Abstract
Most of the species of the Teucrium genus are known for their strong anti-inflammatory and antioxidant activities due to their richness in phenolic compounds. Thus, one species of the Teucrium genus belonging to the Lamiaceae family named Teucrium pseudochamaepitys L. was chosen in this study. The main objective of this study was to establish the pharmacological properties of T. pseudochamaepitys L. aqueous decoctions using both in vitro and in vivo experiments. This research represents the first attempt to validate the medicinal effects of this plant. The chemical composition was determined using SPE LC-MS/MS analysis, revealing the presence of flavonoids and phenolic acids. The biological activity and chemical composition of the aqueous extract of T. pseudochamaepitys (TpAE) were investigated for the first time. Scavenging of DPPH·, ABTS·+, reducing power, and phenanthroline assays was employed in order to measure the antioxidant activity of TpAE. The results showed that TpAE exhibited significant antioxidant activity, with the best performance of the phenanthroline method. In order to evaluate in vitro anti-inflammatory effect, the inhibition of albumin denaturation by TpAE was investigated. TpAE confers high potential anti-inflammatory activity with a percentage of inhibition very close to that of the reference diclofenac. Acute toxicity, anti-inflammatory, and analgesic activities were performed in order to investigate in vivo biological activity of TpAE. Oral administration of TpAE at a single dose of 2000 mg/kg did not cause any symptoms of intoxication in all treated animals. On the other hand, the oral administration of TpAE at different concentrations inhibits the development of paw edema and produces significant inhibition in acetic acid-induced writhing test. Our study revealed that TpAE is not toxic but contains bioactive compounds with antioxidant, anti-inflammatory, and analgesic activity.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous wild plant species across the globe possess medicinal properties and continue to be utilized within the realm of alternative medicine. The natural world has long been acknowledged and continues to be regarded as the oldest and most abundant source of a diverse array of medicinal resources. The primary factor driving the widespread interest in herbal medicines is the perception among individuals that these alternative treatments are devoid of adverse effects, offer cost-effective solutions, and are significantly safer compared to conventional medications (Muhammad et al. 2012). These sources are of significant importance as they contain numerous bioactive compounds that can be utilized in specific therapeutic applications owing to their analgesic and anti-inflammatory properties, among other beneficial attributes (Karaköse 2022; Şen et al. 2022). Inflammation is a significant global issue, given its debilitating symptoms, which lead to widespread pain and reduced productivity. In contemporary times, there has been a notable surge in the attention garnered by the pharmaceutical and scientific communities toward utilizing medicinal plants as analgesic agents. This heightened interest is accompanied by a pressing requirement for novel anti-inflammatory medications, particularly those sourced from natural substances (Boujbiha et al. 2023). Furthermore, the inclusion of experimental research is imperative in order to authenticate the ethnopharmacological understanding pertaining to herbal medicine. Phytochemical and pharmacognostic evaluations are essential procedures for assessing the safety and efficacy of medicinal plant utilization.
Teucrium genus is one of the large and important genera of Lamiaceae family. It includes more than 300 species, widely distributed in the world, especially in the Mediterranean area that contains about 96% species of this genus (Moreira et al. 2014). Based on the ethnomedicine practices observed in various nations, Teucrium is recognized as a prominent resource for alleviating a range of female ailments. Its therapeutic properties encompass anti-nociceptive, anti-inflammatory, anti-bacterial, hypolipidemic, anti-rheumatoid, hypertensive, and hypoglycemic effects (Abdollahi et al. 2003). The therapeutic properties of Teucrium have been ascribed to specific bioactive substances that were isolated from them. These substances include phenolic acids and their derivatives (Mihailović et al. 2020), iridoids and flavonoids (Rizk et al. 1986), volatile oils (such as myrtenal, α-pinene, and sabinene)) (Kabouche et al. 2007). Furthermore, the inclusion of experimental research is imperative in order to authenticate the ethnopharmacological understanding pertaining to herbal medicine. Phytochemical and pharmacognostic evaluations are essential procedures for assessing the safety and efficacy of medicinal plant utilization.
Teucrium pseudochamaepitys, is a specie of Teucrium genus that is growing spontaneously in Algeria. Its leaves are mucronate, divided into three–five linear segments. Its inflorescences are terminal with two flowers per whorl, which have white color, spotted with pink or purple color (Quézel et al. 1962). According to the literature, no study has reported the chemical composition and biological activities of Teucrium pseudochamaepitys extracts; therefore, we chose this specie in our study. Furthermore, the inclusion of experimental research is imperative in order to authenticate the ethnopharmacological understanding pertaining to herbal medicine. Teucrium pseudochamaepitys L. has been used traditionally for the treatment of several illnesses. However, there needs to be more available data on investigating its phytocomponents in relation to their potential anti-inflammatory and antipyretic properties. Thus, the aim of this study was to investigate the chemical analysis, the acute toxicity, analgesic, anti-inflammatory, and antioxidant properties of the aqueous extract obtained from Teucrium pseudochamaepitys (TpAE). The antioxidant activity of the TpAE was determined using four different methods, which are: DPPH, ABTS, reducing power, and phenanthroline tests. In vitro anti-inflammatory activity was performed on TpAE using inhibition of albumin denaturation method. In order to evaluate in vivo biological activity of TpAE, acute toxicity, anti-inflammatory, and analgesic activities were carried out on male and female albino mice. Chemical composition of TpAE was determined in this study using SPE LC-MS/MS analysis.
Experimental
Plant and decoction preparations
The aerial part of the plant Teucrium pseudochamaepitys was harvested from the northeast of Algeria Setif, Bougaa region (36° 09′ 00″ north, 5° 26′ 00″ east), during its flowering season (May 2021). The scientific identification of the plant has been performed by Dr. Halis Youcef researcher in Scientific and Technical Research Centre for Arid Areas Touggourt-Algeria. The plant was dried in the shade, protecting it from moisture and light, at room temperature for 15 days, and a voucher specimen has been deposited in the herbarium of our laboratory under the code: 202105Bo/TeuPs. In order to prepare the aqueous extract of Teucrium pseudochamaepitys (TpAE) following the decoction method, 106.281 g of the aerial part of the plant material was cut into small pieces and boiled in 1000 mL of distilled water for 30 min. After that, the decoction was frozen and lyophilized, and the resulting powdered extract was labeled TpAE. The final lyophilized powder was redissolved in water and stored at 4 °C until phytochemical and pharmacological tests.
