Abstract
Long-term treatment with haloperidol is associated with a number of extrapyramidal side effects, particularly the irregular movements of chorionic type. This limitation presents a marked therapeutic challenge. The present study investigates the molecular etiology of haloperidol neurotoxicity and the role of curcumin, a well-known anti-oxidant, in ameliorating these adverse effects. The redox status of haloperidol-treated brains along with NO, TNF-α, NF-kappaB p65 subunit, caspase-3, and monoamine neurotransmitters were measured in the striatum of rat brain. Chronic treatment with haloperidol (5 mg/kg, i.p., 21 days) produced orofacial dyskinetic movements which were coupled with marked increase in oxidative stress parameters, TNF-α, caspase-3 activity in cytoplasmic lysate and active p65 sub unit of NF-kappaB in nuclear lysates of the striatum. Neurochemically, chronic administration of haloperidol resulted in a significant decrease in the levels of norepinephrine, dopamine, and serotonin. The prototype atypical anti-psychotic, clozapine (10 mg/kg, i.p., 21 days) produced mild oxidative stress but did not alter any other parameters. Interestingly, co-administration of curcumin (25 and 50 mg/kg, i.p., 21 days) dose-dependently prevented all the behavioral, cellular, and neurochemical changes associated with the chronic administration of haloperidol. Curcumin per se (50 mg/kg) did not show any side effects. Co-administration of piperine significantly enhanced the effect of curcumin (25 mg/kg) but not of curcumin (50 mg/kg). Collectively, the data indicated the potential of curcumin as an adjunct to haloperidol treatment and provided initial clues to the underlying molecular mechanisms in haloperidol neurotoxicity. This study also provides a rationale for the combination of piperine and curcumin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haloperidol is a widely used neuroleptic drug for the treatment of acute and chronic psychosis. Haloperidol belongs to butyrophenone group and is thought to exert its therapeutic effects by blocking the cerebral dopamine D2 receptors (Creese et al. 1976) and sigma-1 receptors (Vilner et al. 1995). Use of neuroleptics, especially that of butyrophenones, is limited by their potentials to produce a range of extrapyramidal movement disorders such as Parkinsonism, akathisia, dystonia, and finally chronic tardive dyskinesia (Sagara 1998; Lohr et al. 2003).
Pathophysiologically, several lines of evidences suggest that the neuronal changes in the basal ganglia produced by increased oxidative stress and glutamate excitotoxicity may play a role (Tsai et al. 1998; Burger et al. 2005) in haloperidol-associated neurotoxicity. Chronic blockade of dopamine D2 receptors in the striatum results in persistently enhanced release of glutamate which kills striatal neurons (Tsai et al. 1998). Repeated administrations of haloperidol also cause an increase in extracellular glutamate in the brain tissues of rodents (See and Lynch 1995; See et al. 1995). Increased release of glutamate is associated with increased free radicals generation as well as an increase in the influx of calcium, rendering the neurons more vulnerable to oxidative damage (Tsai et al. 1998). Furthermore, a disruption of the mitochondrial energy production by haloperidol (Burkhardt et al. 1993) may also lead to increased production of reactive oxygen species (ROS) and make the neurons more susceptible to both oxidative stress and the pathological effects of glutamate (Coyle and Puttfarcken 1993). In addition to their direct damaging effects, reactive oxygen species can induce the activation and expression of certain transcriptional factors and other relevant genes. TNF-α and nuclear transcription factor NF Kappa B are among the factors which respond directly to the oxidative stress (Sethi et al. 2006). There is also a direct relationship between the increase in oxidative stress and increase in the levels and expression of TNF-α and nuclear transcription factor NF-kappaB (Aggarwal 2000; Aggarwal et al. 2002). The activation of these transcriptional factors and cytokines may lead to initiation of cell signaling cascade that leads to cellular death.
Curcumin, a yellow pigment from Curcuma longa, is a major component of turmeric and is commonly used as a spice and food-coloring agent (Sharma et al. 2005). Due to its powerful antioxidant and anti-inflammatory properties, curcumin has drawn the attention of the scientific community (Shishodia et al. 2005). Since free-radical-mediated peroxidation of membrane lipids and oxidative damage of DNA and proteins are believed to be associated with a variety of chronic pathological complications such as cancer, atherosclerosis, and neurodegenerative diseases, curcumin is thought to play a vital role against these pathological conditions (Sharma et al. 2007a, b; Aggarwal and Shishodia 2004; Shishodia et al. 2005). Earlier studies have shown that curcumin inhibits ROS production (Quiles et al. 2002) as well as calcium entry (Balasubramanyam et al. 2003). Curcumin also inhibited hydrogen peroxide-induced cell damage (Priyadarsini et al. 2003). Curcumin manganese complex and acetyl-curcumin manganese complex showed much greater SOD activity and an inhibitory effect on lipid peroxidation (Mahakunakorn et al. 2003). It can also affect other cellular processes such as activation of apoptosis, inhibition of inflammatory cytokine production, and inhibition of cyclooxygenase and lipoxygenase isoenzymes (Sethi et al. 2006; Shishodia et al. 2007; Sandur et al. 2007a). Pharmacokinetic properties of curcumin indicate that 1 h after its oral administration; it attains maximum concentration in intestine followed by spleen, liver and kidney, respectively, whereas only a trace amount is found in brain tissue (Pan et al. 1999), which can limit its therapeutic potential. The transformation of curcumin into its metabolites like curcumin-glucuronoside, dihydrocurcumin-glucuronoside, tetrahydrocurcumin (THC)—glucuronoside, and THC (Garcea et al. 2004; Hoehle et al. 2006; Pan et al. 1999) in the liver are probably responsible for its low concentration particularly in blood and brain. Black pepper (Piper nigrum L.) and long pepper (Piper longum L.) have been in use as spices from ancient times throughout the world. Piperine (l-piperoylpiperidine) is a major component of the piper species. It has been reported to enhance the bioavailability of drugs by inhibition of glucuronidation in the liver and small intestine (Atal et al. 1985; Shoba et al. 1998). Literature has reported that piperine enhances the serum concentration and bioavailability of curcumin in rats and men probably due to increased absorption and reduced metabolism.
