Abstract
Long fibers, such as asbestos and carbon nanotubes (CNTs), are more potent activators of inflammatory and genotoxicity than short or tangled fibers. Fibrous particles trigger interleukin (IL)-1β secretion and cause inflammatory diseases through NLRP3 inflammasomes in phagocytotic cells. However, the mechanism involved in fibrous particle-induced inflammation has not been well documented. In this study, we focused on GTPase effector Rho-kinases (ROCK1, and 2), which are known to be involved in a wide range of cellular functions such as adhesion, regulation of cytoskeleton, and phagocytosis. We examined whether ROCKs are associated with multi-walled CNT (MWCNT)- or asbestos-induced IL-1β secretion in human monocytic THP-1 cells using a selective inhibitor and small interfering RNA. THP-1 cells were differentiated to macrophages by PMA and were exposed to MWCNTs, crocidolite asbestos or lipopolysaccharide (LPS) in the presence or absence of Y27632 (ROCK inhibitor) or Z-YVAD (caspase-1 inhibitor). Exposure of the cells to MWCNTs or asbestos provoked IL-1β secretion, but this secretion was suppressed by both Y27632 and Z-YVAD, whereas LPS-induced IL-1β secretion was inhibited only by Z-YVAD and not by Y27632. siRNA designed for knockdown of both ROCK1 and ROCK2 suppressed MWCNT- and asbestos-induced IL-1β secretion, but did not change LPS-induced IL-1β secretion. Moreover, Y27632 suppressed pro-IL-1β protein levels and the release of activated-cathepsin B and activated-caspase-1 induced by MWCNTs or asbestos. In contrast, LPS-induced pro-IL-1β protein was not suppressed by Y27632. These results suggest that ROCKs are involved in fibrous particle-induced inflammasome responses in THP-1 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inhalation exposure to airborne pollutants is associated with several adverse health effects. Exposure to asbestos can result in pulmonary fibrosis, lung cancer, and mesothelioma in humans (Delgermaa et al. 2011; Mossman et al. 2011). Interleukin (IL)-1β, a pro-inflammatory cytokine, has been reported to be involved in the pathogenesis of asbestos-induced mesothelioma (Dostert et al. 2008; Shukla et al. 2009). Recently, it was reported that asbestos increased IL-1β secretion through the NOD-like receptor pyrin domain containing three (NLRP3) inflammasomes. NLRP3 inflammasome activation has been reported to be a key factor in the harmful health effects of particulate matter (Dostert et al. 2008).
The NLRP3 inflammasome is a large multimolecular complex composed of NLRP3, the adaptor apoptosis-associated speck-like protein containing a CARD domain (ASC), and caspase-1. The NLRP3 inflammasome is activated by a wide range of signals including pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Lipopolysaccharide (LPS) and lipoteichoic acid (LTA), a component of the outer membrane of bacteria, are known PAMPs (Freche et al. 2007). Certain endogenous DAMPs such as ATP and monosodium urate (MSU) crystal (Martinon et al. 2006), silica (Dostert et al. 2008), and asbestos (Dostert et al. 2008 Li et al. 2012) are recognized as activators of the NLRP3 inflammasome.
Carbon nanotubes (CNTs) are one of the most promising nanomaterials in many industrial and biomedical applications. With the increasing use of CNTs, considerable concern has been raised in view of their environmental and human health effects (Hirano et al. 2012; Meunier et al. 2012; Palomaki et al. 2011). Though CNTs offer unique physiological properties, such as a nanoscale diameter, high aspect ratio, and large surface area in comparison with large particles, their fibrous shape suggests that they may have toxic properties like asbestos (Nagai and Toyokuni 2010; Pacurari et al. 2010). Recent publications reported that single-walled CNTs (SWCNTs) induced a strong acute inflammatory reaction through the secretion of pro-inflammatory cytokines in mice (Inoue et al. 2010; Shvedova et al. 2008). More recently, it has been reported that double-walled CNTs (DWCNTs)- and needle-like CNT-induced IL-1β secretion are linked to NLRP3 inflammasome activation in human monocytes, in a manner similar to DAMPs such as asbestos and MSU (Dostert et al. 2008; Meunier et al. 2012; Palomaki et al. 2011). Long CNTs and asbestos activate the NLRP3 inflammasome through a similar mechanism because of their similar needle-like shape and physicochemical characteristics (Palomaki et al. 2011). However, the underlying inflammatory mechanisms triggered by fibrous materials remain to be elucidated.
