2019) Fast and direct determination of catechol-3, 6-bis (methyleiminodiacetic acid) prototype in beagle dog plasma using liquid chromatography tandem mass spectrometry : a simplified and high throughput in-vivo method for the metal chelator. J Chromatogr A 1596:84–95. https://doi.org/10.1016/j.chroma.2019.03.002
Yang D, He Y, Sui Y, Chen F (2017) Determination of catechol in water based on gold nanoclusters-catalyzed chemiluminescence. J Lumin 187:186–192. https://doi.org/10.1016/j.jlumin.2017.02.067
Han S, Liu B, Liu Y, Fan Z (2016) Silver nanoparticle induced chemiluminescence of the hexacyanoferrate-fluorescein system, and its application to the determination of catechol. Microchim Acta 183:917–921. https://doi.org/10.1007/s00604-015-1704-4
Rao H, Liu Y, Zhong J et al (2017) Gold Nanoparticle/Chitosan@N,S co-doped multiwalled carbon nanotubes sensor: fabrication, characterization, and electrochemical detection of catechol and nitrite. ACS Sustain Chem Eng 5:10926–10939. https://doi.org/10.1021/acssuschemeng.7b02840
Rao MM, Settu R, Chen S, Alagarsamy P (2018) Electrochemical Determination of Catechol Using Functionalized Multiwalled Carbon Nanotubes modified Screen Printed Carbon Electrode. Int J Electrochem 13:6126–6134. https://doi.org/10.20964/2018.06.121
Vicentini FC, Garcia LLC, Figueiredo-filho LCS et al (2015) A biosensor based on gold nanoparticles, dihexadecylphosphate, and tyrosinase for the determination of catechol in natural water. Enzyme Microbial Technol. https://doi.org/10.1016/j.enzmictec.2015.12.004
Erogul S, Bas SZ, Ozmen M, Yildiz S (2015) A new electrochemical sensor based on Fe3O4 functionalized graphene oxide-gold nanoparticle composite film for simultaneous determination of catechol and hydroquinone. Electrochim Acta 186:302–313. https://doi.org/10.1016/j.electacta.2015.10.174
Wang H, Qu J, Wang Y et al (2017) Analytical methods nanocomposites supported on reduced graphene. Anal Methods 9:338–344. https://doi.org/10.1039/c6ay02814d
Nazari M, Kashanian S, Moradipour P, Maleki N (2018) A novel fabrication of sensor using ZnO-Al2O3 ceramic nanofibers to simultaneously detect catechol and hydroquinone. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2018.01.058
Shen M, Zhang Z, Ding Y (2016) Synthesizing NiAl-layered double hydroxide microspheres with hierarchical structure and electrochemical detection of hydroquinone and catechol. Microchem J 124:209–214. https://doi.org/10.1016/j.microc.2015.08.011
George KJ, Halali VV, Sanjayan CG et al (2020) Perovskite nanomaterials as optical and electrochemical sensors. Inorg Chem Front 7:2702–2725. https://doi.org/10.1039/D0QI00306A
Wang B, Gu S, Ding Y et al (2013) A novel route to prepare LaNiO3 perovskite-type oxide nanofibers by electrospinning for glucose and hydrogen peroxide sensing. Analyst 138:362–367. https://doi.org/10.1039/C2AN35989H
Petrie JR, Cooper VR, Freeland JW et al (2016) Enhanced bifunctional oxygen catalysis in strained LaNiO3 Perovskites. J Am Chem Soc 138:2488–2491. https://doi.org/10.1021/jacs.5b11713
Forslund RP, Mefford JT, Hardin WG et al (2016) Nanostructured LaNiO3 perovskite electrocatalyst for enhanced urea oxidation. ACS Catal 6:5044–5051. https://doi.org/10.1021/acscatal.6b00487
Mcbean CL, Liu H, Sco ME et al (2017) Generalizable, electroless, template-assisted synthesis and electrocatalytic mechanistic understanding of perovskite LaNiO3 nanorods as viable, supportless oxygen evolution reaction catalysts in alkaline media. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.7b06855
Mao M, Xu J, Li L et al (2019) High performance hydrogen production of MoS2-modified perovskite LaNiO3 under visible light. Ionics 25:4533–4546. https://doi.org/10.1007/s11581-019-03210-2
Sales HBE, Inocêncio CVM, Varga E et al (2017) CO2 reforming of methane over supported LaNiO3 perovskite-type oxides. Appl Catal B. https://doi.org/10.1016/j.apcatb.2017.09.022
Xu J, Sun C, Wang Z et al (2018) Perovskite oxide LaNiO3 nanoparticles for boosting H2 evolution over commercial CdS with visible light. Chem Eur J 1:18512–18517. https://doi.org/10.1002/chem.201802920
Sun M, Zhang Q, Chen Q et al (2022) Coupling LaNiO3 Nanorods with FeOOH Nanosheets for Oxygen Evolution Reaction. Catalysts 12:594
Qiu Y, Gao R, Yang W et al (2020) Understanding the enhancement mechanism of a-site-deficient LaxNiO3 as an oxygen redox catalyst. Chem Mater 32:1864–1875. https://doi.org/10.1021/acs.chemmater.9b04287
Qian Y, Ma C, Zhang S et al (2017) High performance electrochemical electrode based on polymeric composite film for sensing of dopamine and catechol. Sens Actuators B Chem. https://doi.org/10.1016/j.snb.2017.08.174
Elancheziyan M, Manoj D, Saravanakumar D (2017) Amperometric sensing of catechol using a glassy carbon electrode modified with ferrocene covalently immobilized on graphene oxide. Microchimica Acta 184:2925–2932. https://doi.org/10.1007/s00604-017-2312-2
Chetankumar K, Kumara Swamy BE, Sharma SC, Hariprasad SA (2021) An efficient electrochemical sensing of hazardous catechol and hydroquinone at direct green 6 decorated carbon paste electrode. Sci Rep 11:15064. https://doi.org/10.1038/s41598-021-93749-w
Song Y, Zhao M, Wang X et al (2019) Simultaneous electrochemical determination of catechol and hydroquinone in seawater using Co3O4/MWCNTs/GCE. Mater Chem Phys 234:217–223. https://doi.org/10.1016/j.matchemphys.2019.05.071
Acknowledgements
K. Krishnamoorthy would like to thank CSIR-UGC, New Delhi for financial support. We wish to acknowledge the Central Instrumentation Facility (CIF), CSIR-CECRI, Karaikudi for characterization studies. Manuscript number: CECRI/PESVC/Pubs/2023-062.
Funding
K. Krishnamoorthy would like to thank CSIR-UGC, New Delhi for financial support.
Author information
Authors and Affiliations
Contributions
KK-writing—original draft, and investigation, SN-writing—review and editing, VS-writing—review and editing, SMSK-investigation and formal analysis, RT-supervision, conceptualization and review and editing.
Corresponding author
Ethics declarations
Competing interest
The authors declared that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishnamoorthy, K., Sivaraman, N., Sudha, V. et al. Electrochemical sensing of phenolic pollutant catechol on LaNiO3 perovskite nanostructure platform. J Appl Electrochem 54, 1637–1645 (2024). https://doi.org/10.1007/s10800-023-02055-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-02055-y