Abstract
The ethanol extract of tuber part of Colocasia fallax Schott (Araceae) was tested for its potential analgesic and antioxidant activities. Acetic acid induced writhing method was used to evaluate the analgesic activity at the concentration of 250 and 500 mg/kg body weight. In order to investigate the antioxidant activity, four complementary test systems, namely, 2,2-diphenyl-1-picryl-hydrazil (DPPH) free radical scavenging, ferric reducing power assay, total phenolic and flavonoid contents were used. Polyphenolic compounds were also detected by HPLC analysis. The extract showed significant (P < 0.005) analgesic activity with 32.14 and 53.57 % writhing inhibition at the concentrations of 250 and 500 mg/kg body weight, respectively that was comparable to standard diclofenac sodium (78.57 % at 25 mg/kg). In DPPH radical scavenging assay, the extract showed the IC50 value of 78.94 μg/ml. Reducing power of the extract was comparable to that of butylated hydroxy toluene (BHT). In HPLC analysis of polyphenols, epicatechin, myricetin, trans-cinnamic, syringic acid, vanillin, p-coumaric acid, trans-ferulic acid and rosmarinic acid were detected in the extract (95.95, 52.14, 20.39, 8.32, 8.43, 6.36, 4.18 and 6.73 mg/100 g of dry extract, respectively). Presence of polyphenols in the extract might have attributed towards the observed bio-activity. Present finding also suggests the traditional use of this plant in the management of pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Botanicals provide an extensive source of new drugs derived from nature with various chemical entities (Sharma and Singh 2014). Extensive studies have been conducted on plant extracts based on traditional uses to find out therapeutically active constituents to be used as new drugs (Rates 2001). Bangladesh is a rich source of medicinal plants. Mass population of Bangladesh use vegetables as food as well as to modify several pathological conditions. Vegetables contain important classes of phytochemicals that are accountable for different pharmacological activities (Rodriguez et al. 2006; Williams et al. 2013).
Colocasia fallax Schott, belongs to the family of Araceae, is locally known as buno kochu. It is an annual or perennial edible herb grown in aquatic area. It is native to Bangladesh, Bhutan, China, India, Nepal, Thailand, Vietnam (Allen 2011). C. fallax is available in northern districts of Bangladesh. The edible part, known as taro, is traditionally used to mitigate pain and to treat wound infection (Hosne and Abul 2006). The taro is used as a vegetable and a good source of minerals and vitamins (Brukil 1966). To the best of our knowledge, no pharmacological studies have been reported so far on C. fallax. As a part of the continuation of our research on bioactivity screening of Bangladeshi medicinal edible herbs, present study was conducted to evaluate antioxidant and analgesic activities of ethanol extract of C. fallax. HPLC analysis was performed to detect the existence of some bioactive polyphenols commonly occurring in plants.
Materials and methods
Chemicals and reagents
DPPH (2,2-diphenyl-1-picryldydrazyl), Folin-Ciocalteu reagent, all polyphenolic acids and other standard chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA). HPLC grade acetic acid, acetonitrile, methanol and ethanol were obtained from Merck (Darmstadt, Germany). Unless otherwise specified, all other chemicals were of analytical grade.
Experimental animals
Young Swiss albino mice were procured from the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B) for the present study. They were used under normal laboratory settings for standard environmental conditions with water and food ad libitum. They were kept in the laboratory for 2 weeks before experiment for acclimatisation. The selected animals were treated under the ethical guidelines for animal experimentation authorised by Pharmacy Discipline, Khulna University.
Plant materials and extraction
The tuber part of C. fallax, was collected from Jessore, Bangladesh and identified by experts at Bangladesh National Herbarium, Bangladesh. A voucher specimen (DACB 41153) has been submitted there for future reference. Collected plant material was cut into small pieces for proper drying. Shade dried taro was ground into coarse powder that was stored in an airtight container kept in a cool, dark and dry place. About 500 g powder was soaked in 800 ml of 98 % of ethanol in a glass container for 7 days accompanying regular shaking and stirring. Extract was separated from the plant debris by filtration using a piece of clean, white cotton plug and dried using rotary vacuum evaporator (Bibby RE200, Sterilin Ltd., UK) at 45 °C to get dried ethanol extract (16.16 g, yield =3.23 %). Then the crude extract was stored in a refrigerator at 4 °C.