It has been reported that phagocytosis of fibrous particles is required as an initial step for inflammasome activation (Meunier et al. 2012). Phagocytosis of fibrous particles leads to leakage of the lysosomal cysteine protease and cathepsin B, into the cytoplasm, and is associated with the activation of the NLRP3 inflammasome (Meunier et al. 2012). Activation of the NLRP3 inflammasome triggers activated-caspase-1-dependent proteolytic processing of immature pro-inflammatory cytokine IL-1 family members, such as IL-1β and IL-18, and enhances the secretion of mature pro-inflammatory cytokines (Qu et al. 2007). The mature IL-1β is released from secretary lysosomes through K+-dependent mechanisms (Kahlenberg and Dubyak 2004; Perregaux and Gabel 1994) and promotes inflammatory responses (Qu et al. 2007). Inflammasome-mediated secretion of IL-1 cytokines is associated with the simultaneous secretion of inflammasome components into the cell culture supernatant (Palomaki et al. 2011).
Rho-kinases (ROCK1 and ROCK2) are the effectors of Rho GTPase and have a molecular mass of ~160 kDa (Ishizaki et al. 1996; Leung et al. 1995). ROCKs are known to be involved in a wide range of fundamental cellular functions, such as adhesion, regulation of cytoskeleton, and phagocytosis (Kanno et al. 2013; Olazabal et al. 2002; Shi and Wei 2007). Activated ROCK1 induces myosin light chain (MLC) phosphorylation and cellular F-actin and activates the actin-myosin contractile system (Coleman et al. 2001), whereas ROCK2 is required for myosin-2 dependent phagocytosis (Yoneda et al. 2005). Previous studies have suggested that cytoskeletal proteins, such as tubulin and actin, are required in the production of fibrous particle-induced IL-1β through the NLRP3 inflammasome (Meunier et al. 2012; Martinon et al. 2006; Misawa et al. 2013). More recently, it has been reported that microtubules mediate the assembly of the NLRP3 inflammasome (Misawa et al. 2013). Rho/ROCK signaling has been reported to be associated with the stability or active role of microtubules (Takesono et al. 2010). Therefore, we considered that ROCKs possibly contribute to the fibrous particle-induced NLRP3 inflammasome. However, the mechanism whereby phagocytosed fibrous particles trigger IL-1β secretion through the NLRP3 inflammasome is poorly understood.
In this study, we examined whether IL-1β secretion by fibrous particles is inflammasome-dependent. We report that Rho-kinases (ROCKs) are associated with the NLRP3 inflammasome pathway in differentiated THP-1 cells using a ROCKs inhibitor and siRNA of ROCKs.
Materials and methods
Chemicals
Y27632, cytochalasin D, LPS from Escherichia coli, and phorbol myristate acetate (PMA) were purchased from Sigma (St. Louis, MO, USA). Z-YVAD-fmk was purchased from BioVision (Mountain View, CA, USA). A human IL-1β/IL-1F2 Quantikine ELISA Kit was purchased from R&D Systems (Minneapolis, MN, USA). Cytochalasin D (1 mg/ml) and PMA (100 μM) stock solutions were prepared in dimethyl sulfoxide (DMSO) and were used at a final DMSO concentration of 0.1 %. Y-27632 (5 mM) and LPS (1 mg/ml) stock solutions were prepared in phosphate-buffered saline (PBS, WAKO, Osaka, Japan). Lipofectamine® RNAiMAX Reagent and AlamarBlue® were purchased from Life Technologies (Carlsbad, CA, USA). All chemicals were of analytical grade.
MWCNTs and asbestos
MWCNTs (XNRI WMVT-7, Lot# 05072001K28) were obtained from Bussan Nanotech Research (Ibaraki, Japan). Crocidolite asbestos was obtained from UICC (Union Internationale Contre le Cancer). The characteristics and the preparation method for MWCNTs and UICC crocidolite suspensions have been described previously (Hirano et al. 2008). The nominal physicochemical data for the MWCNTs were as follows: average diameter, 67 nm; surface area, 26 m2/g; carbon purity, 99.79 wt%; iron impurity, ca. 2,000 ppm; fiber length, not specified. UICC crocidolite asbestos has been shown that average fiber length, 2.5 μm; average width, 0.33 μm (Kohyama et al. 1996), reactive surface area measured by BET method, 8 m2/g (Zalma et al. 1987). Both MWCNTs and crocidolite asbestos were heat-treated at 250 °C for 2 h in an electric furnace to remove any possible contamination endotoxins. MWCNTs were suspended in 10 % endotoxin-free Pluronic F68 (Sigma). MWCNT suspension was added to the culture so that the final Pluronic F68 concentration was 1 % in both the experimental and control wells. Asbestos was suspended in PBS. MWCNTs and asbestos were ultrasonicated in culture medium for 10 s immediately before use in the experiments. Representative microscopic images of MWCNTs and crocidolite asbestos added to the culture medium are shown in Supplemental Fig. 1.