Experimental animals
In this investigation, we used two 20–30-day-old albino mice sexes (Mus musculus) (25 g ± 5 g). These mice were procured from Pasteur Institute in Algeria (Elevage Centre, Kouba, Algeria). The mice were housed in cages under standard conditions of the pet shop of ASSB faculty (FSB/USTHB) with a temperature of 20–24 °C; humidity of 50–65%; and 12 h of lighting per day, mice had free access to water and standard rodent chow for 16 h prior to experiments. All in vivo trials were conducted following preset guidelines of National Research Council (NRC, 1996), Washington, DC, USA. Every effort was made to minimize the number of animals used. The experimental procedures were approved by the University Animal Experimentation Ethics Committee (Approval Ref No. 162/2011/8).
SPE LC-MS/MS analysis
A qualitative analysis of constituents present in aqueous fractions of Teucrium pseudochamaepitys (TpAE) was performed using UPLC–ESI-MS–MS Shimadzu 8040 Ultra-High sensitivity with UFMS technology equipped with binary bump Nexera XR LC-20AD.
Separation was achieved with an Ultra‐force C18 column (I.D. 2.5 mm × 100 mm, 1.8 μm particle size; Restek), and the oven temperature was 25 °C. The chromatographic separation was carried out using a mixture of 30% (water, 0.1% formic acid) as mobile phase A and 70% methanol as mobile phase B. The flow rate was established at 0.3 mL/min, and the injection volume was 6 μL passed through a Millex‐LCR (PTFE) filter with 0.22 mm pore sizes. The separation was performed at room temperature, and the run lasted for 25 min.
The MS/MS used the ESI conditions as follows: 230 KPs CID gas; − 6.00 kV conversion dynode; 350 °C interface temperature; 250 °C DL temperature; 3.00 L/min nebulizing gas flow, 400 °C heat block; 15.00 L/min drying gas flow. The ion trap mass spectrometer was used in both negative and positive ions with MRM mode (multiple reaction monitoring). To achieve high extraction efficiency for all target analytes, the process of isolating and preconcentrating polyphenolic compounds from TpAE was carried out using a previously established solid-phase extraction (SPE) method. Accurate identification was made according to their typical fragments and through comparison with standards available in the developed database.
Phenolic compounds analysis
Total phenolic content
The spectrophotometric measurement of TPC in the TpAE was taken using the Folin–Ciocalteu procedure with a few modifications (Singleton and Rossi 1965). A concise depiction of the experimental procedure involved the addition of 100 µL of Folin–Ciocalteu reagent (diluted at a ratio of 1:10) and 75 µL of sodium carbonate solution (7.5%) to 20 µL of each extract. Following a 2-h incubation period in a light-restricted environment at ambient temperature, the absorbance at a wavelength of 765 nm was quantified using a microplate reader (PerkinElmer, Enspire), with a control serving as the control. The quantification of the polyphenol content was conducted using gallic acid equivalents (μg GAE/mg extract).
Total flavonoid content
The estimation of TFC was conducted using the aluminum colorimetric method with certain modifications (Topçu et al. 2007). A total of 130 μL of methanol was carefully dispensed into a microplate consisting of 96 wells. Subsequently, 50 μL of TpAE was added to the microplate. Additionally, 10 µL of potassium acetate (1 M) and 10 μL of aluminum nitrate at a concentration of 10% were introduced into the microplate. Following a 40-min incubation period at ambient temperature, the absorbance was quantified using a microplate reader (PerkinElmer, Enspire) set to a wavelength of 415 nm. TFC concentration was determined by employing a standard calibration curve for “quercetin” across various concentrations. The findings were quantified in terms of micrograms of quercetin equivalent per gram of extract (μg QE/ mg extract).
Antioxidant activity
DPPH radical trapping test
The assessment of the effect of Teucrium pseudochamaepitys aqueous extract on the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical was conducted using the methodology outlined by Blois (1958). In a 96-well microplate, 40 µL of TpAE and standards with varying concentrations were added to 160 μL of a pre-prepared DPPH solution. Standard antioxidants, namely butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and α-tocopherol, were employed in the study under identical experimental conditions. The measurement of absorbance was taken at a wavelength of 517 nm using a blank solution consisting of DPPH dissolved in methanol. This measurement was taken after a 30-min incubation period. The results were subsequently quantified as the percentage inhibition of DPPH, which was determined using the equation provided below.
where AbsC is the absorbance of the control and AbsE is the absorbance of the extract.
ABTS·+ scavenging activity
The ABTS·+ scavenging activity was assessed using the methodology outlined by Re et al. (1999). The cation ABTS·+ was generated through a reaction involving 7 mM ABTS and 2.45 mM potassium persulfate. Following a 24-h period, the absorbance of the ABTS·+ solution was manipulated in order to attain a specific absorbance value of 0.700 ± 0.020 at a wavelength of 734 nm. Subsequently, a mixture of 160 μL of ABTS·+ solution and 40 μL of samples (TpAE, BHT, BHA, and Trolox) at varying concentrations were prepared. The measurement of absorbance was taken at a wavelength of 734 nm following a duration of 10 min in a lightless environment.
Reducing power assay
According to Oyaizu (1986), the reducing power assay was carried out with potassium ferricyanide, and the absorbance was subsequently measured at a wavelength of 700 nm. Various concentrations of TpAE and standards were combined with a 0.2 M phosphate buffer solution at a pH of 6.6, followed by the addition of 1% potassium ferricyanide (K3Fe(CN)6). The resulting mixture was then incubated at a temperature of 50 °C for a duration of 20 min. Subsequently, a solution containing 10% trichloroacetic acid and 10 μL of ferric chloride FeCl3 (0.1%) was introduced. The outcomes were denoted as A0.5, which represents the concentration that yields an absorbance of 0.5.
Phenanthroline assay
In this experiment, 10 μL of samples with varying concentrations was introduced into a solution containing 50 μL of a 0.2% ferric chloride (FeCl3) solution and 30 μL of a 0.5% phenanthroline solution. Subsequently, the absorbance of the mixture was determined at a wavelength of 510 nm following an incubation period of 20 min at a temperature of 30 °C (Szydłowska-Czerniak et al. 2008). The absorbance levels were reported as A0.5 μg/mL.
Acute toxicity study.
In vivo assessment of acute toxicity of TpAE was conducted in mice of both sexes (male mice and female mice) adopting the experimental protocol described by the guidelines of Organization for Economic Cooperation and Development (OECD) 423 for 14 days acute oral toxicity tests. All animals were fed with TpAE at doses 2000 mg/kg (per oral) (OECD 2001). Initially after TpAE administration, an examination of each animal was performed periodically after 4 h for any change in behavior and then daily for 14 days.