Phytochemical screening
Phytochemical screening represents the identification of different phytochemical groups present in a plant extract. Different chemical groups such as carbohydrates, alkaloids, glycosides, phenolic compounds, flavonoids, tannins, steroids, gums, saponins, proteins, amino acids and acidic compounds were identified by characteristic colour change using standard chemical tests (Ghani 2005).
Analgesic activity
Analgesic activity of the extract was tested using the model of acetic acid induced writhing in mice (Koster et al. 1959; Islam et al. 2005). The experimental laboratory mice were randomly divided in four groups each containing five mice. The first group, treated as control group, was administered orally with 1 % (v/v) Tween-80 in distilled water at the dose of 10 ml/kg body weight. The second group received standard diclofenac sodium (25 mg/kg). Third and fourth groups were treated with the extract at the doses of 250 and 500 mg/kg body weight, respectively. Test samples, standard drug and control vehicle were administered orally 30 min before intraperitoneal administration of 0.7 % of acetic acid. After an interval of 5 min, the number of writhes was counted for a period of 15 min. The number of writhes in the second, third and fourth groups was compared to that of the control group to calculate the percent inhibition of writhing calculated using the formula:
Where, W1 and W0 represent the mean writhing of the control and standard or sample groups, respectively.
DPPH radical scavenging assay
The radical scavenging activity of the extract was quantitatively estimated on the basis of its ability to scavenge the stable free radical 2,2-diphenyl-1-picryl hydrazyl (DPPH) (Uddin et al. 2004; Islam et al. 2014). From the stock solution, different concentrations (512–1 μg/ml) of sample were prepared. In 1 ml of each concentration, 3 ml of instantly prepared 0.1 mM alcoholic DPPH solution was added. After 30 min of incubation in dark at room temperature, absorbance was taken at 517 nm. Ascorbic acid, a natural antioxidant, was used as standard. The percentage of DPPH free radical scavenging activity of each plant extracts and standard drug were calculated as:
Where, A0 is the absorbance of the control solution containing all reagents except plant extracts, A is the absorbance of DPPH solution containing plant extract. Finally, the concentration of sample required to scavenge 50 % DPPH free radical (IC50) was calculated from the plot of inhibition (%) against the concentration of the extract. BHT was used as positive control in this experiment.
Reducing power assay
The reducing power of C. fallax extract was determined according to method described by Dehpour et al. with slight modification (Dehpour et al. 2009; Saha et al. 2013; Oyaizu 1986). In 1 ml of different concentrations (0.1–1 mg/ml) of sample, 2.5 ml phosphate buffer (200 mM, pH 6.6) and 2.5 ml potassium ferricyanide (10 g/L) was added sequentially. The mixture was incubated at 50 °C for 20 min. At room temperature, 2.5 ml trichloroacetic acid (10 % w/v) was added to the mixture and centrifuged at 3000 rpm for 10 min. In an aliquot of 2.5 ml supernatant, 2.5 ml distilled water and 0.5 ml ferric chloride (1 g/L) were mixed. Ten minutes later, absorbance of resultant complex was measured at 700 nm. Butylated hydroxy toluene (BHT) was used as standard to compare the reducing power of the extract.
Total phenolic content
The total phenolic content of the extract was determined by the modified Folin-Ciocalteu’s method (Hemayet et al. 2013). Ethanol solution of the extract was mixed with 5 ml of 10 % Folin-Ciocalteu reagent. Then an aliquot of 4 ml sodium carbonate (75 g/L) was added to the mixture. It was kept at 40 °C for 30 min. Absorbance of the reaction mixture was measured at 765 nm. Different concentrations (0.1–0.5 mg/ml) of gallic acid were used for the preparation of standard calibration curve from where total phenol content was determined and expressed as mg gallic acid equivalent (GAE) per gram of dry extract.
Total flavonoids content
Total flavonoid content was estimated using a simple and well known aluminum chloride colorimetric assay (Shah et al. 2012). In the extract solution, 4 ml distilled water was added followed by 0.3 ml 5 % w/v sodium nitrate. After 5 min, 0.3 ml of 10 % w/v aluminum chloride was added. At the sixth min, 2 ml of 1 M sodium hydroxide was added and the volume was adjusted up to 10 ml. Then absorbance was measured at 510 nm against blank solution. For this assay, quercetin (0.1–0.5 mg/ml) was used to prepare standard calibration curve and total flavonoid content of the extract was expressed in terms of mg quercetin equivalent (QE)/g of dried extract.