The aim of the current study was to investigate the effect of haloperidol administration on oxidative stress, cellular death cascade mediators, and striatal neurotransmitter levels so as to find whether any correlation exists between these parameters and abnormal motor changes. Further, we also investigated the preventive role of curcumin and its combination with piperine against haloperidol-induced behavioral and molecular and biochemical disruptions in rat striatum.
Materials and Methods
Animals
Male Wistar rats (180–220 g; 10–12 rats/group) bred in the Central Animal House facility of Panjab University were used. The animals were housed under standard laboratory conditions, maintained on a normal light–dark cycle and free access of food and water. Animals were acclimatized to laboratory conditions before the test. Each animal was used only once in the experiment. All the experiments were carried out between 0900 and 1,500 h. The experimental protocols were approved by the Institutional Animal Ethics Committee and conducted according to the guidelines of Indian National Science Academy for the use and care of experimental animals.
Drugs and Treatment Schedule
In a series of different experiments, rats received following treatments:
-
Group 1: Control,
-
Group 2: Haloperidol (5 mg/kg, i.p., 21 days) treated,
-
Group 3: Clozapine (10 mg/kg, i.p., 21 days) treated,
-
Group 4: Curcumin (50 mg/kg, p.o., 21 days) per se treated,
-
Group 5–6: Curcumin (25, 50 mg/kg, p.o. 21 days) + haloperidol (5 mg/kg, i.p., 21 days),
-
Group 7: Piperine (2.5 mg/kg, p.o., 21 days) treated,
-
Group 8–9: Curcumin (25, 50 mg/kg, p.o., 21 days) + piperine (2.5 mg/kg, p.o, 21 days) + Haloperidol (5 mg/kg, i.p., 21 days).
Haloperidol (Serenace, Searle, India) was diluted with distilled water and was administered intraperitoneally. Clozapine (Sun Pharmaceuticals, India) was dissolved in slightly acidic solution (0.1 M hydrochloric acid with pH in between 4 and 5) distilled water and administered intra-peritoneally. Curcumin (Sigma-Aldrich, USA) and piperine (Sigma-Aldrich, USA) were suspended in vegetable oil and administered per orally. All the drugs were administered in a constant volume of 0.5 ml per 100 g of bodyweight of rat. In combination studies, haloperidol and curcumin were administered simultaneously once daily at 0900 for a period of 21 days and behavioural assessments were done 24 h after the last dose (Naidu et al. 2003).
Dissection and Homogenization
After behavioural assessment, animals were divided in two groups on the basis of severity and susceptibility (high vs. low orofacial dyskinetic movements) of behavioural assessments. Equal number of animals from both high and low orofacial dyskinetic movements was selected for each assay so that there should be minimum variation. The brains were removed; striatum was dissected out, pooled and weighed. A 10% (w v−1) tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4). Homogenate were centrifuged at 2,500×g for 10 min, at 4°C, and supernatant was used for estimation of lipid peroxidation, superoxide anion, and non-protein thiols. Tissue homogenate was also used for caspase-3 colorimetric detection. Further, cytoplasmic fractions were prepared to study quantification of total nitric oxide and TNF-alpha. Nuclear fractions were prepared for the quantification of NF-kappaB active p65. In another set of animals, the brains were removed; striatum was dissected out and was stored at −80°C for HPLC studies to estimate different neurotransmitters and their metabolites.
Biochemical Assessment
The quantitative measurement of lipid peroxidation in striatum was performed according to the method of Wills (1966). The results were expressed as nmol of malondialdehyde/mg protein using the molar extinction coefficient of chromophore (1.56 × 105 M−1 cm−1). The superoxide anion levels were measured with method devised by Babior (Babior et al. 1973). Results were calculated as nmoles of cytochrome-c reduced/minute using molar extinction coefficient of chromophore (2.1 × 104 M−1 cm−1) and expressed as percentage of control taking control values as 100%. Non-protein thiols (NPSH) in the striatum were estimated according to the method of Ellman (1959). The results were expressed as nmol of NPSH per mg protein (Bishnoi et al. 2007a, b).
Assessment of Inflammatory Mediators
The quantification of total nitric oxide was done by the help and instructions provided by R&D Systems Total nitric Oxide Assay Kit which involves the conversion of nitrate to nitrite by the enzyme nitrate reductase (Bishnoi et al. 2007a, b). The quantification of TNF-alpha was done by the help and instructions provided by R&D Systems Quantikine Rat TNF-alpha immunoassay kit (Guerin-Marchand et al. 2001). The nuclear levels of p65 may correlate positively with the activation of NF-κB pathway. The NF-κB/p65 ActivELISA (Imgenex, San Diego, USA) kit was used to measure NF-κB free p65 in the nuclear lysate of the rat striatum (Schaaf et al. 2006).
Caspase-3 Colorimetric Assay
Caspase-3, also known as CPP-32, Yama or Apopain, is an intracellular cysteine protease that exists as a pro-enzyme, becoming activated during the cascade of events associated with apoptosis. The tissue homogenates can then be tested for protease activity by the addition of a caspase-specific peptide that is conjugated to the color reporter molecule p-nitroanaline (pNA). The cleavage of the peptide by the caspase releases the chromophore pNA, which can be quantitated spectrophotometrically at a wavelength of 405 nm. The level of caspase enzymatic activity in the cell lysate/homogenate is directly proportional to the color reaction. The enzymatic reaction for caspase activity was carried out as using R & D systems caspase-3 colorimetric kit (User guide: R & D systems caspase-3 colorimetric kit) (Guthmann et al. 2005).
Protein Estimation
The protein content of the brain tissue was measured according to the method of Lowry et al. (1951) using bovine serum albumin as standard.
