Abstract
Amaranthus spinosus has been consumed traditionally to prevent various diseases including abdominal pain. In this study, the phytochemical composition, antioxidant and analgesic activities of an ethyl acetate extract of A. spinosus leaves (ASEA) were evaluated. The ASEA had the highest concentrations of total phenols (462.2 mg GAE/g DW), condensed tannin (5.01 mg CE/g DW) and total flavonoid contents (30.07 mg CE/g DW) compared to the chloroform, n-hexane, n-butanol and water extracts. Similarly, ASEA showed the most effective total antioxidant activity (45.45 µg/mL), DPPH scavenging activity (27.32 µg/mL) and hydrogen peroxide scavenging activity (30.60 µg/mL). ASEA with the doses of 200–600 mg/kg (p.o.) clearly demonstrated antinociceptive effects by reducing acetic acid-induced abdominal contortions with a maximal inhibition of 79.57% at 600 mg/kg and increasing latencies of the hot-plate paw-licking response. The tested doses also significantly (p < 0.001) decreased the reaction time in the formalin test at the neurogenic and inflammatory phases. ASEA contained ten polyphenols with caffeic acid being the predominant polyphenol. Overall, this study gave evidence that A. spinosus is a new antioxidant and analgesic agent, and justified its traditional use for the treatment of pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consumption of natural products affords excellent health benefits due to their significant action in the prevention of many human diseases. These health benefits are assigned to their diverse pharmacological abilities including inflammatory, analgesic and antioxidant activities. The antioxidant activities of edible plants have been attributed to their redox properties, metal chelating ability and their capacity to quench 1O2 (Carocho and Ferreira 2013).
Pain and inflammatory reactions in the peripheral and central nervous systems have fundamental roles in the occurrence of numerous pathological pain conditions. The treatment of pain requires analgesics, including non-steroidal anti-inflammatory drugs (NSAIDS). However, extended use of these drugs is frequently associated with several adverse effects the most serious being gastrointestinal bleeding and ulceration (Miller 1983). Therefore, many researchers have intensified the search to characterize new antioxidant and analgesic compounds from plants useable as therapeutic alternatives.
Amaranthus spinosus is a medicinal plant of the Amaranthaceae family, cultivated and eaten as a green vegetable throughout India and tropical countries. Traditionally, this plant is widely used to avoid stomachache and to treat fevers, urinary troubles, and diarrhea. Modern pharmacological studies showed that A. spinosus possess various pharmaceutical properties such as anti-inflammatory, anti-diabetic, anti-cholesterolemic and diuretic activities (Tanmoy et al. 2014). A. spinosus has been reported to protect against paracetamol- (Kumar et al. 2010) and carbon tetrachloride (Zeashan et al. 2008, 2009)-induced liver injury in rats. In our previous studies, we have demonstrated that A. spinosus seed extracts have protective effects against deltamethrin-induced liver injury in rats through reducing hepatic lipid peroxidation and restoring the levels of serum biochemical markers and the activity of the antioxidant enzymes (Rjeibi et al. 2016). Moreover, the phytochemical investigation of seeds showed that caffeic acid, cinnamic acid, epicatechin, gallic acid, vanillic acid and protocatechuic acid are the main phenolic compounds present in A. spinosus. However, no report about the impact of different solvent types on the biological activities and the phytochemical composition of this plant is available in the literature. Therefore, the goal of the present research was to assess and compare the antioxidant activity, the total phenolic, total tannin and total flavonoid contents of A. spinosus using solvents of increasing polarity. The potential antinociceptive effects and phytochemical profile of the ethyl acetate extract from A. spinosus were also performed.
Materials and methods
Plant material and extraction solvents
The leaves of A. spinosus were sampled from the northern Tunisia (36°51′36.43″N latitude and 10°11′36.13″E longitude) in June 2015 and deposited at the herbarium in the Faculty of Sciences Gafsa, Tunisia. Leaves (100 g) were dried and ground into powder through a mechanical blender. They were extracted for three times with ethanol 80%. After 24 h of agitation, the solution was filtered and then lyophilized with a freeze-dry system to obtain the ethanol extract (ASE). The n-hexane, chloroform, ethyl acetate, n-butanol and water extracts were obtained using the same procedure to give ASH, ASC, ASEA, ASB and ASW extracts, respectively.