HPLC detection and quantification of polyphenolic compounds
HPLC analysis was carried out for the detection and quantification of phenolic compounds in the extract using DionexUltiMate 3000 system equipped with quaternary rapid separation pump (LPG-3400RS) and photodiode array detector (DAD-3000RS). Separation was performed using C18 (5 μm) Dionex column (4.6 × 250 mm) at 30 °C with a flow rate of 1 ml/min and an injection volume of 20 μl. The mobile phase consisted of acetonitrile (solvent A), acetic acid solution pH 3.0 (solvent B), and methanol (solvent C) with the gradient elution program of 5 % A/95%B (0–5 min), 10 % A/90 % B (6–9 min), 15 % A/75 % B/10 % C (11–15 min), 20 % A/65 % B /15 % C (16–19 min), 30 % A/50 % B /20 % C (20–29 min), 40 % A /30 % B/30 % C (30–35 min) and 100 % A (36–40 min). In order to prepare a calibration curve, standard stock solution was prepared in methanol containing arbutin, (−)-epicatechin (5 μg/ml each), gallic acid, hydroquinone, vanillic acid, rosmarinic acid, myricetin (4 μg/ml each), caffeic acid, syringic acid, vanillin, trans-ferulic acid (3 μg/ml each), p-coumaric acid, quercetin, kaempferol (2 μg/ml each), (+)-catechin hydrate, ellagic acid (10 μg/ml each), trans-cinnamic acid (1 μg/ml), rutin hydrate (6 μg/ml) and benzoic acid (8 μg/ml). A solution of the extract was prepared in methanol having the concentration of 10 mg/ml. All the solutions were filtered through 0.2 μm syringe filter and degassed in an ultrasonic bath prior to HPLC analysis (Himangsu et al. 2014).
Results
Phytochemical screening
Phytochemical studies showed that carbohydrate, alkaloids, glycosides, phenolic compounds, flavonoids, tannins, gum and acidic compounds were present, while steroids, proteins, amino acids and saponins were absent in the extract.
Analgesic activity
The extract showed significant (P < 0.005) and dose dependent analgesic activity and revealed 32.14 and 53.57 % writhing inhibition at the doses of 250 and 500 mg/kg body weight, respectively whereas standard diclofenac sodium (25 mg/kg) showed 78.57 % writhing inhibition (Table 1).
DPPH radical scavenging assay
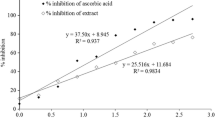
In the DPPH radical scavenging assay, antioxidant activity was gradually increased with increasing concentration of the extract with the IC50 value of 78.94 μg/ml while that of ascorbic acid was 14.68 μg/ml (Fig. 1).
Reducing power assay
Reducing power of the extract was found to be concentration dependent. The maximum absorbance of 0.564 was observed at the highest conc of the extract, i.e., 1 mg/ml. BHT, used as the positive control showed maximum absorbance of 1.472 at the same concentration (Fig. 2).
Total phenolic content
The absorbance values obtained in the test using different concentrations of gallic acid were plotted against respective concentrations. A standard calibration curve was obtained with the equation y = 8.043× + 0.213 (R2 = 0.985) (Fig. 3) Total phenolic content of the extract was calculated using the equation and found to be 16.37 mg GAE/g dry extract.
Total flavonoid content
Different concentrations of quercetin were used to obtain a standard calibration curve (y = 0.475 + 0.006; R2 = 0.991) (Fig. 4) from where total flavonoid content of the extract was calculated as11.44 mg QE/g dry extract.
Results of HPLC analysis of C. fallax extract
Identification and quantification of individual phenolic compounds in the ethanol extract of C. fallax were analysed by HPLC. The chromatographic separations of polyphenols in standard and ethanol extract are shown in Figs. 5 and 6, respectively. The content of each phenolic compound was calculated from the corresponding calibration curve and presented as the mean of five determinations as shown in Table 2.