Cell culture
THP-1, a human monocyte cell line, was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). THP-1 cells were cultured at 37 °C in a 5 % CO2 atmosphere in RPMI1640 medium (Life Technologies). The culture media contained 10 % heat-inactivated fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 μg/ml of streptomycin. For experiments, cells were plated in six-well (for Western blot analyses) or 96-well (for analyses by ELISA) plates and differentiated with 100 nM PMA for 24 h. For priming, the cells were incubated with 10 ng/ml LPS for the last 3 h of differentiation with PMA. The cells were washed with Hanks’ balanced salt solution (HBSS, Life Technologies) and used for the experiments.
Preparation of MSU crystals
MSU crystals were prepared according to McCarty et al. (McCarty and Faires 1963; Sakamaki et al. 2008). Briefly, to 336 mg of uric acid, 80 ml of distilled water and 0.08 g of NaOH were added. After boiling to dissolve the uric acid, the solution was allowed to cool at room temperature to 45 °C and was sterilized by filtration (Steriflip, Millipore, Hayward, CA, USA). The solution was stored at 25 °C to precipitate the MSU crystals.
The obtained MSU crystals were centrifuged and washed with ethanol twice. After drying, the MSU crystals were suspended in PBS as the stock solution (10 mg/ml). The MSU crystal suspension was ultrasonicated (Bioruptor UCD-250, Cosmo Bio, Tokyo, Japan) for 0, 20, 60, or 120 s to create short length-MSUs. The length of the MSU crystals without ultrasonication was about 100 μm and the length following ultrasonication for 20, 60, or 120 s was about 50, 10–20, and 5–15 μm, respectively (Supplemental Fig. 3A).
Determination of IL-1β in culture supernatants
The differentiated THP-1 cells were washed with HBSS and exposed to 2–10 μg/ml MWCNTs, 20–100 μg/ml asbestos, 1 mg/ml MSU crystal, or 0.1 μg/ml LPS in the presence or absence of 10 μM Z-YVAD, 10 μM Y27632, or 1 μg/ml cytochalasin D for 12 h. The concentration of IL-1β in the culture supernatant was measured using an IL-1β/IL-1F2 ELISA kit according to the manufacturer’s instructions. These experiments were replicated three or four times.
Isolation of proteins in cell culture supernatants
The differentiated THP-1 cells were exposed to 2–10 μg/ml MWCNTs, 100 μg/ml asbestos, or 0.1 μg/ml LPS for 5 h in the presence or absence of 10 μM Y27632. The cells were then washed with HBSS and cultured with Opti-MEM® (Life Technologies) without FBS for 15 h. Proteins in the cell supernatants were extracted by methanol/chloroform precipitation. The proteins in the cell culture supernatants were dissolved in PBS and were used for Western blot analyses.
Western blot analysis
The differentiated and LPS-primed THP-1 cells were washed with HBSS and exposed to 2–10 μg/ml MWCNTs, 100 μg/ml asbestos, or 0.1 μg/ml LPS in the presence or absence of 10 μM Y27632 for 15 h. The cells were lysed with RIPA buffer containing the protease inhibitor, PMSF, and sodium orthovanadate (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The whole lysate were ultrasonicated for 1 min and was used for Western blotting. Protein concentrations were determined using Bradford ULTRA® reagent (Novexin, Cambridge, UK). Proteins in the whole lysate or concentrated culture supernatant were resolved using SDS-PAGE under reducing conditions, then electroblotted onto a PVDF membrane. The membrane was blocked with PVDF Blocking Reagent (TOYOBO, Osaka, Japan) and probed with anti-cathepsin B (Santa Cruz Biotechnology), anti-proIL-1β (Santa Cruz Biotechnology), anti-caspase-1 (Cell Signaling Technology, Danvers, MA, USA), anti-ROCK1, or anti-ROCK2 (BD Biosciences), followed by peroxidase (POD)-tagged anti-mouse IgG antibody (Santa Cruz Biotechnology). The immunoreactions on the membrane were visualized using enhanced chemiluminescence (ECL, GE Healthcare, Buckinghamshire, UK). After probing with each antibody, the membrane was incubated with stripping buffer (2 % SDS and 100 mM 2-mercaptoethanol in Tween-PBS) at 56 °C for 20 min to remove the antibodies, and re-probed with POD-tagged anti-α-tubulin antibody (MBL, Nagoya, Japan). These experiments were repeated three times.
To examine ROCK protein level changes according to differentiation, the cells were plated into a six-well culture dish and treated with or without 100 nM PMA for 24 h. The cells were washed with HBSS and lysed as described above. Each lysate was centrifuged at 10,000×g for 5 min at 4 °C, and the supernatant was used for Western blot analyses. The intensities of the bands were quantified using a chemiluminescence densitometer (Lumino Imaging Analyzer, FAS-1100, TOYOBO, Osaka, Japan).