Total phenolic content (TPC)
TPC was measured using a modified colorimetric Folin–Ciocalteu method previously reported by Tlili et al. (2013). Briefly, 10% of Folin–Ciocalteu reagent (5 mL) was added to 1 mg/mL of different sample (1 mL). After 5 min of incubation, 7.5% of Na2CO3 (2 mL) was added to the mixture and re-incubated for 60 min at room temperature in the dark. The absorbance was measured at 760 nm in UV–Vis spectrophotometer (Shimadzu, 1240 model, Tokyo, Japan).
Total flavonoid content (TFC)
TFC was performed according to the colorimetric assay previously published (Dewanto et al., 2002). One milliliter of sample (1 mg/mL) was mixed with 0.75 mL of 5% sodium nitrite solution. After 5 min, 10% aluminum chloride solution was added and the mixture was left standing for 5 min, and then 0.5 ml of 1 M sodium hydroxide was added to the solution. The volume of the mixture was adjusted to 2.5 mL with distilled water and mixed well. The absorbance was measured at 510 nm.
Total condensed tannin (TCT)
TCT was determined using the method of Sun et al. (1998). To each tested sample (50 µL), 1.5 mL of vanillin solution (4%) and 0.750 µL of concentrated H2SO4 were added. Then the mixture was incubated for 20 min in the dark. Finally, the absorbance was read at 500 nm.
Antioxidant activities
Total antioxidant capacity (TAC)
The TCA assay was carried using the method described by Prasad et al. (2009) with slight modifications. Different concentrations (10–100 µg/mL) of the sample were prepared. Then, 0.1 mL of each extract was added to 1 mL of the reagent solution of sulfuric acid, sodium phosphate and ammonium molybdate at concentrations of 0.6 M, 28 and 4 mM, respectively. The tubes were incubated in a boiling water bath at 95 °C for 90 min. The absorbance of each solution was measured at 695 nm. Vitamin C was used as positive control.
DPPH radical scavenging assay
The effect of different fractions on DPPH radical was determined following the method reported by Bounatirou et al. (2007) with slight modifications. Different concentrations (5–100 µg/mL) of each extract were mixed with 2 mL of a freshly prepared DPPH methanolic solution (0.1 mM). After 30 min of incubation in the dark, the absorbance was measured at 515 nm.
Hydrogen peroxide (H2O2) scavenging assay
This test was done using the method of Liu et al. (2010). One milliliter of the sample with different concentration (10–200 µg/mL) was mixed with 2.4 mL of phosphate buffer (0.1 M, pH 7.4) and 0.6 mL of H2O2 solution (40 mM). The mixture was shaken vigorously and incubated at room temperature for 10 min; vitamin C was used as positive control. The absorbance was measured at 230 nm.
Antinociceptive activity
Experimental animals
Swiss albino mice, about 22–25 g body weight (BW), were purchased from the Central Pharmacy (Tunisia) and were maintained for a 2-week adaptation period in a clean environment at ambient temperature with an alternating 12 h light–dark cycle and were fed with standard chow diet and water ad libitum. Animals were cared according to the Tunisian code of practice for the Care and Use of Animals for Scientific Purposes and the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (Council of Europe No 123, Strasbourg, 1985). Approval for these experiments was obtained from the Medical Ethical Committee for the Care and Use of Laboratory Animals of Pasteur Institute of Tunis (approval number: LNFP/Pro 152012). The number of animals and intensity of noxious stimuli used were the minimum necessary to demonstrate consistent effects of the drug treatments.
Toxicity study of the extract
Four groups (n = 6) received different doses of ASEA (50, 100, 500, 1000 mg/kg of BW) in 1% Tween 80 and diluted in normal saline; while the control group was orally treated with normal saline solution (10 mL/kg) and observed for toxic symptoms and death rate within 12 and 24 h. All the behavioral testing procedures were conducted blind with regard to the treatment groups, and tests were performed between 9:00 A.M. and 1:00 P.M.
Based on the results of the preliminary toxicity testing, the doses of the extract for further pharmacological studies were decided to be 200 and 600 mg/kg, BW.