Neurotransmitters Quantification
Biogenic amines (dopamine, serotonin, and their metabolites (HVA and 5-HIAA)) were estimated by HPLC with electrochemical detector. Waters standard system consisting of a high pressure isocratic pump, a 20 μl sample injector valve, C18 reverse phase column (Type: Waters symmetry C18 (5 μm); Length and diameter: 4.6 × 250 mm), and electrochemical detector were used. Data was recorded and analyzed with the help of empower software. Mobile phase consisting of 2% citric acid, 2% KHPO4, 1 mM EDTA, 1.2% MeOH, and 70 mg/ml of sodium octyl sulphate. pH of the mobile phase was adjusted to 3 with the help of HCl (6 N). Electrochemical conditions for the experiment were +0.800 V, sensitivity ranges from 5 to 50 nA. Separation was carried out at a flow rate of 1 ml/min. Samples (20 μl) were injected manually.
On the day of experiment, frozen striatum samples were thawed and they were homogenized in homogenizing solution containing 0.1 M perchloric acid. After that the samples were centrifuged at 12,000×g for 5 min. The supernatant was further filtered through 0.25 micron nylon filters before injecting in the HPLC injection pump. Data was recorded and analyzed with the help of empower software (Church 2005; Bishnoi et al. 2007a, b).
Behavioural Assessment of Orofacial Dyskinesia
On the test day, rats were placed individually in a small (30 × 20 × 30 cm) Plexiglas cage for the assessment of oral dyskinesia. Animals were given 10 min to get acclimatized to the observation chamber before behavioural assessments. To quantify the occurrence of oral dyskinesia, hand operated counter was used employed to score number of tongue protrusions, facial jerkings and vacuous chewing frequencies (VCMs). In the present study, VCM are referred to as single mouth openings in the vertical plane not directed toward physical material. If tongue protrusion or VCM occurred during a period of grooming, they were not taken into account. Counting was stopped whenever the rat began grooming, and restarted when grooming stopped. The observation chamber has mirrored flooring and the back wall of the chamber also has a mirror so that no observation is missed when the animal was faced away from the observer. The behavioural parameters of oral dyskinesia were recorded continuously for a period of 5 min. In all the experiments, the scorer was unaware of the treatment given to the animals (Naidu et al. 2003).
Statistical Analysis
All data are expressed as means ± S.E.M. The data were analyzed by using one-way analysis of variance (ANOVA) followed by Dunnett’s test. In all tests, the criterion for statistical significance was P < 0.05. Pearson correlation analysis was used to examine the correlation between two independent factors.
Results
Biochemical Assessment
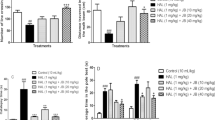
Chronic administration of haloperidol (5 mg/kg i.p. for 21 days) resulted in a marked increase in the amount of thiobarbituric acid reactive substances (TBARS) formed (lipid peroxidation), superoxide anion levels and marked decrease in Non-protein thiols (NPSH) in the striatal homogenates. Chronic administration of clozapine (10 mg/kg i.p. for 21 days) also resulted in an increase in TBARS formed (lipid peroxidation) and superoxide anion levels as well as decrease in NPSH in the striatal homogenates but the effect was markedly less pronounced than haloperidol. Pretreatment with curcumin (25 and 50 mg/kg) dose-dependently prevented the chronic haloperidol-induced increase the amount of TBARS levels, superoxide anion levels, and NPSH (no significant effect at lower doses) in the striatal homogenates. Curcumin (50 mg/kg) per se did not show any significant change as compared to control (Fig. 1).
Effects of haloperidol (5 mg/kg, i.p. 21 days), clozapine (10 mg/kg, i.p. 21 days), curcumin (25 and 50 mg/kg) + Haloperidol (5) on the altered oxidative damage parameters (a) lipid peroxidation (control (100%) = 1.52 ± 0.08 nmoles TBARS/mg protein), (b) superoxide anion, (c) non-protein thiols (control (100%) = 0.008 ± 0.0003 nmoles NPSH/mg protein) in rat brain striatum homogenates. Data has been expressed as % control (taking control values as 100) (n = 6–8/group) (HAL haloperidol, CUR curcumin). a P ≤ 0.05 as compared to control group, b P ≤ 0.05 as compared to haloperidol group, c P ≤ 0.05 as compared to curcumin (25 mg/kg) + Haloperidol(5) group
Assessment of Inflammatory and Apoptotic Markers
Compared to control group, chronic administration of haloperidol (5 mg/kg i.p. for 21 days) resulted in increase in the levels of total nitric oxide, caspase-3, TNF-alpha, and active p65 subunit of NF-kappaB in the tissue lysates of rat brain striatum whereas these parameters except total nitric oxide (significantly decreased) and NF-kappaB p65 active sub unit (significantly increased) were not amenable to any change after clozapine administration. Pretreatment with curcumin (25 and 50 mg/kg) dose-dependently prevented the chronic haloperidol-induced increase in the levels of total nitric oxide, caspase-3, TNF-alpha, and active p65 subunit of NF-kappaB in the tissue lysates of the striatal region of rat brain. Curcumin (50 mg/kg) per se did not show any significant change as compared to control (Fig. 2a–d).
Effects of haloperidol (5 mg/kg, i.p. 21 days), clozapine (10 mg/kg, i.p. 21 days), curcumin (25 and 50 mg/kg) + Haloperidol (5) on (a) total nitric oxide (NO) levels, (b) TNFalpha levels, (c) active p65 NF-kappaB unit (nuclear tissue lysate), (d) % caspase activity (control = 100) in rat brain striatum (n = 6–8/group). (HAL haloperidol, CUR curcumin). a P ≤ 0.05 as compared to control group, b P ≤ 0.05 as compared to haloperidol group, c P ≤ 0.05 as compared to curcumin (25 mg/kg) + Haloperidol(5) group
Assessment of Neurochemical Milieu
The striatal homogenates of haloperidol treated rats demonstrated a significant decrease in the levels of norepinephrine, dopamine, and serotonin. Chronic administration of clozapine (10 mg/kg i.p. for 21 days) did not result in significant change in the levels of nor epinephrine, dopamine but serotonin levels were significantly increased. Pretreatment with curcumin significantly prevented the chronic haloperidol-induced decrease in the levels of norepinephrine, dopamine, and serotonin in the striatal homogenates. Curcumin (50 mg/kg) per se did not show any significant change as compared to control (Table 1).