Quantitative measurements of uptake of MWCNTs or asbestos by differentiated THP-1 cells
Cells were cultured with 100 nM PMA for 1 or 3 days in six-well culture dishes. The differentiated THP-1 cells were washed with HBSS and further cultured with or without 10 μg/ml MWCNTs or 100 μg/ml asbestos in the presence or absence of 10 μM Y-27632 for 12 h. The concentration of cellular-associated MWCNTs or asbestos was evaluated according to a method described previously (Hirano et al. 2010). Briefly, the cells were washed twice with HBSS and lysed with 0.2 M NaOH (0.4 ml). Dimethyl sulfoxide (0.2 ml) was added to each well and the lysate was pipetted thoroughly. A 250-μl sample was transferred to a 96-well culture dish, and the O.D. (640 nm) was measured with a spectrofluorometer (POLARstar OPTIMA, BMG Labtech, Offenburg, Germany) to quantify the amount of cell-associated MWCNTs or asbestos. Standard MWCNT and asbestos samples were prepared similarly without the cells.
Cytotoxicity
Cells were plated at a concentration of 2 × 104 cells/well into a 96-well culture dish and cultured with 100 nM PMA for 24 h. The cells were washed with HBSS and cultured with MWCNTs (0.1–100 μg/ml) or asbestos (1–1,000 μg/ml) in the presence or absence of 10 μM Y27632 for 16 h. Cell viability was evaluated using AlamarBlue® according to the manufacturer’s instructions. The fluorescence intensity of the medium was measured with a spectrofluorometer (POLARstar OPTIMA) using the excitation and emission wavelengths of 540 and 588 nm, respectively. The experiments were replicated four times.
ROCK1 or ROCK2 knockdown with small interfering RNA (siRNA)
Cells were cultured in the presence of 100 nM PMA for 24 h. The differentiated THP-1 cells were washed with HBSS and transfected with three independent antisense siRNAs of ROCK1 (S1–3) and ROCK2 (S4–6) (Life Technologies) or with control siRNA (Stealth RNAi Negative Control Duplex®, Life Technologies) using Lipofectamine® RNAiMAX Reagent according to the manufacturer’s protocol. Primer sequences for ROCK1 and ROCK2 siRNA are shown in Supplemental Fig. 4A. After transfection, the protein level of ROCK1 or ROCK2 was examined by Western blotting. We selected two antisense and used them for exposure experiments. After transfection with siRNA or control siRNA for 48 h, the cells were further cultured in fresh RPMI1640 complete medium with 10 μg/ml MWCNTs, 100 μg/ml asbestos, or 0.1 μg/ml LPS for 12 h. The concentration of IL-1β in the culture supernatant was measured by ELISA as described above.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses were performed by ANOVA with Bonferroni/Dunn’s post hoc analysis (GraphPad Prism 4.0, GraphPad Software, San Diego, CA, USA). The statistical significance level was set at p < 0.05.
Results
Changes in ROCK1 and ROCK2 protein levels according to differentiation
It is known that THP-1 cells differentiate into macrophages by PMA (Tsuchiya et al. 1982). Thus, we examined whether expression of ROCKs is affected by differentiation with PMA. Treatment with PMA increased expression of ROCK1, whereas that of ROCK2 was not changed in THP-1 cells (Fig. 1). These results indicate that ROCKs are associated with the functional characteristics of macrophages.
Western blot analyses for the detection of ROCK1 and ROCK2 in differentiated and undifferentiated THP-1 cells. Cells were cultured in the presence or absence of 100 nM PMA for 24 h to differentiate THP-1 cells into macrophages. After washing twice with HBSS, cells were lysed with lysate buffer. The lysate proteins were resolved by SDS-PAGE and electroblotted onto a PVDF membrane. The blot was probed with anti-ROCK1 and anti-ROCK2 antibodies, followed by a corresponding POD-tagged secondary antibody. The membranes were stripped and re-probed with POD-tagged anti-tubulin antibody. The intensities of the bands were quantified by chemiluminescence densitometry. Data are presented as mean ± SD (N = 3)
Effects of caspase-1 inhibitor on IL-1β secretion induced by MWCNTs, asbestos, or LPS
Caspase-1 is a key protease activated by the NLRP3 inflammasome complex involved in the maturation of IL-1β. Thus, we confirmed whether caspase-1 is involved in MWCNT-, asbestos-, and LPS-induced IL-1β secretion in THP-1 cells. IL-1β secretion was markedly induced by MWCNTs and asbestos, as well as by LPS, and the induced IL-1β secretion was significantly suppressed by Z-YVAD, a caspase-1 selective inhibitor, in both primed (Fig. 2a) and unprimed (Fig. 2a; Supplemental Fig. 2) THP-1 cells. These results indicate that IL-1β maturation in response to MWCNTs, asbestos, or LPS was indeed NLRP3 inflammasome-driven.