Acetic acid-induced writhing
The test was conducted according to the method of Collier et al. (1968). Animals (six per group) were pretreated orally with ASEA (200 and 600 mg/kg) for 1 h and then acetic acid (1%, v/v in saline, 10 mL/kg) was injected intraperitoneally. Control animals received 0.9% saline solution (10 mL/kg) 1 h before acetic acid injection. Paracetamol (100 mg/kg) was administrated as reference drug for antinociception. All efforts were made to minimize animal suffering. The number of abdominal writhes observed within the first 20 min of treatment was enumerated.
Hot-plate test
The test was conducted according to the method Vaz et al. (1997). Animals (six per group) were pretreated orally (p.o.) with ASEA (200 and 600 mg/kg, p.o.) for 1 h and then were placed into a glass beaker on the heated plate at 47 ± 1 °C for maximum 60 s to prevent paw lesions. The control group (normal saline, 10 mL/kg) and positive groups (paracetamol, 100 mg/kg) were orally pretreated 60 min before submitted on a hot plate. The basal reaction time was noted when the mice licked their paws and that during 0, 15, 30, 45, 60 and 90 min after sample injection.
Formalin test
The test was performed as described by Hunskaar and Hole (1987). Mice (six per group) received ASEA (200 and 600 mg/kg, p.o.) and indomethacin (10 mg/kg) for 60 min prior to injecting 20 μL formalin (1%) into the right hind paws. The time spent for licking the injected paw was timed. The negative control animals were treated with normal saline (0.9%). Readings were determined in two separate time ranges after the formalin administration: from 0 to 5 min (neurogenic phase caused by direct stimulation of the nociceptors) and from 15 to 30 min (inflammatory phase caused by release of inflammatory mediators).
High-performance liquid chromatography (HPLC) analysis
The ASEA fraction showing the strongest antioxidant and analgesic activities was analyzed using HPLC. The analyses were performed in HPLC–DAD with a Varian ProStar HPLC System (Varian 330/Vis Detector and Varian 230 SDM). In the analyses, we used reverse phase chromatography performed under gradient conditions with C18 column (4.6 mm × 250 mm) and packed with 5-µm diameter particles; the mobile phase was containing solvent A: acetic acid at 2% in water and Solvent B: 40% acetonitrile, 2% acetic acid, and 58% water. The gradient was composed of 0–80% B for 25 min, 80–100% B for 10 min and 100–0% B for 5 min. The ASEA fraction was utilized in the concentration of 1 mg/mL. The flow rate was 0.9 mL/min and the volume injected was 40 µL. The detected compounds were identified by comparing with authentic standards injected under the same conditions and the use of DAD spectra (200–600 nm). Thus, twelve polyphenols standards were used: gallic acid, catechin, caffeic acid, epicatechin, vanillic acid, rutin, quercetin, luteolin, ferulic acid, coumaric acid, kaempferol and cinnamic acid.
Statistical analysis
Statistical analysis was performed using the SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA). All data were analyzed using ANOVA followed by Tukey test. All values are expressed as mean ± standard deviation (SD) from three different experiments. Differences were considered significant at p < 0.05.
Results and discussion
The yields and phytochemical analysis of various solvent extracts
Plant tissues are characterized by the presence of different antioxidant components, which make them relatively difficult to be quantified separately. Therefore, several extraction steps are necessary to guarantee the maximum separation of antioxidants (Khoudja et al. 2014).
The extraction yields of A. spinosus leaves using different solvents are shown in Table 1. The percentage yields ranged from 0.24 to 15.88% with a decreasing order of ethanol > water > butanol > ethyl acetate > chloroform > hexane extracts. The highest yields obtained in ethanol and water extracts can be explained by their high polarity.