HPLC chromatogram of a standard mixture of polyphenolic compounds (Peaks 1: arbutin, 2:gallic acid, 3: hydroquinone, 4: (+)-catechin, 5: vanillic acid, 6: caffeic acid, 7: syringic acid, 8: (−)-epicatechin, 9: vanillin, 10: p-coumaric acid, 11: trans-ferulic acid, 12: rutin hydrate, 13: ellagic acid, 14: benzoic acid, 15: rosmarinic acid, 16: myricetin, 17: quercetin, 18: trans-cinnamic acid, 19: kaempferol)
The experimental results indicated that ethanol extract of C. fallax contain a high concentration of epicatechin (95.95 mg/100 g of dry extract). Myricetin and trans-cinnamic acid were also detected at moderate concentrations (52.14 and 20.39 mg/100 g of dry extract respectively). In addition, syringic acid, vanillin, p-coumaric acid, trans-ferulic acid and rosmarinic acid were also detected in lower concentrations (8.32, 8.43, 6.36, 4.18 and 6.73 mg/100 g of dry extract, respectively).
Discussion
Medicinal plants, as source of remedies help our body to fight against free radicals, pain, germs and toxins. These pharmacological effects are due to the presence of important classes of phytochemicals. Preliminary phytochemical analysis of the extract indicated the presence of some pharmacologically important secondary metabolites such as alkaloids, glycosides, phenolic compounds and flavonoids.
Acetic acid induced writhing is a reliable method to evaluate peripherally acting analgesic activity of plant extract. Acetic acid-induced writhing model represents pain sensation by triggering localised inflammatory response. Acetic acid triggers synthesis of several local hormones namely prostaglandins, bradykinin, serotonin, histamine etc. that excite the pain nerve endings (Shilpi et al. 2006). It is well known that analgesic and antioxidant activities are correlated to ameliorate pain sensation (Gülçin et al. 2004, Areekul and Phomkaivon 2015). Plant materials containing polyphenols, flavonoids and phenolic acids exert analgesic activity (Guan-Jhong et al. 2012; Kumar and Pandey 2013). Myricetin, syringic acid, epicatechin, vanillin, p-coumaric acid and trans-cinnamic acid exert analgesic activity by inhibiting neutrophil degranulation subsequently decreasing the release of local hormone arachidonic acid (Ismet et al. 2014; Hoult et al. 1994). The experimental extract significantly (p < 0.05) inhibited acetic acid induced pain sensation in a dose dependent manner. The extract might have exerted analgesic effect by interfering synthesis, release or antagonising local hormones. Flavonoids, as well as phenolic acids may be the active components of C. fallax that relates to its analgesic activity.
Ethanoic extract of C. fallax was screened for evaluation of antioxidant activity using four complementary assays, namely DPPH free radical scavenging, reducing power assay, total phenolic and flavonoid contents. DPPH test is a direct and reliable method based on the ability of DPPH, a stable free radical, to decolorise in presence of antioxidant. This method is widely used to predict antioxidant properties of plant extracts (Yen and Duh 1994; Kulisica et al. 2004). The free radical inhibitory effect was found to be dose dependent and the IC50 was calculated as 78.94 μg/ml.
In addition to DPPH test, we performed reducing power assay to support the results. There is a direct correlation between antioxidant capacity and reducing power of certain plant extracts (Tanaka et al. 1988). The reducing properties are generally linked with the presence of reductones, which have been reported to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (Duh et al. 1999). Reducing power of the extract was also found to be concentration dependent. The maximum absorbance was noted 0.564 at 1 mg/ml. BHT, which was used as positive control showed maximum absorbance 1.472 at the same concentration (Fig. 2). Secondary metabolites of plant origin, especially phenolic compounds such as flavonoids, tannins, phenolic acids etc. are very important components for the free radical scavenging activities. Having similar chemical properties, these polyphenolic compounds react as hydrogen donors and neutralise free radicals (Miliauskas et al. 2004; Atoui et al. 2005). The antioxidant activity depends on both the quantity and type of polyphenols present in the extract. In this study we quantified the total flavonoid and phenolic contents. The C. fallax extract showed high level of polyphenols as estimated by Folin-Ciocalteu reagent. HPLC analysis of C. fallax was also carried out to identify some selected polyphenols that are abundant in plants. It showed the presence of syringic acid; (−)-epicatechin; vanillin; p-coumaric acid; trans-ferulic acid; rosmarinic acid; myricetin; trans-cinnamic acid. Among different groups of flavonoids, flavonols is a major group to contribute towards antioxidant activity (Inmaculada et al. 2015). Myricetin, member of flavonols is a major polyphenol present in the extract. Antioxidant activity of epicatechin, coumaric acid, vanillin, trans-cinnamic acid are well established (Inmaculada et al. 2015; Monika et al. 2014; Emira et al. 2014; Sina et al. 2014; Harleen et al. 2011). Four complementary assays of ethanol extract provided evidence that the antioxidant activities of C. fallax may be due to the presence of good amount of natural antioxidants and their synergistic effect.