Behavioural Assessment
Chronic administration of haloperidol (5 mg/kg i.p. for 21 days) resulted in a significant increase in the number of vacuous chewing movements, tongue protrusions and facial jerkings during 5 min. However, 21 days treatment of clozapine (10 mg/kg) also showed significant increase in these orofacial movements but they were much less than haloperidol-treated group. Pretreatment with curcumin (25 and 50 mg/kg) dose-dependently prevented the chronic haloperidol-induced increase in vacuous chewing movements (36 and 63% at 25 and 50 mg/kg, respectively), tongue protrusions (30 and 55% at 25 and 50 mg/kg, respectively) and facial jerkings (39 and 62% at 25 and 50 mg/kg, respectively) during 5 min. Curcumin (50 mg/kg) per se did not show any significant change as compared to control (Table 2).
Pearson’s Correlation Analysis
Pearson correlation analysis also suggested that the oxidative stress markers (TBARS levels, superoxide anion levels and NO levels) are positively correlated (P ≤ 0.0001–0.0002, r = 0.90–0.95) with the TNF-α production which in turn positively correlated with NF-kappaB p65 unit activation and caspase-3 activation. NF-kappaB p65 unit activation is also positively correlated with caspase-3 activation. Further, Pearson correlation analysis revealed that vacuous chewing movements induced by haloperidol (marker of orofacial dyskinetic movements) has a positive correlation with TBARS levels (P ≤ 0.0001, r = 0.907), TNFalpha levels (P ≤ 0.0002, r = 0.728), NF-kappaB p65 unit activation (P ≤ 0.00015, r = 0.885), and caspase-3 activation (P ≤ 0.0003, r = 0.650).
Effect of Co-Administration of Piperine (Bioavailability Enhancer) with Curcumin on Various Biochemical, Neurochemical and Behavioural Parameters
As shown in Figs. 3 and 4, co-administration of piperine with curcumin significantly potentiated the protective effect of curcumin alone (25 mg/kg) on orofacial dyskinetic movements, oxidative damage parameters in striatal homogenates, inflammatory and apoptotic mediators in striatum, neurotransmitter levels in striatal homogenates induced by haloperidol. Co-administration of piperine with curcumin (50 mg/kg) significantly potentiated the protective effect of curcumin alone (50 mg/kg) against only some of the parameters (lipid peroxidation, superoxide anion and NF-kappaB p65 subunit). Rest of the parameters was not significantly changed in case of co-administration of piperine and curcumin (50) as compared to curcumin (50) alone (Figs. 3, 4).
Comparative protective effect of curcumin (25), curcumin (50), curcumin (25) + piperine (2.5), and curcumin (50) + piperine (2.5) against haloperidol induced a orofacial dyskinetic movements, b oxidative stress parameters, c inflammatory mediators and apoptotic markers (CUR curcumin, PIP piperine). a P ≤ 0.05 as compared to curcumin (25 mg/kg), b P ≤ 0.05 as compared to curcumin (50 mg/kg) group
Comparative increase in the striatal levels of neurotransmitter (norepinephrine, dopamine, and serotonin) levels by curcumin (25), curcumin (50), curcumin (25) + piperine (2.5), and curcumin (50) + piperine (2.5) (CUR curcumin, PIP piperine). a P ≤ 0.05 as compared to curcumin (25 mg/kg), b P ≤ 0.05 as compared to curcumin (50 mg/kg) group
Discussion
In the present study, chronic administration of haloperidol resulted in a significant increase in oxidative stress parameters, inflammatory mediators and pro-apoptotic markers as well as decrease in the neurotransmitter (norepinephrine, dopamine, and serotonin) levels in the striatum. Atypical antipsychotic, clozapine (10 mg/kg, i.p. for 21 days), also produced a significant but less pronounced effect on all these parameters. Our findings are in accordance with previous reports which suggested that haloperidol and clozapine can induce oxidative damage and possible cell death in the striatum and hippocampal areas of rat brain. However, clozapine is reported to produce significantly less oxidative damage as compared to haloperidol (Reinke et al. 2004; Polydoro et al. 2004). Similarly, there was a noticeable difference in the degree of orofacial behavioural dyskinetic movements produced by haloperidol as compared to clozapine. This is a generally accepted observation that clozapine has low propensity to cause tardive dyskinesia (Tamminga et al. 1994). Further curcumin attenuated all these alterations induced by haloperidol.
Curcumin is a potent antioxidant, comparable to Vitamin C and E and it inhibits lipid peroxidation in liver microsomes, erythrocyte membranes, and brain homogenates (Araujo and Leon 2001). It protects hemoglobin from oxidation at the concentration as low as 0.08 micromoles (Araujo and Leon 2001). It is also capable of scavenging oxygen free radicals such as superoxide anions, singlet oxygen, NO, and hydroxyl radicals which are important for the initiation of lipid peroxidation and causing oxidative damage (Quiles et al. 2002; Rajeswari 2006). All these observations support the present results that it dose-dependently inhibited the increase in the levels of lipid peroxides, superoxide anion levels, and decreased the levels of NPSH in rat striatum. Besides, it is believed that rise in glutamate dependent intracellular calcium levels represent the final common pathway of several injurious stimuli. It is the critical calcium concentration that is responsible for triggering the pro-oxidants and protease systems that are ultimately responsible for neuronal death. Hence, lowering of intracellular calcium by curcumin through inhibition of calcium influx (Balasubramanyam et al. 2003) leading to a decrease in the glutamate release might have produced protective effects. In one of our previous studies, we explained that curcumin prevent haloperidol-induced (at 1 mg/kg, i.p. for 21 days) behavioural and biochemical changes by virtue of its anti-oxidant effect (Bishnoi et al. 2008a).