The healing effects of plants are believed to be derived from bioactive substances that are named secondary metabolites. For these reasons, polyphenols from A. spinosus were investigated in this study. It was also important to note that, until now, there are no reports on total phenolics (TPC), total flavonoids (TFC) and total condensed tannin (TCT) contents, of the studied species, by the different extraction methods. Results of TPC, TFC, and TCT contents in various solvent extracts are displayed in Fig. 1. Significant differences were observed depending on the polarity of the solvent (p < 0.05). The highest TPC was found in ASEA (462.2 mg GAE/g DW), whereas ASE had the lowest value (45.2 mg GAE/g DW). The highest TCT was obtained in the ethyl acetate extract (5.01 mg CE/g DW). The amount of TFC differed greatly between samples, and this value was once again higher in ethyl acetate (30.07 mg CE/g DW) followed by chloroform > butanol > hexane > water > ethanol extracts. Our results confirmed the previous studies which showed that the solvents used for extraction have significant effects on the content of phenolic compounds (Huang et al. 2011) and the solubility of those bioactive compounds (Naczk and Shahidi 2006). Our results clearly demonstrated that the ethyl acetate is the most suitable solvent to attain the highest amount of TPC, TFC, and TCT. The ethyl acetate solvent was frequently used for the extraction of phenolic compounds with low and high molecular weight (Mariod et al. 2009). Moreover, Joana Gil-Chávez et al. (2013) reported that the ethyl acetate may be used in food products as food colorants.
Total phenolic content (a), condensed tannin content (b) and total flavonoid content (c) of extracts from Amaranthus spinosus obtained by various solvents. Means with different letters were significantly different at the level of p < 0.05. Each value is expressed as the mean ± SD of triplicate measurements
In vitro antioxidant activity of various solvent extracts
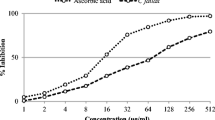
In this study, the antioxidant activity of A. spinosus using various solvent has been determined by the measurement of the total antioxidant activity, the scavenging ability towards DPPH radical and hydrogen peroxide scavenging activity. The total antioxidant activity was dependent on the extract concentration (Fig. 2). The ethyl acetate extract at a concentration of 90 µg/ml indicated the highest absorbance value (A695 = 0.81 ± 0.021), which suggested the strongest total antioxidant activity. As shown in Table 1, ASEA exhibited the highest total antioxidant activity due to their efficient EC50 value (45.45 ± 0.31 µg/mL) compared to that of vitamin C (35.71 ± 0.24 µg/mL). The EC50 values of the total antioxidant activity of various solvent extracts can be presented in the following order ethyl acetate > chloroform > butanol > water > ethanol > hexane. Correlation analyses indicate that the total antioxidant activity was significantly correlated to TPC (r2 = 0.908) and TFC (r2 = 0.883) (Figure 1S, supplementary information). However, the insignificant correlation was noted between condensed tannin and the total antioxidant activity (r2 = − 0.257).
The DPPH scavenging activity of all studied extracts increased in a manner dependent on the concentration (5–100 µg/mL) (Fig. 3a). At the concentration of 100 µg/mL, ASEA showed the highest scavenging ability compared to other extracts (83.25%). ASEA showed the highest activity due to their efficient EC50 value (27.32 ± 0.83 µg/mL) compared to that of vitamin C (15.67 ± 0.87 µg/mL). The EC50 values of scavenging ability of various solvent extracts can be presented in the following order: ASEA > ASB > ASC > ASW > ASE > ASH (Table 1). The correlation coefficient between TPC and TFC and EC50 values of radical scavenging activity with DPPH was highly significant (r2 = − 0.979 and -0.862, respectively). In accordance with previous studies, our results indicated the direct contribution of phenolics and flavonoids to DPPH radical scavenging activity (Hlila et al. 2015).
Antioxidant activity of various solvent extracts from Amaranthus spinosus. Vitamin C used as positive control. DPPH free radical scavenging activity (a) and hydrogen peroxide scavenging activity (b). Values are means of three replications ± SD. ASH, ASC, ASEA, ASB, ASW and ASE extracts were respectively, n-hexane, chloroform, ethyl acetate, n-butanol, water and ethanol extracts from Amaranthus spinosus leaves
The hydrogen peroxide scavenging activity of various solvent extracts from A. spinosus is presented in Fig. 3b. Similarly, all studied solvent extracts exhibited dose-dependent (H2O2) scavenging ability. At the concentration of 200 µg/mL, ASEA showed the highest radical scavenging activity value (93.68%) but significantly lower than vitamin C (97.63%). The sequence of EC50 values of scavenging ability was ethyl acetate > butanol > chloroform > water > ethanol > hexane (Table 1). The antioxidant activity in the H2O2 scavenging activity was correlated with TPC (r2 = − 0.827) and TFC (r2 = − 0.705) (Figure 1S, supplementary information). In general, results from this study demonstrated that A. spinosus is a good source of natural antioxidant. This is in agreement with the findings of Amin et al. (2006) emphasizing that antioxidant capacity of Amaranthus plants is due to their richness in bioactive components.