Effects of caspase-1 inhibitor (a) or ROCK inhibitor (b) on MWCNT-, asbestos- or LPS-induced IL-1β secretion in THP-1 cells. Cells were cultured with 100 nM PMA for 24 h. For priming, the cells were incubated with 10 ng/ml LPS for the last 3 h of differentiation with PMA. a The cells were washed with HBSS and cultured with 10 μg/ml MWCNTs, 100 μg/ml asbestos, or 0.1 μg/ml LPS in the presence or absence of 10 μM Z-YVAD for 12 h. The concentration of IL-1β in each culture supernatant was measured by ELISA. Data are presented as mean ± SD (N = 4). *p < 0.05, compared to Z-YVAD-untreated group. b The cells were washed with HBSS and cultured with or without 2–10 μg/ml MWCNTs, 20–100 μg/ml asbestos, or 0.1 μg/ml LPS in the presence or absence of 10 μM Y27632 for 12 h. The concentration of IL-1β in each culture supernatant was measured by ELISA. Data are presented as mean ± SD (N = 4). # p < 0.05, compared to Y27632-untreated group; *p < 0.05, compared to the priming-alone control group
Effects of ROCKs inhibitor on IL-1β secretion induced by MWCNTs, asbestos, or LPS
To understand the induction of inflammasomes by MWCNTs, asbestos, and LPS in more detail, we focused on ROCKs which are known to be involved in a wide range of fundamental cellular functions, such as adhesion, regulation of cytoskeleton, and phagocytosis. We investigated the effect of Y27632, a selective ROCKs inhibitor, on IL-1β secretion to examine whether ROCKs are involved in IL-1β secretion induced by MWCNTs, asbestos, or LPS. Exposure to MWCNTs and asbestos increased IL-1β secretion in a dose-dependent manner (Fig. 2b). Treatment with Y27632 suppressed slightly but significantly the production of IL-1β evoked by fibrous particles in both primed (Fig. 2b) and unprimed (Supplemental Fig. 2) THP-1 cells, although it had less effect on IL-1β in response to LPS (Fig. 2b; Supplemental Fig. 2).
Effects of ROCKs inhibitor on IL-1β secretion induced by MSU crystals
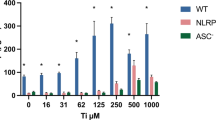
MSU crystals are recognized as endogenous DAMPs which activate NLRP3 inflammasomes. We examined the effects of ROCKs inhibitor on MSU-induced IL-1β secretion. As shown in Supplemental Fig. 3A, the original MSU crystals were disrupted by ultrasonication. Ultrasonication for longer time periods generated shorter MSU crystals of non-uniform length. A small amount of the MSU crystals dissolved rapidly in culture medium even in the absence of cells. The undissolved MSU looked like needle-like crystals in the culture medium without an apparent loss of mass at a concentration of 1 mg/ml. Exposure to each group of MSU crystals increased IL-1β secretion, and this IL-1β secretion was inhibited by Y27632 (Supplemental Fig. 3B). ROCKs inhibitor suppressed IL-1β secretion induced not only by MWCNTs and asbestos, but also by MSU.
Effects of knockdown of ROCK1 and ROCK2 on IL-1β secretion induced by MWCNTs, asbestos, and LPS
To further examine the effect of ROCK1 and ROCK2 on MWCNT- and asbestos-induced IL-1β secretion, differentiated THP-1 cells were transfected with siRNA to knockdown ROCKs. The expression was down-regulated by three independent antisense siRNAs for each of ROCK1 (S1–S3) or ROCK2 (S4–S6) compared to that of a non-targeting control siRNA (Supplemental Fig. 4B). siRNA designed for either ROCK1 or ROCK2 decreased MWCNT- and asbestos-induced IL-1β secretion significantly, but did not change LPS-induced IL-1β secretion (Fig. 3). These results demonstrate that both ROCKs are implicated in IL-1β secretion induced by fibrous particles, but that LPS is not implicated.
Effects of ROCK1 and ROCK2 siRNA on MWCNT-, asbestos- or LPS-induced IL-1β secretion in THP-1 cells. Cells were cultured in the presence of 100 nM PMA for 24 h. The differentiated cells were washed with HBSS and transfected for 48 h with antisense siRNAs of ROCK1 (S1 and 3) or of ROCK2 (S4 and 6), or with control siRNA. After transfection, the cells were further cultured in fresh RPMI1640 complete medium with 10 μg/ml MWCNTs, 100 μg/ml asbestos, or 0.1 μg/ml LPS for 12 h. The concentration of IL-1β in each culture supernatant was measured by ELISA. Data are presented as mean ± SD (N = 3). # p < 0.05, compared to each treatment group
Cellular association of MWCNT and asbestos
Phagocytosis is a key event in initiating macrophage-derived inflammatory responses. Therefore, the effects of ROCKs inhibitor on cell-associated MWCNTs or asbestos were assayed by turbidimetry. Exposure to MWCNTs or asbestos increased the cellular association of both fibrous particles in a time-dependent manner; interestingly, treatment with Y27632 had no effect on the amount of cellular-associated particles (Fig. 4). The measured amounts of fibrous particles include both cellular phagocytosed and membrane-bound particles, as it is difficult to distinguish between them using conventional methods.