Anti-inflammatory activity
Inhibition of bovine serum albumin (BSA) denaturation
In this experiment, a volume of 100 μL of the sample solution (at concentrations of 97.65, 195.31, 390.62, and 1562.5 μg/mL) was combined with 100 μL of a 0.2% w/v BSA solution that was prepared in Tris-buffered saline with a pH of 6.6. The control group was comprised of a solution containing 100 μL of a 0.2% BSA solution mixed with 100 μL of water. On the other hand, the standard group consisted of a solution containing 100 μg/μL of diclofenac sodium dissolved in water, combined with 500 μL of BSA solution. The test tubes were subjected to incubation at a temperature of 37 °C for a duration of 15 min, followed by immersion in a water bath maintained at a temperature of 72 °C for a period of 5 min. The absorbance values of the solutions were measured using a microplate reader (PerkinElmer, Enspire) at a wavelength of 660 nm (Kandikattu et al. 2013). The measurement of the inhibition against denaturation of BSA was taken using the following equation:
ACN: 100 µL H2O + 100 µL BSA, ATpAE: 100 µL TpAE + 100 µL BSA, Awhite: 100 µL TpAE + 100 µL Tris–phosphate (pH 6.8).
The control group in this study consists of denatured proteins, representing 100% of the sample. The obtained results are then compared to those of diclofenac sodium.
In vivo anti-inflammatory activity
Carrageenan-induced paw inflammation activity of TpAE was carried out in strain of male albino mice according to the method of Winter et al. (1962). In this study, the mice used were fasted for 16 h before the experiment. Then, the animals were divided into four groups each group comprised of 3 animals (n = 3). Animals in group 1 (control): mice were fed with normal saline. Animals in group 2 (positive control): the mice received standard Diclofenac® (500 mg/kg, b.w). Animals in group 3 and 4 were fed with TpAE 100 mg/kg and 500 mg/kg, separately. All mice were injected with carrageenan suspension (0.05 mL, 1% w/w) into the sub-plantar tissues of the right hind paw one hour after oral administration of all treatments. Following 3 h of anesthesia with diethyl ether, the animals were killed. Two posterior legs were severed at the tarsal joint and weighed on an analytical scale. The percentage of increase in paw weights (%Edema) and the percentage of reduction in edema (%inhibition) of treated mice were calculated using following formulas:
where WA LP is the weight average of left paw; WA RP is the weight average of right paw
where %Edema C: %Edema of control; %Edema E: %Edema of extract.
Analgesic activity
The assessment of analgesic efficacy was performed through the use of chemical stimulation-induced pain, utilizing two distinct models, namely the writhing.
Writhing test
The current study was carried out utilizing the torsion approach (manifesting as abdominal cramps) as expounded by Koster (1959) and afterward modified by Vogel (2002). Before beginning the experiment, the mice were subjected to a 16-h fasting period and subsequently divided into 4 groups, each consisting of 3 mice. Each group of mice was administered 0.5 mL of the respective substance. The substances used were divided into two groups: the negative control (physiological water) and the positive control (500 mg/kg Aspirin). Groups 3 and 4 were administered (TpAE at 100 mg/kg and 500 mg/kg). Following oral administration of the appropriate dosage, all mice were subjected to an intraperitoneal injection of 0.6% (1 mL/kg) acetic acid after a lapse of 30 min to elicit pain characterized by cramping. After 5 min, the frequency of cramps in each mouse was assessed through direct observation of the animal for a duration of 15 min. The analgesic effect of TpAE was evaluated by determining the percentage reduction in cramps (percentage protection), which was determined for each group using the formula:
where WC is the mean value of writhes in treated groups and WD is the mean value of writhes in control groups.
Statistical analysis
The data pertaining to phytochemical studies and biological activity tests are presented as the average values obtained from three independent analyses. Values represent the average results of three assays, with the standard deviation indicated. The one-way analysis of variance (ANOVA) test, specifically utilizing the XLSTAT software, was employed to conduct a statistical comparison of the mean values associated with each treatment. The experiments were conducted using concentrations exceeding four distinct values. The IC50 and A0.50 values were determined through the application of linear regression analysis, while the detection of significant differences was accomplished using one-way computation of variance (ANOVA) with a value level set at p < 0.05.
Results and discussion
SPE LC-MS/MS analysis
Once the optimal conditions for UPLC–ESI-MS–MS were determined, the TpAE was subjected to analysis using the MRM mode, employing both negative and positive ions. Figure 1A, B, C, D, E, and F depicts the UPLC–ESI-MS–MS and Mass spectra of the chemicals that have been identified from TpAE. Table 1 presents a comprehensive overview of the retention time (Rt), the mass-to-charge ratio (m/z), and the chemical formula of the identified compounds. These compounds were detected based on their characteristic fragments and were further confirmed by comparing them with the standards stored in the established database.
Chromatograms profiles of Teucrium pseudochamaepitys (TpAE). A Chromatogram of standard chrysin in TpAE; B chromatograms of standard quercetin in TpAE; C chromatograms of standard chlorogenic acid in TpAE; D chromatograms of standard gallic acid in TpAE; E chromatograms of standard ferulic acid in TpAE; F chromatograms of standard rutin in TpAE
According to Table 1 and Fig. 1, six of the compounds identified in TpAE are categorized as polyphenols based on the available standards in the existing database: chrysin, quercetin, salicylic acid, gallic acid, naringenin, and rutin (Fig. 2).
The outcomes of a phytochemical investigation conducted on the aerial parts of Teucrium pseudochamaepitys were analyzed using LC-MS–MS methodology. The obtained data, presented in Table 1, allowed for the preliminary identification of eight compounds out of a total of eleven. This identification was achieved by comparing the obtained results with standards that are currently accessible in the established database (Table 1). Both full and fragmentation analyses were utilized for this purpose. The electron spray ionization mass spectrometry (ESI-MS) technique is commonly employed to analyze ion peaks in the form of [M−H]−/[M + H]+. The following is an extensive list of the phytoconstituents that have been found: rutin (C15H12O5), chrysin (C15H10O4), quercetin (C15H10O7), salicylic acid (C7H6O3), gallic acid (C7H6O5), chlorogenic acid (C16H18O9), ferulic acid (C10H10O4), and naringenin (C15H12O5). Since four of them are constituents of the flavonoid family, the Teucrium pseudochamaepitys aqueous fraction has a heavy amount of flavonoids. Some of these compounds are being investigated for their potential to treat diabetes (rutin) (Habtemariam and Lentini 2015), reduce inflammation and exhibit higher antioxidant capacity (chrysin, naringenin, and salicylic acid) (Zeinali et al. 2017; Venkateswara et al. 2017; Randjelović et al. 2015), and protect cells from free radical damage (quercetin) (Baghel et al. 2012).