Analgesic activities of the ethyl acetate extract of A. spinosus in mice
Our results revealed that the ethyl acetate extract is rich in polyphenols and has strong antioxidant activity. For these reasons, ASEA was selected to carry out the analgesic activity in mice.
Toxicity study
The oral treatment with the ethyl acetate extract of A. spinosus at doses of 50, 100, 500, 1000 mg/kg of BW did not promote mortality, deleterious effects and behavior alterations. The result suggests that the extract has an LD50 of greater than 1000 mg/kg. Thus, based on these findings, the doses of 200 and 600 mg/kg BW were chosen to investigate the pharmacological activities in experimental animals.
Peripheral antinociceptive activity
Acetic acid-induced writhing is a perfect in vivo paradigm in evaluating the peripheral analgesic properties of medicinal plants. Previous studies have indicated that the peripheral analgesic effect of polyphenolic extract may be realized by inhibition of cyclooxygenase COX-synthesized prostaglandins. The injection of acetic acid (1%, p.i.) produced a typical model of writhing behavior in mice (Table 2). However, both doses of ASEA induced an attenuation of the painful stimuli in a dose-dependent manner in comparison with the normal control. After the administration of the ethyl acetate extract of A. spinosus at the dose 200 and 600 mg/kg, the percent inhibition values were 28.64 and 79.57%, respectively. On the other hand, paracetamol (reference drug; 100 mg/kg) showed a potent analgesic effect (p < 0.001) in relation to the control group. Collier et al. (1968) showed that the injection of the acetic acid in mice causes abdominal pain sensation and body elongation due to the stimulation of production and liberation of pro-inflammatory mediators and cytokines such as interleukine IL-8, tumor necrosis factor-α, prostaglandins, and bradykinins. Accordingly, the antinociceptive action of ASEA could be inhibiting the synthesis and release of these last one. These chemical mediators stimulate peripheral nociceptive neurons and induce dilatation of arterioles and venules with contraction and separation of endothelial cells, resulting in increased vascular permeability.
Central antinociceptive activity
The hot plate test was used for testing the central analgesic activity by measuring the reaction time of the perception of pain. The heat stimulation sensitizes peripheral nerve endings and the impulses generated propagate to the brain via the spinal cord. Hence, this test was conducted to examine the possible central antinociceptive action of ASEA. Compared to saline control, the analgesic effect of ASEA significantly increased the latency time in a dose-dependent manner at the different intervals tested. The ethyl acetate extract at dose 600 mg/kg showed maximum analgesic activity at 60 min with an increase in reaction time of 8.17 ± 0.15 s (p < 0.001) when compared with the response of normal saline groups (4.03 ± 0.11 s) (Table 3). Paracetamol (reference drug; 100 mg/kg) on the other hand, produced a very potent analgesic effect at 60 min with an increase in reaction time of 8.78 ± 0.22 s when compared to control animals (4.03 ± 0.11 s). This increasing of the latency times was due to the central analgesic effect of ethyl acetate extract; this means that the opioid-like receptors are involved. Schmauss and Yaksh (1984) demonstrated that central antinociceptive effects were mediated by opioid receptors, namely kappa and delta receptors.
Formalin-induced paw licking
The test of formalin-induced hyperalgesia in the mice was evaluated to better understand the antinociceptive effect of ASEA. This assay is well studied to evaluate the neurogenic pain (0–5 min of the test) and the inflammatory pain (15–30 min of the test). The first phase is caused by the direct stimulation of the nociceptors by formalin (Hunskaar and Hole 1987; Coderre et al. 1990). The late phase is due to the liberation of pro-inflammatory molecules such as prostaglandin (Wheeler-Aceto et al. 1990). Some findings have reported that others species in the family Amaranthaceae, such as Cyathula prostrata (Ibrahim et al. 2012), A. graecizans (Ishtiaq et al. 2017) and A. viridis (Jayaprakasam et al. 2004) inhibited nociception in experimental animals.