Conclusion
The present study inferred that the ethanol extract of Colocasia fallax possesses potent analgesic and antioxidant activity. These pharmacologic actions are dose dependent. These potentials may be due to the polyphenols present in the extract. Further, pharmacological investigation and bioactivity guided studies are required to isolate the active principle(s) responsible for these activities.
References
Allen DJ (2011) Colocasiafallax. The International Union for Conservation of Nature and Natural Resources (IUCN) Red List of Threatened Species. Version 2015.2
Areekul V, Phomkaivon N (2015) Thai indigenous plants: focusing on Total phenolic content, antioxidant activity and their correlation on medicinal effects. KMITL Sci Tech J 15(1)
Atoui AK, Mansouri A, Boskou G, Kefalas P ( 2005) Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem 89:27–36
Brukil H (1966) A Dictionary of the economic products of malay peninsula, Governments of Malaysia & Singapour by ministry of Agriculture and cooparetives; kualalampur, Malaysia. Vol-I (A-H) and vol-II(I-Z); p 2444
Dehpour AA, Ebrahimzadeh MA, Nabavi SF, Nabavi SM (2009) Antioxidant activity of methanol extract of Ferula asafoetida and its essential oil composition. Grasas Aceites 60(4):405–412
Duh PD, Tu YY, Yen GC (1999) Antioxidant activity of the aqueous extract of harng Jyur (Chrysanthemum morifolium Ramat). Lebensm Wiss Technol 32:269–277
Emira N, Mejdi S, Inès N, Eulogio V, Mahjoub A, Abdulbasit A (2014) Comparative study on the antifungal and antioxidant properties of natural and colored Juglansregia L. barks: A high activity against vaginal Candida strains. Life Sci J 11(8):327–335
Ghani A (2005) Practical phytochemistry. Parash Publishers, Dhaka, pp. 8–20
Guan-Jhong H, Ming-Hsing H, Chuan-Sung C, Shyh-Shyun H, Pei-Hsin S, Ming-Tsung Y, Bor-Sen W (2012) Analgesic and anti-inflammatory activities of aqueous extracts of Fructus LigustriLucidi. J Food Drug Anal 20(3):617–627
Gülçin I, Küfrevio I, Oktay M, Büyükokuro ME (2004) Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 90:205–215
Harleen KS, Bimlesh K, Sunil P, Prashant T, Manoj S, Pardeep S (2011) A review of phytochemistry and pharmacology of flavonoids. Int Pharm Sci 1(1):25–41
Hemayet H, Ismet AJ, Sariful IH, Jamil AS, Shubhra KD, Arpona H, Arif A (2013) Anti- inflammatory and antioxidant activities of ethanolic leaf extract of Brownlowiatersa (L.) Kosterm. Orient Pharm Exp Med 13(3):181–189
Himangsu M, Sanjib S, Khalijah A, Hemayet H, Abdulwali A, Khirul MI, Ismet AJ, Samir KS, Golam MH, Jamil AS, Shaikh JU (2014) Central-stimulating and analgesic activity of the ethanolic extract of Alternantherasessilis in mice. BMC Complement Altern Med 14:398
Hosne A, Abul MH (2006) Three new records of Aroids (Araceae) for Bangladesh. Bangladesh J Plant Taxon 13(2):83–91
Hoult JRS, Moroney MA, Paya M (1994) Actions of flavonoids and coumarins on lipoxygenase and cyclooxygenase. Methods Enzymol 234:443–454
Inmaculada N, Rocío G, Verónica G, Ana Belén B, María JP (2015) Nutritional composition and antioxidant capacity in edible flowers: characterisation of phenolic compounds by HPLC-DAD ESI/MSn. Int J Mol Sci 16:805–822
Islam MA, Ahmed F, Das AK, Bachar SC (2005) Analgesic and anti-inflammatory activity of Leonurus sibiricus. Fitoterapia 76:359–362
Islam MK, Nripendra NB, Sanjib S, Hemayet H, Ismet AJ, Tanzir AK, Khalijah A, Jamil AS (2014) Antinociceptive and antioxidant activity of Zanthoxylumbudrunga Wall (Rutaceae) seeds. Sci World J. doi:10.1155/2014/869537