Numerous studies have affirmed the presence of phenolic acid and flavonoid compounds in different Teucrium species, notably T. botrys, T. compactum, T. montanum, T. aragonense, T. haensekri, and all the subspecies of T. polium. These compounds act as the main contributors to the antioxidant potential and to many other biological activities of plant extracts. Flavones and their glycosides are the most common class of secondary metabolites within the genus of Teucrium (Harborne, et al. 1986), notably rutin and quercetin which are the most common flavonoids found in our subjected species (T. pseudochamaepitys). Diverse flavanols such as cirsiliol, apigenin, pectolinarigenin, and naringenin were identified to be components of T. chamaedrys (Labbe et al. 1989) salvigenin, cirsimaritin, 3’-0-methyleupatonin from T. heterophyllum (Fraga et al. 1995). Furthermore, several additional secondary metabolites originating from the molecular identification of different Teucrium species in previous studies included caffeoylated phenylpropanoid, terpenoids, and derivatives, volatiles, and phenolic compounds (Bahramikia et al. 2022). Volatile compounds of T. polium and T. montanum consisted mainly of sesquiterpenes with germacrene D and δ-cadinene as the main constituents (Radulović et al. 2012); nevertheless, they were not found in our plant.
Total phenolics and flavonoids
Table 2 presents the quantitative analysis of secondary metabolites found in the aqueous extract of T. pseudochamaepitys. The quantification of TPC and TFC was conducted by determining their respective concentrations in μg GAE/mg and μg QE/mg of the extract. This was achieved by employing a calibration curve for each compound (phenol: y = 0.0034x + 0.1044, R2 = 0.9972; flavonoid: y = 0.0048x, R2 = 0.997), as illustrated in Fig. 3 and Fig. 4. The findings of our study indicate that the plant under investigation exhibits a high concentration of polyphenols, as evidenced by the total phenol content of TpAE, which was measured to be 187.32 ± 2.39 μg GAE/mg of extract. Regarding the overall flavonoid content, the total flavonoids content extract (TpAE) demonstrates a significantly higher concentration, measuring 152.98 ± 1.96 μg QE/mg of extract.
The analysis of total phenolic content and total flavonoid content has revealed that the TpAE exhibits a significant presence of phenolic and flavonoid compounds, surpassing the levels reported in previous studies on the Teucrium species (Bendjabeur et al. 2018; Al-Otaibi and AlMotwaa 2022).
Antioxidant activity
The findings related to the antioxidant activity, as assessed through the DPPH, ABTS, RP, and O-phenanthroline tests, are summarized in Table 3. The IC50 and A 0.5 values assess the concentration of TpAE required to achieve a 50% inhibition of free radicals. Nevertheless, there exists an inverse correlation between the antioxidant capacity of a compound and its IC50 value. Consequently, a compound with a lower IC50 value demonstrates a higher level of antioxidant activity.
It is noteworthy to mention that the aqueous fraction of T. pseudochamaepitys demonstrated a sustainable antioxidant activity in all four tests. However, it is important to acknowledge that there exists a correlation between the concentration of polyphenols and the activity of antioxidants. This correlation serves to validate the notion that polyphenols possess significant antioxidant properties, as they are able to effectively hinder the formation of free radicals and counteract the oxidation of macromolecules.
The potential antioxidant capacity of a biochemical substance could be assessed through various in vitro tests that measure its ability to inhibit stable free radicals. In pursuit of this objective, we have conducted an array of antioxidant tests employing various methodologies such as direct, indirect, or competitive procedures, as well as principles of reduction, chelation, or inhibition (Cantos et al. 2002). It is our contention that the implementation of the four examined techniques may effectively guarantee the antioxidant attributes of TpAE, notwithstanding its moderate potential in one of these strategies. The TpAE has demonstrated significant antioxidant activity, as evidenced by the relatively low A0.5 and IC50 presented in Table 3. These concentrations were comparable to the standard values, with the exception of the ABTS scavenging assays.
It can be inferred that the amount of the compound responsible for the observed effect is relatively lower. This inference is made under the assumption that the measured activity resulted from the combined effect of multiple molecules. However, despite this, the TpAE still exhibited significant antioxidant capabilities comparable to those of the standard chemicals.
The TpAE exhibited a notable antioxidant capacity, as evidenced by its lower DPPH IC50 and reducing power A0.5 values in comparison with extracts derived from Teucrium species, Teucrium montanum, Teucrium chamaedrys (Kadifkova et al. 2005), Teucrium orientale (Çakir et al. 2006), whereas the methanolic extract of Teucrium polium showed the highest antioxidant activity as measured by DPPH and FRAP assays with IC50 values of 0.41 ± 0.03 mg/mL and 0.21 ± 0.002 mg/mL, respectively (El Atki et al. 2019), which confirms that our findings are more significant than those of previous studies. Indeed, DPPH IC50 from of the essential oil of Teucrium pseudochamaepitys was 0.77 ± 0.05 mg/mL (Hammami et al. 2015). Concerning previous antioxidant studies of Teucrium pseudochamaepitys, none have utilized four distinct antioxidant techniques to demonstrate how potent and powerfully bioactive the target extract.
The TpAE has demonstrated significant antioxidant activity, suggesting its potential as a promising intervention for addressing various illnesses associated with imbalances in oxidant-antioxidant levels and oxidative stress, which can contribute to the onset of severe diseases.
Acute toxicity
During a 14-day study of acute toxicity evaluation, we found no toxicity, body weight changes, behavioral alterations, or death among rats when TpAE was administered at a dosage of 2000 mg/kg. Therefore, the LD50 of this extract is greater than 2000 mg/kg, which indicated that TpAE is considered as relatively safe according to the Globally Harmonized System (GHS) of Classification and Labelling of Chemicals (Europe 2005). This finding is consistent with the results of Chabane et al. (2021), which reported that the oral administration of methanolic extract of Teucrium polium at the doses of 1000 to 5000 mg/Kg did not cause any toxicity symptoms.