As shown in Fig. 4, the orally administrated ASEA (200 and 600 mg/kg) significantly inhibited (p < 0.001) paw licking, indicating analgesic effects on formalin-induced pain. The inhibition values for the neurogenic phase in the respective doses of 200 and 600 mg/kg and were 23.49 and 37.20%. Whereas, for the inflammatory phase, the percentage of inhibition for ASEA was 44.01 and 63.10% at 200 and 600 mg/kg, respectively. These obtained results also were in agreement with the central analgesic effects (neurogenic phase) in the hot-plate test and peripheral analgesic effects (inflammatory phase) in the acetic acid-induced writhing test. The possible mechanism of the antinociceptive activity of ASEA may be linked to the stimulation of nociceptors and release of many pro-inflammatory mediators (Ymele et al. 2013). Moreover, previous studies have reported that the anti-inflammatory effect of Amaranthus is contributed to the antinociceptive effects by their cyclooxygenase-1 (COX-1) and -2 (COX-2) enzymes inhibitory activities (Jayaprakasam et al. 2004).
Effects of the ethyl acetate extract of Amaranthus spinosus leaves (ASEA) and indomethacin (10 mg/kg) on formalin-induced pain in mice. 200 and 600 mg/kg represent the dose of ASEA. Values are means of three replications ± SD for the paw-licking time measured in first phase (0–5 min) and second phase (15–30 min) for each experimental group (n = 6). **p < 0.01 and ***p < 0.001 compared to the control group
Characterization of bioactive compounds by HPLC
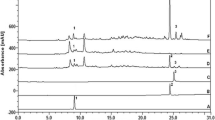
The ethyl acetate extract was analyzed by HPLC (Fig. 5). Six phenolic acids and four flavonoids were identified by comparing the retention time and UV spectra of compounds from different samples with those of standards in the same conditions.
HPLC–DAD profile of ethyl acetate extract of Amaranthus spinosus leaves (ASEA). a Phenolic acids at λ = 280 nm and b flavonoids at λ = 360 nm. Peaks (1) gallic acid, (2) catechin, (3) caffeic acid, (4) ferulic acid, (5) coumaric acid, (6) cinnamic acid, (7) rutin, (8) quercetin, (9) luteolin and (10) kaempferol
As shown in Table 4, the ethyl acetate extract was characterized by the predominance of caffeic acid (65.23%) followed by cinnamic acid (13.73%), catechin (5.89%), gallic acid (5.12%), luteolin (2.81%), coumaric acid (1.52%), rutin (1.08%), kaempferol (1.02%), quercetin (0.57%) and ferulic acid (0.57%). In recent years, many studies have shown that flavonoids contributed to the anti-inflammatory response through different mechanisms, principally modulation of pro-inflammatory gene expressions such as cyclooxygenase and nitric oxide synthase (Kim et al. 2004; Okoli et al. 2007). Mehrotra et al. (2011) reported that caffeic acid can inhibit the action of noxious stimuli producing a peripheral analgesic effect in rodents. In addition, caffeic acid is already known to have anti-inflammatory effects by inhibiting the activity of both COX-1 and COX-2 enzymes (Jayaprakasam et al. 2006). Moreover, caffeic acid was evinced to have antinociceptive action by promoting the inhibition of cytoplasmic protein kinase C (PKC) and nuclear factor-κB (NF-κB) activation (Nardini et al. 2001). Cinnamic acid and coumaric acid have been reported to have analgesic activity in vivo and anti-inflammatory action (Nwidu et al. 2011). Furthermore, Xu et al. 2016 demonstrated the antinociceptive action of the ferulic acid on neuropathic pain by targeting opioid receptors. Moreover, Kupeli and Yesilada (2007) reported the strong antinociceptive activity of quercetin and kaempferol. Therefore, we assume that these active metabolites showing antinociceptive activity might act synergically or individually to contribute to the analgesic activity of the A. spinosus.