Neurotransmitter quantification studies reveal decreased levels of monoamines in striatal regions following chronic haloperidol administration. Chronic administration of haloperidol may increase the number of dormant receptors as well as increase the receptor supersensitivity (Bishnoi et al. 2007a, b; Cara et al. 2001). Haloperidol-induced depolarization block of dopamine and other monoaminergic neuronal activity is also responsible for causing a decrease in monoamine levels in extracellular spaces in the brain. The decrease in monoamine levels may be due to increase in their metabolism. Increased metabolism of monoamine is associated with increased generation of reactive oxygen species (Terland et al. 2006). Clozapine treatment failed to affect the levels of dopamine and norepinephrine but significantly increased the levels of serotonin, suggesting the involvement of serotonin in its atypical action. This differential affect may account for the difference in the degrees of oxidative damage observed with haloperidol and clozapine, respectively. Serotonin displays a neuroprotective role as evident by recent reports that 5-HT (1A) receptor activation protects against KA-induced striatal lesions (Cosi et al. 2005; Madhavan et al. 2003; Ramos et al. 2004). 5-HT depletion following intrastriatal quinolinic acid exposure adversely affects neuronal survival and lends support to the hypothesis that increasing 5-HT level during NMDA receptor-mediated excitotoxicity may spare neurons destined to degenerate (Cummings and Walker 1996). Even the genetic studies are also suggesting the involvement of dopamine and serotonin receptors in haloperidol-induced tardive dyskinesia (Müller et al. 2004). In particular, there is important evidence suggesting association between dopamine 2 receptor (D2) polymorphisms (Taq I and -141-C Ins/Del) and a dopamine 3 receptor (D3) polymorphism (Ser9Gly) with antipsychotic response and drug-induced tardive dyskinesia (Lerer et al. 2002; Müller et al. 2004; De Leon et al. 2005). It has been suggested that in case of serotonin, 5HT2A and 5HT2C receptor-related genes are involved (Müller et al. 2004; Reynolds et al. 2005). Curcumin prevented this depletion of norepinephrine, dopamine, and serotonin hence limited the excitotoxicity and oxidative damage in the brain. Curcumin has also been reported to have a direct action on the central monoaminergic system. In several animal models such as forced swimming and olfactory bulbectomy, decrease in the levels of serotonin, noradrenaline, 5-hydroxyindoleacetic acid, and 4-dihydroxyphenylacetic acid in the hippocampus were observed, and were completely reversed by curcumin administration (Xu et al. 2005a). Moreover, curcumin was found to inhibit monoamine oxidase activity in the mouse brain (Xu et al. 2005b). These findings suggest that the neuroprotective effects of curcumin may involve the modulation of central monoaminergic neurotransmitter systems and even genetic modulation in the protective effect of curcumin.
Chronic administration of haloperidol also resulted in a significant increase in the striatal levels of TNF-α and total nitric oxide. Overproduction of NO may also lead to the generation of highly reactive species, such as peroxynitrite and stable nitrosothiols that may cause damage to proteins (3-nitrotyrosine, 3-NT) and lipids (4-hydroxynonenal, 4-HNE) (Liñares et al. 2006) and thus may contribute to neuronal damage. Recently, Liou et al. (2006) found that the haplotype T-4b-Glu, one of the genetic variant of NOS3 gene represents a protective haplotype against TD after long-term antipsychotic treatment. This finding suggests that human NOS3 gene may be involved in the pathogenesis of TD. Interestingly, curcumin was shown to inhibit the production of NO and various cytokines, including TNF-α (Cho et al. 2007). Curcumin exhibits activities similar to recently discovered tumor necrosis factor blockers (e.g., HUMIRA, REMICADE, and ENBREL) (Shishodia et al. 2007). TNF-mediated NF-kappaB and AKT activation were also reported to be inhibited by curcumin (Shishodia et al. 2007). Recently, we have also shown a beneficial effect of curcumin and its combination with insulin in attenuating diabetic neuropathy, possibly through its NO and TNF-α inhibitory activity (Sharma et al. 2007a, b). In the present study, curcumin dose-dependently prevented increase in the levels of both TNF-α and total nitric oxide, suggesting an important role of curcumin in regulating inflammatory cascade in haloperidol-induced neurotoxicity.
Earlier studies have shown the implication of NF-kappaB in the pathophysiology of haloperidol-induced toxicity (Post et al. 1998, 2002; Saldaña et al. 2006). The levels of p53 are reported to be upregulated by NF-kappaB activation in the rat striatum (Qin et al. 1999). Upregulation of p53 is associated with direct inhibition of anti-apoptotic (Bcl) genes and upregulation of pro-apoptotic (Bax and caspase-3) genes and proteins. Several stimuli including oxidative stress and TNF—α also induce the activation of NF-kappaB by promoting the degradation of IkB and nuclear translocation of dimer released from the IkB (Kikumori et al. 2001). The present observations are in line with these studies, i.e., an increase in the NF-kappaB expression coupled with increase in the striatal caspase-3 activity suggesting the role of NF-kappaB in the haloperidol-induced neurodegeneration and neuronal cell death. In our study, chronic administration of haloperidol but not clozapine (less activation) increased the p65 subunit of NF-kappaB which is positively correlated with the oxidative damage markers and caspase-3 activation, suggesting that it is an important mediator of the neuronal cell death pathway. Co-administration of curcumin significantly and dose-dependently inhibited NF-κB p65 unit levels in the nuclear fraction of rat brain striatal homogenate. Several studies involving systemic administration of curcumin have shown its beneficial effects on a variety of inflammatory conditions by modulating NF-κB activity (Singh et al. 1981). The results from the recent studies indicate that the relative potency for suppression of tumor necrosis factor (TNF)-induced nuclear factor-kappaB (NF-kappaB) activation was curcumin > demethoxycurcumin > bisdemethoxycurcumin > tetrahydrocurcumin (Sandur et al. 2007a, b).