A prior investigation utilized four Teucrium species: T. carthaginenses Linn. (chloroform extract), T. flavum Linn. (methanolic extract), T. pumilum Linn. (chloroform extract), and T. buxifolium Lin to establish acute toxicity. Male albumin was used to conduct acute toxicity experiments with a constant volume of 1 mL/kg administered intraperitoneally (ip). In this study, the dose tested ranged from 130 to 200 mg/kg. Most of the extracts exhibited limited toxicity, consistent with our findings (Ulubelen et al. 2000).
Anti-inflammatory activity
Inhibition of bovine serum albumin denaturation
The findings of the in vitro anti-inflammatory effectiveness of the aqueous extract of T. pseudochamaepitys are presented in Table 4. It is observed that the level of inhibition of BSA denaturation (0.2%) exhibits a direct relationship with the concentration of various plant extracts. The concentration of 1562.5 μg/mL yielded the highest percentage inhibition, measuring 71.93 ± 2.78. Nevertheless, these values are close to that obtained for diclofenac which demonstrated 86.52% inhibition at 1.5 mg/mL (Fig. 5), commonly used as a standard anti-inflammatory drug, effectively inhibited the denaturation of BSA at an equivalent concentration.
Effect of Teucrium pseudochamaepitys aerial parts aqueous extracts on albumin denaturation. The variance analysis of our results was performed on XLSTAT Version 2016 using ANOVA, and the significance of differences was evaluated using Tukey’s HSD test. Values with different subscripts (a, b) in the same column were significantly different compared with the control (*p ≤ 0.001; very highly significant, **p ≤ 0.01; highly significant, *p ≤ 0.05; significant), and those with the same subscripts were not significantly different (p > 0.05)
In vivo anti-inflammatory effect
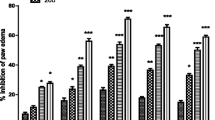
The anti-inflammatory effects of two doses of TpAE (100 mg/kg and 500 mg/kg) were evaluated in an in vivo environment. Carrageenan was used to induce experimental paw inflammation in mice. The induction of paw edema by carrageenan is a widely recognized acute model of inflammation that is commonly employed to evaluate the anti-inflammatory efficacy of various compounds. Table 5 represents the percentage of edema (%Edema), and the percentage of its reduction using TpAE and diclofenac sodium (%inhibition). The distinct phases of paw edema induced by carrageenan are evident. The initial phase, occurring approximately one hour after the stimulus, is characterized by the liberation of histamine, serotonin, bradykinin, and to a lesser extent, prostaglandins. In contrast, the subsequent phase (occurring after one hour) is marked by the infiltration of polymorphonuclear (PMN) leucocytes and the sustained synthesis of prostaglandins (Sadeghi et al. 2011).
The present study aimed to evaluate the in vivo anti-inflammatory activity of TpAE by examining its ability to inhibit paw edema induced by carrageenan in albino mice. Conversely, the in vitro anti-inflammatory activity was assessed using the albumin denaturation method. Based on the findings derived from our in vivo experiments, it can be concluded that there exists anti-inflammatory activity (Table 5); the present experiment employs a widely recognized experimental model for investigating acute inflammation, utilizing carrageenan, a chemically approved substance known for its ability to induce the release of mediators of inflammation. The results of this study indicate that TpAE demonstrates a beneficial anti-inflammatory effect, as it displayed significant activity during the later stage of the inflammatory response process. The outcomes of our study indicate that the administration of TpAE at a dosage of 500 mg/kg resulted in a substantial decrease in carrageenan-induced paw edema when compared with diclofenac sodium, a standard drug administered at an equivalent concentration. Table 5 demonstrates a noticeable reduction in paw weight edema, reaching 68.28%, when juxtaposed with diclofenac sodium, which exhibited an inhibition rate of 70.23%. Therefore, the results acquired in this study provide confirmation of the extract's efficacy as a remedy for inflammation. This effect can be attributed to the combined effect of its various naturally occurring phenolic substances. Moreover, our in vivo anti-inflammatory effect data align with the findings collected on the genus Teucrium using carrageenan-induced inflammation method, found by Tariq et al. (1989), Farshchi el al. (2010), Pourmotabed et al. (2010) and El-Ashmawy (2018).
Moreover, in order to preserve 3D proteins structure, the principal mechanism of albumin denaturation consist of maintain of the electrostatic, hydrophobic, hydrogen, and disulfide links (Mizushima and Kobayashi 1968; Barros et al. 2008). Most proteins lose their biological roles after denaturation, which leads to the creation of autoantigens that trigger a variety of autoimmune dysfunctions, including inflammatory illnesses. Thus, it is thought that anti-inflammatory medications that inhibit protein denaturation are efficient (Lekmine et al. 2021). Inflammation is a multifaceted physiological reaction that is contingent upon various contributing factors. The denaturation of cell proteins is considered to be an important contributor (Khan et al. 2022). Therefore, the inhibitory effects of various concentrations of TpAE were investigated to evaluate the in vitro anti-inflammatory properties of the plant. The findings demonstrated a significant inhibitory effect of TpAE on the denaturation of proteins. Based on our results of the in vitro anti-inflammatory activity of TpAE (Table 3), which is show strong anti-inflammatory ability of this extract with an inhibition percentage 71.93 ± 2.78 at concentration of 1562.5 µg/mL, this protective capacity was very closer to the Diclofenac®. Compared to the results found for hydroalcoholic extract of the plant Teucrium polium reported by Benchikha et al. (2022), at a 2 mg/mL concentration, the evaluated substance exhibited a noteworthy inhibitory potential for BSA denaturation, estimated to be approximately 97.53%, also different concentration of this plant shows strong anti-inflammatory activity higher than the diclofenac sodium. Thus, these studies suggesting that Teucrium plants could be potential candidates as anti-inflammatory agents.