Measurement of cell-associated MWCNTs (a) and asbestos (b). Cells were cultured with 100 nM PMA for 1 or 3 days. The differentiated THP-1 cells were washed with HBSS and further cultured with 10 μg/ml MWCNTs or 100 μg/ml asbestos in the presence or absence of 10 μM Y-27632 for 12 h. The cells were lysed with the lysis solution. A 250-μl sample was transferred to a 96-well culture dish, and the O.D. (640 nm) was measured to quantify the amount of cell-associated MWCNTs or asbestos. Data are presented as mean ± SD (N = 3)
Effect of ROCKs inhibitor on cell viability
Figure 5 demonstrates the effects of Y27632 on the cytotoxicity of MWCNTs and asbestos in differentiated THP-1 cells. Y27632 diminished both MWCNT- and asbestos-induced cytotoxicity. The concentration of MWCNTs (0–10 μg/ml) and asbestos (0–100 μg/ml) used for other experiments in this study did not affect the viability of THP-1 cells.
Cytotoxicity of MWCNTs (a) and asbestos (b). Cells were cultured in the presence of 100 nM PMA for 24 h. The cells were washed with HBSS and cultured with MWCNTs or asbestos in the presence or absence of 10 μM Y27632 for 16 h. Cell viability was evaluated using AlamarBlue®. Data are presented as mean ± SD (N = 4)
Effects of cytochalasin D on IL-1β secretion induced by MWCNTs, asbestos and LPS
Cytochalasin D is a disruptor of actin polymerization and is used commonly as an inhibitor of phagocytosis. We examined whether phagocytosis is associated with IL-1β secretion induced by MWCNTs, asbestos, and LPS. Treatment of cytochalasin D suppressed MWCNT- and asbestos-induced IL-1β secretion, but not LPS-induced IL-1β secretion (Fig. 6). Microscopic examination revealed that treatment with cytochalasin D caused cell morphological changes (data not shown).
Effects of cytochalasin D on MWCNT-, asbestos- or LPS-induced IL-1β secretion in THP-1 cells. Cells were cultured with 100 nM PMA for 24 h. For priming, the cells were incubated with 10 ng/ml LPS for the last 3 h of differentiation with PMA. The cells were washed with HBSS and cultured with 5, 10 μg/ml MWCNTs, 50, 100 μg/ml asbestos or 0.1 μg/ml LPS in the presence or absence of 1 μg/ml cytochalasin D for 12 h. The concentration of IL-1β in each culture supernatant was measured by ELISA. Data are presented as mean ± SD (N = 4). # p < 0.05, compared to the cytochalasin D-untreated group; *p < 0.05, compared to the priming-alone control group
Pro-IL-1β, caspase-1 and cathepsin B responses in THP-1 cells exposed to MWCNTs, asbestos, or LPS
To understand in more detail how ROCKs regulate fibrous particle-induced NLRP3 inflammasomes, we investigated the effects of Y27632 on the protein levels of pro-IL-1β, activated-caspase-1, and activated-cathepsin B in THP-1 cells exposed to MWCNTs, asbestos, or LPS. Figure 7 indicates that exposure to MWCNTs or asbestos increased pro-IL-1β, and that their effect was decreased to about 65 % by Y27632 as densitometric analyses of pro-IL-1β/tubulin, whereas Y27632 did not affect the LPS-induced increase in pro-IL-1β. These results are consistent with the results of mature IL-1β secretion using ELISA (Fig. 2b).
Western blot analyses for the detection of proIL-1β in THP-1 cells exposed to MWCNTs, asbestos, or LPS. Cells were cultured in the presence of 100 nM PMA for 24 h. For priming, the cells were incubated with 10 ng/ml LPS for the last 3 h of differentiation with PMA. The cells were washed with HBSS and cultured with 10 μg/ml MWCNTs, 100 μg/ml asbestos, or 0.1 μg/ml LPS for 5 h in the presence or absence of 10 μM Y27632. After washing twice with HBSS, cells were further cultured in fresh Opti-MEM® for 15 h in the presence or absence of 10 μM Y27632. The lysate and concentrated supernatants were electroblotted onto a PVDF membrane. The blot was probed with anti-proIL-1β, followed by a corresponding POD-tagged secondary antibody. The membranes were stripped and re-probed with POD-tagged anti-α-tubulin antibody. Data are representative of three experiments
Caspase-1 activation involves autocatalytic processing of the 45 kDa pro-caspase-1 into two subunits, p20 and p10 (Osuka et al. 2012). We next examined activated-caspase-1 at p20 in cell lysates and the release of activated-caspase-1 into cell culture supernatants. As shown in Fig. 8a, Western blot analyses of concentrated supernatants revealed that activated-caspase-1 was released from THP-1 cells exposed to MWCNTs and asbestos, but not from cells exposed to LPS. The release of activated-caspase-1 induced by MWCNTs and asbestos was reduced by treatment with Y27632.