Caspase-3 represents the predominant caspase in the CNS, both in normal neurodevelopmental and in neuropathological states (Jarskog et al. 2007; Yuan and Yankner 2000). In the previous studies using western blotting, 40–55% increase in immunoreactivity of activated caspase-3 bands after the chronic administration of haloperidol was observed. It was also demonstrated that the activation of caspase after the chronic treatment of haloperidol is a delayed effect, only occurs after 3–4 weeks of treatment (Jarskog et al. 2004). In the present study, chronic administration of haloperidol (5 mg/kg i.p. for 21 days) but not atypical antipsychotic clozapine could activate caspase-3 in the rat striatum. The increased expression of caspase results in the cleavage of DNA fragmentation factor and other key cellular substrates. Owing to its association with DNA fragmentation, the presence of activated caspase-3 has been identified as a marker for active apoptosis (Arends et al. 1990). Caspase-3 is also involved in haloperidol-induced changes in higher doses and can cause neuronal degeneration and cell death (Bishnoi et al. 2008a, b). The selective activation of striatal caspase-3 suggests that it may play an important role in the extrapyramidal side effects associated with the chronic administration of haloperidol and related neuroleptics. Co-administration of curcumin significantly and dose-dependently inhibited caspase-3 levels in the cytoplasmic fraction of striatal homogenate.
Once the pharmacodynamic effect of curcumin was confirmed, we focused on the pharmacokinetics of curcumin. Keeping in mind about the low bioavailability of curcumin, we attempted to increase its bioavailability. We thought that increase in the bioavailability will increase the therapeutic effect of curcumin at lower doses, which will help to limit the toxicity as well as dosage required for maximum therapeutic activity. Co-administration of piperine, a known bioavailability enhancer, is a major approach to improve the bioavailability of any drug. Piperine is known to be an inhibitor of hepatic and intestinal glucuronidation. It alters the disposition and bioavailability of a large number of drugs including curcumin (Shoba et al. 1998), ECCG (Lambert et al. 2004; Atal et al. 1985), theophylline (Atal et al. 1985), etc. Shoba et al. (1998) have demonstrated that when piperine was concomitantly administered with curcumin, it increased the plasma concentration and time to maximum peak level (T max), while decreased the elimination half life and clearance of curcumin. Mechanistically, it appears that piperine inhibits small intestinal glucuronidation of curcumin, which may result in increased absorption, and that piperine may also slow the GI transit of curcumin, thus increasing residence time in the intestine and allowing for greater absorption. One can argue that the antioxidant effect of piperine may also be contributing towards the enhanced benefit observed with curcumin (25). However, in our study as well as previous literatures have suggested that piperine does not exert any antioxidant activity at such a low dose, i.e., 2.5 mg/kg. Piperine usually showed its free radical scavenging activity at the dose 20 mg/kg or more than that (40 mg/kg and 100 mg/kg) (Vijayakumar et al. 2004; Selvendiran et al. 2003; Rauscher et al. 2000). However, in our study piperine (2.5) was able to increase the effect of curcumin (25) but not of curcumin (50).
The present study clearly demonstrates the neurotoxic activity of haloperidol (typical neuroleptic) and provided an evidence for its multifactorial nature involving neurotransmitter irregulation, oxidative damage, inflammation, and apoptosis. Further, the effective modulation of molecular targets by curcumin points towards its strong therapeutic potential in haloperidol-induced neurotoxicity and extrapyramidal side effects. This study also provides a rationale for the combination of piperine and curcumin for the amelioration of haloperidol-induced neurotoxicity and tardive dyskinesia.
References
Aggarwal BB (2000) Tumor necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum D 59(Suppl 1):6–16
Aggarwal BB, Shishodia S (2004) Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N Y Acad Sci 1030:434–441
Aggarwal BB, Shishodia S, Ashikawa K, Bharti AC (2002) The role of TNF and its family members in inflammation and cancer: lessons from gene deletion. Curr Drug Targets Inflamm Allergy 1(4):327–341
Araujo CAC, Leon LL (2001) Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz 6(5):723–728
Arends MJ, Morris RG, Wyllie AH (1990) Apoptosis. The role of the endonuclease. Am J Pathol 136:593–608
Atal CK, Dubey RK, Singh JJ (1985) Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. Pharmacol Exp Ther 232:258–262
Babior BM, Kipner RS, Cerutte JT (1973) Biological defense mechanism. The production by leukocytes of superoxide, a potential bacterial agent. J Clin Investig 52:741–744
Balasubramanyam M, Koteswari AA, Kumar RS, Monickaraj SF, Maheswari JU, Mohan V (2003) Curcumin induced inhibition of cellular reactive oxygen species generation: novel therapeutic implications. J Biosci 28:715–721
Bishnoi M, Chopra K, Kulkarni SK (2007a) Possible anti-oxidant and neuroprotective mechanisms of zolpidem in attenuating typical anti-psychotic-induced orofacial dyskinesia—a biochemical and neurochemical study. Prog Neuropsychopharmacol Biol Psychiatry 31(5):1130–1138
Bishnoi M, Kumar A, Chopra K, Kulkarni SK (2007b) Comparative neurochemical changes associated with chronic administration of typical and atypical neuroleptics: implications in tardive dyskinesia. Indian J Exp Biol 45(2):175–179