The Teucrium genus encompasses many species, such as T. polium, T. oliverianum, T. hyrcanicum, and T. chamaedrys. These species are known to possess secondary metabolites that demonstrate a wide range of biological activities, including anti-inflammatory properties both in vitro and in vivo (Stanković 2020). In this study the aqueous extract Teucrium pseudochamaepitys L. aerial part shows strong anti-inflammatory activity through in vivo and in vitro tests. Obviously, the phytochemical compounds found in the aqueous extract of Teucrium pseudochamaepitys L. aerial parts by the LC-MS/MS analysis contribute to the anti-inflammatory activity of this plant. Further, as we mentioned previously within the LC-MS/MS analysis results, the major compounds detected on the TpAE belong to flavonoids and phenolic types of compounds. Therefore, the TpAE anti-inflammatory properties may be attributed to the existence of flavonoids and phenolic compounds within the plant. Previous studies have indicated that flavonoids significantly stabilize lysosomal membranes, both in vitro/in vivo (Kariawasam et al. 2017). The study reported the advantageous impacts of chrysin in the suppression of blood levels of NF-kB (nuclear transcription factor kB) p65 unit, TNF-a (tumor necrosis factor-alpha), and interferon-gamma (IFN-g). Additionally, it has been established that chrysin acts as an NF-kB antagonist and a PPAR-g agonist. This dual action leads to the reduction of crucial pro-inflammatory enzymes, including cyclooxygenase-2, myeloperoxidase, inducible nitric oxide synthase, prostanoids, and phospholipase A2 (Zeinali et al. 2017). Furthermore, naringenin possesses the ability to generate a sufficient amount of -OH (hydroxyl substitutions), thereby endowing it with the capacity to effectively scavenge reactive oxygen species (ROS). Therefore, it is postulated that naringenin possesses the potential to attenuate or enhance pathological states characterized by oxidative stress or inflammation as key contributing factors (Venkateswara et al. 2017). Additionally, it is worth noting that acetyl salicylic acid, a pharmaceutical agent with a history of over a century in clinical application, is commonly regarded as possessing comparable anti-inflammatory properties to salicylic acid (Randjelović et al. 2015). Therefore, this study proposes that TpAE could act as promising candidates for anti-inflammatory drugs.
Analgesic activity
Acetic acid-induced writhing
The study’s findings indicate that administering varying doses of the aqueous extract of Teucrium pseudochamaepitys resulted in a significant reduction (p < 0.001) in abdominal writhing in mice compared to the negative control group. The administration of all doses of TpAE demonstrated a robust and statistically significant analgesic effect in the animal subjects. This effect was observed through the dose-dependent inhibition of acetic acid-induced writhing, resulting in inhibitions of 64.93% and 70.12% at doses of 100 and 500 mg/kg, respectively. In contrast, aspirin administration at a 500 mg/kg dosage resulted in a writhing inhibition effect of only 67.53%, as indicated in Table 6.
The acetic acid-induced writhing test model is widely regarded as a classical peripheral analgesic animal model and is regularly employed to screen analgesic drugs (Negus et al. 2006). Inhibiting cyclo-oxygenases, lipoxygenases, and other inflammatory mediators facilitates the peripheral analgesic impact. On the other hand, the central analgesic action is potentially facilitated by the suppression of central pain receptors. The administration of acetic acid intraperitoneally during the analgesic effect test resulted in the biosynthesis of leukotrienes and prostaglandins, as well as an increase in cyclooxygenase (COX), lipoxygenase (LOX), serotonin, histamine, bradykinin, substance P, interleukin-1β (IL-1β), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α) in the peripheral tissue fluid. The augmentation of indigenous substances in the affected area has been documented as the causative agent for pain perception and contortions induced by acetic acid (Sarkar et al. 2019). Nonsteroidal anti-inflammatory drugs (NSAIDs) like aspirin produce their analgesic effects by inhibiting the biogenesis of prostaglandins and are therefore able to inhibit acetic acid-induced writhing. The extract TpAE demonstrated a noteworthy capacity to inhibit writhing in mice that is comparable to that of conventional aspirin; it is plausible that they exerted their effects via a similar mechanism. As previously discussed, the involvement of flavonoids has been observed in the inhibition of COX-2, as well as the reduction of intracellular calcium levels and mediators of inflammation like TNF-α. Additionally, steroids have been found to inhibit prostaglandins, resulting in the production of an analgesic effect (Imam and Moniruzzaman 2014; Golder et al. 2020). The LC-MS/MS analysis of TpAE indicated the presence of phytochemical constituents, which is chrysin, quercetin, naringenin, ferulic acid, chlorogenic acid, and rutin, as previously mentioned. The previous investigation on phytochemicals in the Teucrium genus indicated the presence of monoterpene compounds. More specifically, (−)-linalool, a major compound identified in Teucrium polium (Vokou and Bessiere 1985), was reported to possess anti-nociceptive potential based on experimental animal studies. As a result, (−)-linalool has been extensively studied in various painful conditions. The observed effects of this particular monoterpene on nociception in various models indicate its potential involvement with muscarinic, opioid, dopaminergic, adenosinergic, and glutamatergic systems, as well as the participation of ATP− sensitive K+ channels. These findings suggest a significant reduction in the nociceptive response (Guimarães et al. 2013). Additionally, it is important to consider the manner in which findings that validate prior research in the respective field are presented, which can be achieved through a thorough examination of the existing literature (Radhakrishnan et al. 2001). The in vivo experiments conducted in this study demonstrate a strong correlation with previous research findings.
Conclusion
The present research represents the first inquiry into the phytochemical and pharmacological features of aqueous decoctions from Teucrium pseudochamaepitys L. collected in Algeria. Hence, it can be asserted that the aqueous decoction derived from Algerian Teucrium pseudochamaepitys L. has exhibited promoting pharmacological properties as evidenced by its successful outcomes in both in vitro and in vivo experiments involving laboratory animals. The administration of orally delivered TpAE did not result in any observed toxicity or mortality in animals at doses up to 2000 mg/kg BW. Additionally, notable analgesic and anti-inflammatory effects were noticed. Based on the results obtained from this study via LC-MS/MS analysis of TpAE, it can be concluded that the biological activity of TpAE could be attributed to the bioactive compounds present in the T. pseudochamaepitys plant, especially flavonoids and phenolic acids. The latter are prospective candidates based on their currently demonstrated biological activity and tolerated non-cytotoxicity. These results open up future horizons for us to separate and purify the compounds present in this extract using various chromatographic techniques to obtain pure compounds, as well as determining the structure of these compounds with the help of several spectroscopic methods, including 1D NMR, 2D NMR, HR-MS, UV–Vis, and IR. Study of the biological activities mentioned in this study of the separated compounds is the most important step to find and determine the responsible molecules for the antioxidant, anti-inflammatory, and analgesic activities.