Conclusion
Our findings demonstrated that A. spinosus ethyl acetate extract possesses in vitro antioxidant activities and in vivo antinociceptive effects. Moreover, this study reveals the peripheral and centrally acting analgesic properties of A. spinosus and justifies the traditional use of this plant for the treatment of pain. From our data, we can speculate that these beneficial effects of A. spinosus are related to the presence of a wide array of bioactive compounds. Nevertheless, further biological assays using isolated metabolites are solicited to confirm these activities, elucidate the mechanism of action and enhance drug discovery.
References
Amin I, Norazaidah Y, Emmy Hainida KI (2006) Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem 94:47–52
Bounatirou S, Smiti S, Miguel MG, Faleiro L, Rejeb MN, Neffati M, Pedro LG (2007) Chemical composition antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link. Food Chem 105:146–155
Carocho M, Ferreira IC (2013) A review on antioxidants prooxidants and related controversy natural and synthetic compounds screening and analysis methodologies and future perspectives. Food Chem Toxicol 51:15–25
Coderre TJ, Vaccarino AL, Melzack R (1990) Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res 535:155–158
Collier HOJ, Dinneen LC, Johnson CA, Schneider C (1968) The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol 32:295–310
Dewanto V, Wu X, Adom K, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Hlila MB, Mosbah H, Mssada K, Jannet HB, Aouni M, Selmi B (2015) Acetylcholinesterase inhibitory and antioxidant properties of roots extracts from the Tunisian Scabiosa arenaria Forssk. Ind Crop Prod 67:62–69
Huang B, Ke H, He J, Ban X, Zeng H, Wang Y (2011) Extracts of Halenia elliptica exhibit antioxidant properties in vitro and in vivo. Food Chem Toxicol 49:185–190
Hunskaar S, Hole K (1987) The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30:103–114
Ibrahim B, Sowemimo A, van Rooyen A, Van de Venter M (2012) Antiinflammatory analgesic and antioxidant activities of Cyathula prostrata (Linn.) Blume (Amaranthaceae). J Ethnopharmacol 141:282–289
Ishtiaq S, Ali T, Ahmad B, Anwar F, Afridi MSK, Shaheen H (2017) Phytochemical and biological evaluations of methanolic extract of Amaranthus graecizans subsp. silvestris (Vill.) Brenan. Br J Pharm Res 15:1–11
Jayaprakasam B, Zhang Y, Nair MG (2004) Tumor cell proliferation and cyclooxygenase enzyme inhibitory compounds in Amaranthus tricolor. J Agric Food Chem 52:6939–6943
Jayaprakasam B, Vanisree M, Zhang Y, Dewitt DL, Nair MG (2006) Impact of alkyl esters of caffeic and ferulic acids on tumor cell proliferation, cyclooxygenase enzyme, and lipid peroxidation. J Agric Food Chem 54:5375–5381
Joana Gil-Chávez G, Villa JA, Fernando Ayala-Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM, González-Aguilar GA (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients an overview. Compreh Rev Food Sci Food Saf 12:5–23
Khoudja NK, Boulekbache-Makhlouf L, Madani K (2014) Antioxidant capacity of crude extracts and their solvent fractions of selected Algerian Lamiaceae. Ind Crop Prod 52:177–182
Kim HP, Son KH, Chang HW, Kang SS (2004) Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci 96:229–245
Kumar BSA, Lakshman K, Jayaveera KN, Shekar DS, Nandeesh R, Velmurugan C (2010) Chemoprotective and antioxidant activities of methanolic extract of Amaranthus spinosus leaves on paracetamol induced-liver damage in rats. Acta Med Salin 39:68–74
Liu J, Luo J, Ye H, Sun Y, Lu Z, Zeng X (2010) In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr Polym 82:1278–1283
Mariod AA, Ibrahim RM, Ismail M, Ismail N (2009) Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem 116:306–312
Mehrotra A, Shanbhag R, Chamallamudi MR, Singh VP, Mudgal J (2011) Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur J Pharmacol 666:80–86
Miller T (1983) Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Ame J Physiol Gastrointest Liver Physiol 245:G601–G623