Bishnoi M, Chopra K, Kulkarni SK (2008a) Protective effect of Curcumin, the active principle of turmeric (Curcuma longa) in haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes in rat brain. Pharmacol Biochem Behav 88:511–520
Bishnoi M, Chopra K, Kulkarni SK (2008b) Activation of striatal inflammatory mediators and caspase-3 is central to haloperidol-induced orofacial dyskinesia. Eur J Pharmacol 590(1–3):241–245
Burger MBE, Fachineto R, Alves A, Callegari L, Rocha JBT (2005) Acute reserpine and sub-chronic haloperidol treatments change synaptosomal brain glutamate uptake and elicit orofacial dyskinesia in rats. Brain Res 1031(2):202–210
Burkhardt C, Kelly JP, Lim YH, Filley CM, Parker WD (1993) Neuroleptic medications inhibit complex II of the electron transport chain. Ann Neurol 33:512–517
Cara DB, Dusticier N, Forni C, Lievens JC, Daszuta A (2001) Serotonin depletion produces long lasting increase in striatal glutamatergic transmission. J Neurochem 78:240–248
Cho JW, Lee KS, Kim CW (2007) Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med 19(3):469–474
Church WH (2005) Column chromatography analysis of brain tissue: an advanced laboratory exercise for neuroscience majors. J Undergrad Neurosci Educ 3(2):A36–A41
Cosi C, Waget A, Rollet K, Tesori V, Newman-Tancredi A (2005) Clozapine, ziprasidone and aripiprazole but not haloperidol protect against kainic acid-induced lesion of the striatum in mice, in vivo: role of 5-HT1A receptor activation. Brain Res 1043(1–2):32–41
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262(5134):689–695
Creese I, Burt D, Snyder SH (1976) Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192:481–483
Cummings TJ, Walker PD (1996) Serotonin depletion exacerbates changes in striatal gene expression following quinolinic acid injection. Brain Res 743(1–2):240–248
De Leon J, Susce MT, Pan RM, Koch WH, Wedlund PJ (2005) Polymorphic variations in GSTM1, GSTT1, PgP, CYP2D6, CYP3A5, and dopamine D2 and D3 receptors and their association with tardive dyskinesia in severe mental illness. J Clin Psychopharmacol 25(5):448–456
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP (2004) Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 90:1011–1015
Guerin-Marchand C, Sénéchal H, Pelletier C, Fohrer H, Olivier R, David B, Berthon B (2001) H2O2 impairs inflammatory mediator release from immunologically stimulated RBL-2H3 cells through a redox-sensitive, calcium-dependent mechanism. Inflamm Res 150:341–349
Guthmann F, Wissel H, Schachtrup C, Tölle A, Rüdiger M, Spener F, Rüstow B (2005) Inhibition of TNFalpha in vivo prevents hyperoxia-mediated activation of caspase 3 in type II cells. Respir Res 6:10
Hoehle SI, Pfeiffer E, Solyom AM, Metzler M (2006) Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J Agric Food Chem 54:756–764
Jarskog LF, Selinger ES, Lieberman JA, Gilmore JH (2004) Apoptotic proteins in the temporal cortex in schizophrenia: high Bax/Bcl-2 ratio without caspase-3 activation. Am J Psychiatry 161:109–115
Jarskog LF, Gilmore JH, Glantz LA, Gable KL, German TT, Tong RI, Lieberman JA (2007) Caspase-3 activation in rat frontal cortex following treatment with typical and atypical antipsychotics. Neuropsychopharmacology 32:95–102
Kikumori T, Kambe F, Nagaya T, Funahashi H, Seo H (2001) Thyrotrophin modifies activation of nuclear factor kappaB by tumor necrosis factor-alpha in rat thyroid cell line. Biochem J 354:573–579
Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS (2004) Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J Nutr 134(8):1948–1952
Lerer B, Segman RH, Fangerau H, Daly AK, Basile VS, Cavallaro R, Aschauer HN, McCreadie RG, Ohlraun S, Ferrier N, Masellis M, Verga M, Scharfetter J, Rietschel M, Lovlie R, Levy UH, Meltzer HY, Kennedy JL, Steen VM, Macciardi F (2002) Pharmacogenetics of tardive dyskinesia: combined analysis of 780 patients supports association with dopamine D3 receptor gene Ser9Gly polymorphism. Neuropsychopharmacology 27(1):105–119
Liñares D, Taconis M, Maña P, Correcha M, Fordham S, Staykova M, Willenborg DO (2006) Neuronal nitric oxide synthase plays a key role in CNS demyelination. J Neurosci 26(49):12672–12681
Liou YJ, Lai IC, Lin MW, Bai YM, Lin CC, Liao DL, Chen JY, Lin CY, Wang YC (2006) Haplotype analysis of endothelial nitric oxide synthase (NOS3) genetic variants and tardive dyskinesia in patients with schizophrenia. Pharmacogenet Genomics 16(3):151–157
Lohr JB, Kuezenski R, Niculescu AB (2003) Oxidative mechanisms and tardive dyskinesia. CNS drugs 17(1):47–62
Lowry OH (1951) Protein measurements with the Folin-phenol reagent. J Biol Chem 193:265–275
Madhavan L, Freed WJ, Anantharam V, Kanthasamy AG (2003) 5-Hydroxytryptamine 1A receptor activation protects against N-methyl-d-aspartate-induced apoptotic cell death in striatal and mesencephalic cultures. J Pharmacol Exp Ther 304(3):913–923
Mahakunakorn P, Tohda M, Murakami Y, Matsumoto K, Watanabe H, Vajaragupta O (2003) Cytoprotective and cytotoxic effects of curcumin: dual action on H2O2-induced oxidative cell damage in NG108-15 cells. Biol Pharm Bull 26:725–728
Müller DJ, Shinkai T, De Luca V, Kennedy JL (2004) Clinical implications of pharmacogenomics for tardive dyskinesia. Pharmacogenomics J 4(2):77–87
Naidu PS, Singh A, Kulkarni SK (2003) Quercetin, a bioflavonoid attenuated haloperidol induced orofacial dyskinesia. Neuropharmacology 44:1100–1106
Pan MH, Huang TM, Lin JK (1999) Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 27:486–494
Polydoro M, Schroder N, Lima MN, Caldana F, Laranja DC, Bromberg E, Roesler R, Quevedo J, Moreira JC, Dal-Pizzol F (2004) Haloperidol- and clozapine-induced oxidative stress in the rat brain. Pharmacol Biochem Behav 78(4):751–756
Post A, Holsboer F, Behl C (1998) Induction of NF-KB activity during haloperidol-induced oxidative toxicity in clonal hippocampal cells: suppression of NF-KB and neuroprotection by antioxidants. J Neurosci 15:8236–8246