References
Abdollahi M, Karimpour H, Monsef-Esfehani HR (2003) Antinociceptive effects of Teucrium polium L. total extract and essential oil in mouse writhing test. Pharmacol Res 48:31–35. https://doi.org/10.1016/S1043-6618(03)00059-8
Al-Otaibi WA, AlMotwaa SM (2022) Chemical composition and antioxidant, antibacterial and cytotoxic properties of essential oil from Teucrium polium L. from Riyadh province. Emir J Food Agric. https://doi.org/10.9755/ejfa.2022.v34.i1.2802
Baghel SS, Shrivastava N, Baghel RS, Agrawal P, Rajput S (2012) A review of quercetin: antioxidant and anticancer properties. World J Pharm Pharm Sci 1:146–160
Bahramikia S, Gavyar PHH, Yazdanparast R (2022) Teucrium polium L: an updated review of phytochemicals and biological activities. Avicenna J Phytomed 12:224. https://doi.org/10.22038/AJP.2021.19155
Barros L, Falcão S, Baptista P, Freire C, Vilas-Boas M, Ferreira ICFR (2008) Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem 111:61–66. https://doi.org/10.1016/j.foodchem.2008.03.033
Benchikha N, Messaoudi M, Larkem I, Ouakouak H, Rebiai A, Boubekeur S, Ferhat MA, Benarfa A, Begaa S, Benmohamed M (2022) Evaluation of possible antioxidant, anti-hyperglycaemic, anti-alzheimer and anti-inflammatory effects of Teucrium polium aerial parts (Lamiaceae). Life 12:1579. https://doi.org/10.3390/life12101579
Bendjabeur S, Benchabane O, Bensouici C, Hazzit M, Baaliouamer A, Bitam A (2018) Antioxidant and anticholinesterase activity of essential oils and ethanol extracts of Thymus algeriensis and Teucrium polium from Algeria. J Food Meas Charact 12:2278–2288. https://doi.org/10.1007/s11694-018-9845-x
Blois M (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200. https://doi.org/10.1038/1811199a0
Boujbiha MA, Chahdoura H, Adouni K, Ziani BEC, Snoussi M, Chakroun Y, Ciudad-Mulero M, Fernández-Ruiz V, Achour L, Selmi B, Flamini G, Mosbah H (2023) Wild Vitex agnus-castus L.: phytochemical characterization, acute toxicity, and bioactive properties. Molecules 28:5096. https://doi.org/10.3390/molecules28135096
Çakir A, Mavi A, Kazaz C, Yildirim A, KÜFREVIOĞLU Öİ (2006) Antioxidant activities of the extracts and components of Teucrium orientale L. var. orientale. Turk J Chem 30:483–494
Cantos E, Espín JC, Tomás-Barberán FA (2002) Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J Agric Food Chem 50:6322–6329. https://doi.org/10.1021/jf020562x
Chabane S, Boudjelal A, Keller M, Doubakh S, Potterat O (2021) Teucrium polium-wound healing potential, toxicity and polyphenolic profile. S Afr J Bot 137:228–235. https://doi.org/10.1016/j.sajb.2020.10.017
El Atki Y, Aouam I, El kamari F, Taroq A, Lyoussi B, Taleb M, Abdellaoui A (2019) Total phenolic and flavonoid contents and antioxidant activities of extracts from Teucrium polium growing wild in Morocco. Mater Today Proc 13:777–783. https://doi.org/10.1016/j.matpr.2019.04.040
El-Ashmawy IM (2018) Anti-inflammatory and phytoconstituents of Teucrium oliverianum Ging. Ex. Benth. Eur J Pharm Med Res 5:406–411
Europe UNECE (2005) Globally harmonized system of classification and labelling of chemicals (GHS). https://unece.org/ghs-rev1-2005
Farshchi A, Ghiasi G, Abdollahuasl A (2010) Antinociceptive and antiinflammatory effects of Teucrium hyrcanicum aqueous extract in male mice and rats. J Physiol Pharmacol 14:78–84
Fraga BM, Hernández MG, Mestres T, Terrero D, Arteaga J (1995) Nor-sesquiterpenes from Teucrium heterophyllum. Phytochemistry 39:617–619. https://doi.org/10.1016/0031-9422(94)00947-R
Golder M, Sadhu SK, Biswas B, Islam T (2020) Comparative pharmacologic profiles of leaves and hypocotyls of a mangrove plant: Bruguiera gymnorrhiza. Adv Tradit Med 20:395–403. https://doi.org/10.1007/s13596-019-00423-8
Guimarães AG, Quintans JS, Quintans-Júnior LJ (2013) Monoterpenes with analgesic activity—a systematic review. Phytother Res 27:1–15. https://doi.org/10.1002/ptr.4686
Habtemariam S, Lentini G (2015) The therapeutic potential of rutin for diabetes: an update. Mini Rev Med Chem 15:524–528. https://doi.org/10.2174/138955751507150424103721
Hammami S, Jmii H, El Mokni R, Khmiri A, Faidi K, Dhaouadi H, El Aouni MH, Aouni M, Joshi RK (2015) Essential oil composition, antioxidant, cytotoxic and antiviral activities of Teucrium pseudochamaepitys growing spontaneously in Tunisia. Molecules 20:20426–20433. https://doi.org/10.3390/molecules201119707
Harborne JB, Tomás-Barberán FA, Williams CA, Gil MI (1986) A chemotaxonomic study of flavonoids from European Teucrium species. Phytochemistry 25:2811–2816. https://doi.org/10.1016/S0031-9422(00)83747-4
Imam MZ, Moniruzzaman MD (2014) Antinociceptive effect of ethanol extract of leaves of Lannea coromandelica. J Ethnopharmacol 154:109–115. https://doi.org/10.1016/j.jep.2014.03.032
Kabouche A, Kabouche Z, Ghannadi A, Sajjadi SE (2007) Analysis of the essential oil of Teucrium polium ssp. aurasiacum from Algeria. J Essent Oil Res 19:44–46. https://doi.org/10.1080/10412905.2007.9699227
Kadifkova-Panovska T, Kulevanova S, Stefova M (2005) In vitro antioxidant activity of some Teucrium species (Lamiaceae). Acta Pharm 55:207–214
Kandikattu K, Kumar PBR, Priya RV, Kumar KS, Rathore RSB (2013) Evaluation of anti-inflammatory activity of Canthium parviflorum by in-vitro method. Indian J Res Pharm Biotechnol 1:729–731
Karaköse M (2022) An ethnobotanical study of medicinal plants in Güce district, north-eastern Turkey. Plant Divers 44:577–597. https://doi.org/10.1016/j.pld.2022.03.005
Kariawasam KWJC, Pathirana RN, Ratnasooriya WD, Handunnetti S, Abeysekera WPKM (2017) Phytochemical profile and in vitro anti-inflammatory activity of aqueous leaf extract of Sri Lankan variety of Psidium guajava L. J Pharmacogn Phytochem 6:22–26