Western blot analyses for the detection of p20 mature-form and 45 kDa pro-form caspase-1 in the culture supernatant and lysate of THP-1 cells exposed to MWCNTs, asbestos or LPS. Cells were cultured in the presence of 100 nM PMA for 24 h. For priming, the cells were incubated with 10 ng/ml LPS for the last 3 h of differentiation with PMA. The cells were washed with HBSS and cultured with 10 μg/ml MWCNTs, 100 μg/ml asbestos, or 0.1 μg/ml LPS (a) or 2–10 μg/ml MWCNTs (b) for 5 h in the presence or absence of 10 μM Y27632. After washing twice with HBSS, cells were further cultured in fresh Opti-MEM® for 15 h in the presence or absence of 10 μM Y27632. The lysate (cells) and concentrated supernatants (SN) were electroblotted onto a PVDF membrane. The blot was probed with anti-caspase-1, followed by a corresponding POD-tagged secondary antibody. The membrane electroblots of the cell lysates were stripped and re-probed with POD-tagged anti-α-tubulin antibody. Data are representative of three experiments
Western blot analyses of concentrated supernatants revealed that activated-cathepsin B with a molecular mass of 27 kDa was released from THP-1 cells after exposure to MWCNTs and asbestos, but not following exposure to LPS (Fig. 9a). Treatment with Y27632 reduced the release of activated-cathepsin B similar to caspase-1. Furthermore, we examined activated-caspase-1 and activated-cathepsin B release from cells exposed to MWCNTs at a concentration of 2–10 μg/ml (Figs. 8b, 9b, respectively). The release of both increased in a dose-dependent manner and were suppressed by Y27632.
Western blot analyses for the detection of cathepsin B in the culture supernatant of THP-1 cells exposed to MWCNTs, asbestos, or LPS. Cells were cultured in the presence of 100 nM PMA for 24 h. For priming, the cells were incubated with 10 ng/ml LPS for the last 3 h of differentiation with PMA. The cells were washed with HBSS and cultured with 10 μg/ml MWCNTs, 100 μg/ml asbestos, or 0.1 μg/ml LPS (a) or 2–10 μg/ml MWCNTs (b) for 5 h in the presence or absence of 10 μM Y27632. After washing twice with HBSS, the cells were further cultured in fresh Opti-MEM® for 15 h in the presence or absence of 10 μM Y27632. The cell culture supernatants were collected, and the concentrated supernatants were electroblotted onto a PVDF membrane. The blot was probed with anti-cathepsin B. Data are representative of three experiments
Discussion
Long fibers, such as asbestos and CNTs, are more potent activators of inflammation and genotoxicity than short or tangled fibers (Keka et al. 2014; Schinwald et al. 2012). It has been reported that long, needle-like CNTs and asbestos induce the secretion of pro-inflammatory cytokines, such as IL-1β and IL-18, through NLRP3 inflammasomes (Palomaki et al. 2011). The shape and size of particulate substances are two of the most important factors affecting their ability to cause NLRP3 inflammasome-mediated pro-inflammatory responses (Li et al. 2012; Palomaki et al. 2011; Sandberg et al. 2012). The NLRP3 inflammasome is implicated in particulate matter-related pulmonary diseases (Dostert et al. 2008). Several studies have shown the interaction between cytoskeletal proteins and NLRP3 inflammasomes in primary human monocytes (Meunier et al. 2012) or THP-1 cells (Dostert et al. 2008). ROCKs are known to be involved in a wide range of fundamental cellular functions, such as adhesion, regulation of cytoskeleton, and phagocytosis (Olazabal et al. 2002; Shi and Wei 2007; Kanno et al. 2013 #162). Therefore, we speculated that Rho/ROCKs are possibly involved in IL-1β secretion through NLRP3 inflammasomes.
We first examined whether ROCKs are associated with IL-1β secretion in THP-1 cells using a ROCKs inhibitor and siRNAs of ROCKs. Interestingly, the ROCKs inhibitor (Fig. 2b) and siRNA designed for both ROCK1 and ROCK2 (Fig. 3) markedly decreased MWCNT- and asbestos-induced IL-1β secretion, but did not affect LPS-induced IL-1β secretion. MSU-induced IL-1β secretion, which was suppressed by ROCKs inhibitor (Supplemental Fig. 3B). To confirm whether MWCNTs, asbestos, or LPS induce IL-1β secretion through the NLRP3 inflammasome, we examined the effect of Z-YVAD, a caspase-1 selective inhibitor, on IL-1β secretion. Caspase-1 is a key protease activated by the NLRP3 inflammasome complex and is involved in the maturation of IL-1β. MWCNT-, asbestos-, and LPS-induced IL-1β secretion was significantly abrogated by Z-YVAD (Fig. 2a). These results suggested that fibrous particles and LPS induced IL-1β secretion by the NLRP3 inflammasome, and that ROCKs are implicated in fibrous particle-, but not LPS-, induced IL-1β secretion.