Post A, Rücker M, Ohl F, Uhr M, Holsboer F, Almeida OF, Michaelidis TM (2002) Mechanisms underlying the protective potential of alpha-tocopherol (vitamin E) against haloperidol-associated neurotoxicity. Neuropsychopharmacology 26(3):397–407
Priyadarsini K, Maity D, Naik G (2003) Role of phenolic O–H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic Biol Med 35:475–484
Qin ZH, Chen RW, Wang Y, Nakai M, Chuang DM, Chase TN (1999) Nuclear factor KB nuclear translocation upregulates c-Myc and p53 expression during NMDA receptor apoptosis. J Neurosci 19:4023–4033
Quiles JL, Mesa MD, Ramirez-Tortosa CL, Aguilera CM, Battino M, Gil A, Ramirez-Tortosa MC (2002) Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler Thromb Vasc Biol 22(7):1225–1231
Rajeswari A (2006) Curcumin protects mouse brain from oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Eur Rev Med Pharmacol Sci 10(4):157–161
Ramos AJ, Rubio MD, Defagot C, Hischberg L, Villar MJ, Brusco A (2004) The 5HT1A receptor agonist, 8-OH-DPAT, protects neurons and reduces astroglial reaction after ischemic damage caused by cortical devascularization. Brain Res 1030(2):201–220
Rauscher FM, Sanders RA, Watkins JB (2000) Effects of piperine on antioxidant pathways in tissues from normal and streptozotocin-induced diabetic rats. J Biochem Mol Toxicol 14(6):329–334
Reinke A, Martins MR, Lima MS, Moreira JC, Dal-Pizzol F, Quevedo J (2004) Haloperidol and clozapine, but not olanzapine, induces oxidative stress in rat brain. Neurosci Lett 372(1–2):157–160
Reynolds GP, Templeman LA, Zhang ZJ (2005) The role of 5-HT2C receptor polymorphisms in the pharmacogenetics of antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry 29(6):1021–1028
Sagara Y (1998) Induction of reactive oxygen species in neurons by haloperidol. J Neurochem 71:1002–1012
Saldaña M, Bonastre M, Aguilar E, Marin C (2006) Role of nigral NF-kappaB p50 and p65 subunit expression in haloperidol-induced neurotoxicity and stereotyped behavior in rats. Eur Neuropsychopharmacol 16(7):491–497
Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB (2007a) Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol Med 43(4):568–580
Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB (2007b) Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 28(8):1765–1773
Schaaf MJ, Willetts L, Hayes BP, Maschera B, Stylianou E, Farrow SN (2006) The relationship between intranuclear mobility of the NF-KB subunit p65 and its DNA-binding affinity. J Biol Chem 281(31):22409–22420
See RE, Lynch AM (1995) Chronic haloperidol potentiates stimulated glutamate release in caudate putamen, but not prefrontal cortex. Neuroreport 6(13):1795–1798
See RE, Lynch AM, Aravagiri M, Nemeroff CB, Owens MJ (1995) Chronic haloperidol-induced changes in regional dopamine release and metabolism and neurotensin content in rats. Brain Res 704(2):202–209
Selvendiran K, Singh JP, Krishnan KB, Sakthisekaran D (2003) Cytoprotective effect of piperine against benzo[a]pyrene induced lung cancer with reference to lipid peroxidation and antioxidant system in Swiss albino mice. Fitoterapia 74(1–2):109–115
Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB (2006) Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J Biol Chem 281(33):23425–23435
Sharma RA, Gescher AJ, Steward WP (2005) Curcumin: the story so far. Eur J Cancer (13):1955–1968
Sharma RA, Steward WP, Gescher AJ (2007a) Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol 595:453–470
Sharma S, Chopra K, Kulkarni SK (2007b) Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha. Phytother Res 21(3):278–283
Shishodia S, Sethi G, Aggarwal BB (2005) Curcumin: getting back to the roots. Ann N Y Acad Sci 1056:206–217
Shishodia S, Chaturvedi MM, Aggarwal BB (2007) Role of curcumin in cancer therapy. Curr Probl Cancer 31(4):243–305
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64(4):353–356
Singh S, Khanna M, Sarin JPS (1981) High pressure liquid chromatographic determination of Curcumin in biological fluids. Indian Drugs 18:207–209
Tamminga CA, Thaker GK, Moran M, Kakigi T, Gao XM (1994) Clozapine in tardive dyskinesia: observations from human and animal model studies. J Clin Psychiatry 55(Suppl B):102–106
Terland O, Almås B, Flatmark T, Andersson KK, Sørlie M (2006) One-electron oxidation of catecholamines generates free radicals with an in vitro toxicity correlating with their lifetime. Free Radic Biol Med 41(8):1266–1271
Tsai G, Goff DC, Wang RW, Flood J, Baer L, Coyle JT (1998) Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. Am J Psychiatry 155:1207–1213
Vijayakumar RS, Surya D, Nalini N (2004) Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep 9(2):105–110
Vilner BJ, Costa BR, Bowen WD (1995) Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. J Neurosci 15:134–137
Wills ED (1966) Mechanism of lipid peroxide formation in animal tissues. Biochem Jour 99:667–676
Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ (2005a) The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol 518(1):40–46
Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ (2005b) Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav 82(1):200–206
Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407:802–809
Acknowledgments
The study was supported by the UGC grant under Centre with Potential for Excellence in Biomedical Sciences (CPEBS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bishnoi, M., Chopra, K., Rongzhu, L. et al. Protective Effect of Curcumin and its Combination with Piperine (Bioavailability Enhancer) Against Haloperidol-Associated Neurotoxicity: Cellular and Neurochemical Evidence. Neurotox Res 20, 215–225 (2011). https://doi.org/10.1007/s12640-010-9229-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-010-9229-4