Khan M, Manzoor Z, Rafiq M, Munawar SH, Waqas MY, Majeed H, Ali Shah SZ, Hussain R, Hussain HI, Tahir T, Kotwica-Mojzych K, Mojzych M (2022) Phytochemical screening, anti-inflammatory, and antidiabetic activities of different extracts from Caralluma edulis plant. Molecules 27:5346. https://doi.org/10.3390/molecules27165346
Koster R (1959) Acetic acid for analgesic screening. Fed Proc 18:412
Labbe C, Polanco MI, Castillo M (1989) 12-epi-teuscordonin and other neoclerodanes from Teucrium bicolor. J Nat Prod 52:871–874. https://doi.org/10.1021/np50064a037
Lekmine S, Boussekine S, Akkal S, Martín-García AI, Boumegoura A, Kadi K, Djeghim H, Mekersi N, Bendjedid S, Bensouici C, Nieto G (2021) Investigation of photoprotective, anti-inflammatory, antioxidant capacities and LC–ESI–MS phenolic profile of Astragalus gombiformis Pomel. Foods 10:1937. https://doi.org/10.3390/foods10081937
Mihailović V, Katanić Stanković JS, Mihailović N (2020) Phenolic compounds diversity of Teucrium species. In: Stanković M (ed) Teucrium species: biology and applications, 1st edn. Springer, Kragujevac, pp 143–177
Mizushima Y, Kobayashi M (1968) Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol 20:169–173. https://doi.org/10.1111/j.2042-7158.1968.tb09718.x
Moreira DdL, Teixeira SS, Monteiro MHD, De-Oliveira ACA, Paumgartten FJR (2014) Traditional use and safety of herbal medicines. Rev Bras 24:248–257. https://doi.org/10.1016/j.bjp.2014.03.006
Muhammad N, Saeed M, Barkatullah IM, Khan H (2012) Pharmacognostic studies of Viola betonicifolia. Afr J Pharm Pharmacol 6:43–47. https://doi.org/10.5897/AJPP11.578
Negus SS, Vanderah W, Brandt MR, Bilsky EJ, Becerra L, Borsook D (2006) Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther 319:507–514. https://doi.org/10.1124/jpet.106.106377
OECD. 2001. OECD guidelines for the testing of chemicals. Oecd
Oyaizu M (1986) Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet 44:307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
Pourmotabed A, Farshchi A, Ghiasi G, Khatabi PM (2010) Analgesic and anti-inflammatory activity of Teucrium chamaedrys leaves aqueous extract in male rats. Iran J Basic Med Sci 13:119–125
Quézel P, Santa S, Schotter O, Emberger L (1962) Nouvelle flore de l'Algérie et des régions désertiques méridionales. Tome II, Paris
Radhakrishnan R, Zakaria MNM, Islam MW, Kamil M, Ismail A, Chan K, Al-Attas A (2001) Analgesic and anti-inflammatory activities of Teucrium stocksianum. Pharm Biol 39:455–459. https://doi.org/10.1076/phbi.39.6.455.5885
Radulović N, Dekić M, Joksović M, Vukićević R (2012) Chemotaxonomy of Serbian Teucrium species inferred from essential oil chemical composition: the case of Teucrium scordium L. ssp. scordioides. Chem Biodivers 9(1):106–122. https://doi.org/10.1002/cbdv.201100204
Randjelović P, Veljković S, Stojiljković N, Sokolović D, Ilić I, Laketić D, Randjelović D, Randjelović N (2015) The beneficial biological properties of salicylic acid. Acta Fac Med Naissensis 32:259–265. https://doi.org/10.1515/afmnai-2015-0026
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Rizk A, Hammouda F, Rimpler H, Kamel A (1986) Iridoids and flavonoids of Teucrium polium herb1. Plant Med 52:87–88. https://doi.org/10.1055/s-2007-969087
Sadeghi H, Hajhashemi V, Minaiyan M, Movahedian A, Talebi A (2011) A study on the mechanisms involving the anti-inflammatory effect of amitriptyline in carrageenan-induced paw edema in rats. Eur J Pharmacol 667:396–401. https://doi.org/10.1016/j.ejphar.2011.05.053
Sarkar KK, Mitra T, Acharyya RN, Sadhu SK (2019) Phytochemical screening and evaluation of the pharmacological activities of ethanolic extract of Argemone mexicana Linn. aerial parts. Orient Pharm Exp Med 19:91–106. https://doi.org/10.1007/s13596-018-0357-3
Şen G, Akbulut S, Karaköse M (2022) Ethnopharmacological study of medicinal plants in Kastamonu province (Türkiye). Open Chem 20:873–911. https://doi.org/10.1515/chem-2022-0204
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158. https://doi.org/10.5344/ajev.1965.16.3.144
Stanković M (2020). Teucrium species: biology and applications. Springer, Kragujevac
Szydłowska-Czerniak A, Dianoczki C, Recseg K, Karlovits G, Szłyk E (2008) Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 76:899–905. https://doi.org/10.1016/j.talanta.2008.04.055
Tariq M, Ageel AM, Al-Yahya MA, Mossa JS, Al-Said MS (1989) Anti-inflammatory activity of Teucrium polium. Int J Tissue React 11:185–188
Topçu G, Ay M, Bilici A, Sarıkürkcü C, Öztürk M, Ulubelen A (2007) A new flavone from antioxidant extracts of Pistacia terebinthus. Food Chem 103:816–822. https://doi.org/10.1016/j.foodchem.2006.09.028
Ulubelen A, Topu G, Sönmez U (2000) Chemical and biological evaluation of genus Teucrium. Stud Nat Prod Chem 23:591–648. https://doi.org/10.1016/S1572-5995(00)80139-8
Venkateswara Rao P, Kiran SDVS, Rohini P, Bhagyasree P (2017) Flavonoid: a review on naringenin pharmacogn. Phytochem 6:2778–2783
Vogel HG (2002) Drug discovery and evaluation: Pharmacological assays. Springer, Germany
Vokou D, Bessiere JM (1985) Volatile constituents of Teucrium polium. J Nat Prod 48:498–499. https://doi.org/10.1021/np50039a032
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med 111:544–547. https://doi.org/10.3181/00379727-111-278
Zeinali M, Rezaee SA, Hosseinzadeh H (2017) An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed Pharmacother 92:998–1009. https://doi.org/10.1016/j.biopha.2017.06.003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belguidoum, M., Harchaoui, L., Khattabi, L. et al. Teucrium pseudochamaepitys L.: chemical composition, acute toxicity, antioxidant, anti-inflammatory, and analgesic properties. Chem. Pap. 78, 1989–2003 (2024). https://doi.org/10.1007/s11696-023-03221-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03221-4