Ismet AJ, Proity NA, Nasima K, Tanzir AK, Mohammad MR, Arpona H, Hemayet H (2014) Comparative study of anti-nociceptive activity and phenolic content of the ethanol extracts of Piper nigrum and Piper longum fruits. Int J Pharm Sci Rev Res 27(1):47–52
Koster R, Anderson M, De-Beer EJ (1959) Acetic acid for analgesic screening. Fed Proc 18:412–418
Kulisica T, Radonicb A, Katalinicc V, Milosa M (2004) Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 85:633–640
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: An overview. ScientificWorldJournal. doi:10.1155/2013/162750
Miliauskas G, Venskutonis PR, Van Beek TA (2004) Screening of radical scavenging activity of some medicinal plants and aromatic plant extract. Food Chem 85:231–237
Monika M, Nomita G, Palak P, Varsha M, Manisha K (2014) Phytochemical evaluation and pharmacological activity of Syzygium aromaticum: a comprehensive review. Int J Pharm Pharm Sci 6(8):67–72
Oyaizu M (1986) Studies on product of browning reaction prepared from glucosamine. Jpn J Nutr Diet 44(6):307–315
Rates SMK (2001) Review: plants as source of drugs. Toxicon 39:603–613
Rodriguez EB, Flavier ME, Rodriguez-Amaya DB, Amaya-Farfán J (2006) Phytochemicals and functional foods. current situation and prospect for developing countries. Segurança Alimentar e Nutricional, Campinas 13(1):1–22
Saha S, Shilpi JA, Mondal H, Gofur R, Billah M, Nahar L, Satyajit DS (2013) Bioactivity studies on Musa seminifera Lour. Pharmacogn Mag 9(36):315–322
Shah M, Behara YR, Jagadeesh B (2012) Phytochemical screening and in vitro antioxidant activity of aqueous and hydroalcoholic extract of Bacopamonnieri Linn. Int J Pharm Sci Res 3(9):3418–3424
Sharma V, Singh M (2014) Antinephrotoxic efficacy of Operculina turpethum and its isolated stigma-5,22 dien-3-O-b-D-glucopyranoside against N-nitrosodimethylamine induced renal carcinogenesis in male mice. Int J Phytopharm 4(2):777779–777756
Shilpi JA, Rouf R, Ferdous MM, Uddin SJ (2006) Central nervous system stimulatingactivity of the ethanolic extract of Fleurya interrupta Gaud.(Urticaceae). Orient Pharm Exp Med 6(1):21–26
Sina C, Ion T, Violeta N, Mira I, Felicia T (2014) Phenolics content, antioxidant activity and color of green walnut extracts for preparing walnut liquor. Not Bot Horti Agrobo 42(2):551–555
Tanaka M, Kuie CW, Nagashima Y, Taguchi T (1988) Applications of antioxidative Maillard reaction products from histidine and glucose to sardine products. Nippon Suisan Gakk 54:1409–14
Uddin SJ, Shilpi JA, Delazar A, Nahar L, Sarker SD (2004) Free radical scavenging activity of some Bangladeshi plant extracts. Orient Pharm Exp Med 4(3):187–195
Williams DJ, Edwards D, Hamernigb I, Jian L, James AP, Johnson SK, Tapsell LC (2013) Vegetables containing phytochemicals with potential anti-obesity properties: a review. Food Res Int 52(1):323–333
Yen GC, Duh PD (1994) Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-Radical and Active-Oxygen Species. J Agric Food Chem 42(3):629–632
Acknowledgments
Authors are grateful to Pharmacy Discipline, Life Science School, Khulna University, Khulna, Bangladesh for providing technical support to carry out the present investigations successfully. We would like thanks Mr. Hemayet Hossain, Scientific Officer, BCSIR, Dhaka, Bangladesh for HPLC analysis of the sample.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
N/A
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zilani, M.N.H., Islam, M.A., Khushi, S.S. et al. Analgesic and antioxidant activities of Colocasia fallax . Orient Pharm Exp Med 16, 131–137 (2016). https://doi.org/10.1007/s13596-016-0222-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-016-0222-1