Naczk M, Shahidi F (2006) Phenolics in cereals fruits and vegetables occurrence extraction and analysis. J Pharm Biomed Anal 41:1523–1542
Nardini M, Leonardi F, Scaccini C, Virgili F (2001) Modulation of ceramide-induced NF-κB binding activity and apoptotic response by caffeic acid in U937 cells: comparison with other antioxidants. Free Rad Biol Med 30:722–733
Nwidu LL, Nwafor PA, Da Silva VC, Rodrigues CM, dos Santos LC, Vilegas W, Nunes-de-Souza RL (2011) Anti-nociceptive effects of Carpolobia lutea G. Don (Polygalaceae) leaf fractions in animal models. Inflammopharmacol 19:215–225
Okoli CO, Akah PA, Nwafor SV, Anisiobi AI, Ibegbunam IN, Erojikwe O (2007) Anti-inflammatory activity of hexane leaf extract of Aspilia africana CD Adams. J Ethnopharmacol 109:219–225
Prasad KN, Yang B, Yang S, Chen Y, Zhao M, Ashraf M, Jiang Y (2009) Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem 116:1–7
Rjeibi I, Saad AB, Hfaiedh N (2016) Oxidative damage and hepatotoxicity associated with deltamethrin in rats: the protective effects of Amaranthus spinosus seed extract. Biomed Pharmacother 84:853–860
Schmauss C, Yaksh TL (1984) In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther 228:1–12
Sun B, Ricardo-da-Silva JM, Spranger I (1998) Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem 46:4267–4274
Tanmoy G, Arijit M, Tanushree S, Jagadish S, Kumar MT (2014) Pharmacological actions and phytoconstituents of Amaranthus spinosus Linn a review. Intern J Pharmacogn Phytochem Res 6:405–413
Tlili N, Elfalleh W, Hannachi H, Yahia Y, Khaldi A, Ferchichi A, Nasri N (2013) Screening of natural antioxidants from selected medicinal plants. Int J Food Prop 16:1117–1126
Vaz ZR, Mata LV, Calixto JB (1997) Analgesic effect of the herbal medicine catuama in thermal and chemical models of nociception in mice. Phytother Res 11:101–110
Wheeler-Aceto H, Porreca F, Cowan A (1990) The rat paw formalin test: comparison of noxious agents. Pain 40:229–238
Xu Y, Lin D, Yu X, Xie X, Wang L, Lian L, Huang X (2016) The antinociceptive effects of ferulic acid on neuropathic pain: involvement of descending monoaminergic system and opioid receptors. Oncotarget 7:20455–20468
Ye Küpeli E, Silada E (2007) Flavonoids with anti-inflammatory and antinociceptive activity from Cistus laurifolius L. leaves through bioassay-guided procedures. J Ethnopharmacol 112:524–530
Ymele EV, Dongmo AB, Dimo T (2013) Analgesic and anti-inflammatory effect of aqueous extract of the stem bark of Allanblackia gabonensis (Guttiferae). Inflammopharmacol 21:21–30
Zeashan H, Amresh G, Singh S, Rao CV (2008) Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem Toxicol 46:3417–3421
Zeashan H, Amresh G, Rao CV, Singh S (2009) Antinociceptive activity of Amaranthus spinosus in experimental animals. J Ethnopharmacol 122:492–496
Acknowledgements
The authors express their gratitude to the Ministry of Higher Education and Scientific Research.
Funding
The authors did not get any fund from any organization for this study.
Author information
Authors and Affiliations
Contributions
IR conducted the experiments and wrote the draft. ABS conducted data analysis and contributed to the writing of the manuscript. JS and AF contributed substantially to the writing of the manuscript. SN participated in experimental design and provided reagents. MSA revised the pharmacological part of the manuscript. NH participated in the direction of all the experimental parts of the manuscript. SS has provided direction during the current investigation and has contributed to the writing of the manuscript. All authors edited, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors have declared no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rjeibi, I., Ben Saad, A., Sdayria, J. et al. HPLC–DAD identification of polyphenols from ethyl acetate extract of Amaranthus spinosus leaves and determination of their antioxidant and antinociceptive effects. Inflammopharmacol 27, 975–984 (2019). https://doi.org/10.1007/s10787-018-0482-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0482-0