We next investigated how ROCKs are involved in the NLRP3 inflammasome pathway. Activation of the NLRP3 inflammasome is subject to several events. Cellular phagocytosis of fibrous particles, such as asbestos, CNTs, or MSU, is required as an initial step for inflammasome activation (Meunier et al. 2012). Phagocytosis of long fibers most likely leads to lysosomal rupture and phagosomal destabilization, and the lysosomal damage results in the release of cathepsin B into the cytosol, which may promote NLRP3 inflammasome activation. It has been reported that long CNT- and asbestos-induced NLRP3 inflammasome activation depends on reactive oxygen species (ROS) production and cathepsin B activity (Palomaki et al. 2011), and that activated-cathepsin B is released from the cells via the P2X7 receptor (Niemi et al. 2011). The inflammasome assembly activates caspase-1, which cleaves pro-IL-1β and causes the secretion of mature IL-1β. Caspase-1 activation involves autocatalytic processing of the 45 kDa pro-caspase-1 into two subunits, p20 and p10 (Osuka et al. 2012). Here we show that THP-1 cells exposed to MWCNTs or asbestos result in increased pro-IL-1β protein levels in cell lysates (Fig. 7) and the release of both activated-caspase-1 (Fig. 8a) and activated-cathepsin B (Fig. 9a) into the culture medium; in contrast, LPS increased only pro-IL-1β (Fig. 7). Our results are consistent with previous reports that fibrous particles increased the protein levels of activated-caspase-1 and activated-cathepsin B in the culture medium (Dostert et al. 2008; Li et al. 2012; Meunier et al. 2012). In addition, ROCKs inhibitor suppressed the release of activated-caspase-1 (Fig. 8a, b) and activated-cathepsin B (Fig. 9a, b), and suppressed the pro-IL-1β protein level (Fig. 7) induced by MWCNTs and asbestos, whereas it did not affect the increase in pro-IL-1β protein level induced by LPS (Fig. 7). ROCKs inhibitor suppressed the protein levels of activated-cathepsin B and its downstream molecules, suggesting that ROCKs act at least partially upstream of lysosomal injury triggered by fibrous particles.
It has been reported that phagocytosis of fibrous particles is required as an initial step for inflammasome activation (Meunier et al. 2012). The actin cytoskeleton is necessary to phagocytose long or large materials or particles (>0.5 μm diameter) (Kanno et al. 2007; Misawa et al. 2013). Indeed, pretreatment with cytochalasin D, a disruptor of actin polymerization, suppressed IL-1β secretion in THP-1 cells exposed to MWCNTs or to asbestos, but not in cells exposed to LPS (Fig. 6). Previous reports have been demonstrated that pretreatment with an actin polymerization disruptor such as cytochalasin D or latrunculin A inhibited the stimulation of IL-1β secretion by asbestos (Dostert et al. 2008), cholesterol crystals (Rajamaki et al. 2010), MSU (Dostert et al. 2008; Yazdi et al. 2010), and DWCNTs (Meunier et al. 2012). Conversely, LPS is a potent bacterial effector triggering NLRP3 inflammasomes following binding with Toll-like receptor 4 (TLR4) (Freche et al. 2007; Mitchell et al. 2007). Cellular internalization of LPS is independent of actin cytoskeleton remodeling in mammalian systems (Soldatos et al. 2003). Consistent with these previous findings, our results indicate that cytochalasin D suppresses phagocytosis-dependent, but not phagocytosis-independent, NLRP3 inflammasome activation, and that phagocytosis is required for inflammasome activation by fibrous particles.
It is of interest to note that ROCKs inhibitor and siRNA against ROCKs suppressed phagocytosis-dependent, but not phagocytosis-independent, IL-1β secretion as well as cytochalasin D, suggesting the involvement of ROCKs in the signal cascade following phagocytosis of fibrous particles. However, the ROCKs inhibitor did not change the amount of cell-associated fibrous particles, as determined by turbidimetry analyses (Fig. 4). Intriguingly, ROCK1 expression increased following differentiation in THP-1 cells (Fig. 1), indicating that ROCKs are associated with the function of macrophages. Furthermore, viability assays demonstrated that ROCKs inhibitor diminished MWCNT- and asbestos-induced cytotoxicity (Fig. 5). Thus, it is plausible that the ROCKs inhibitor suppressed the internalization of fibrous particles by the cells without affecting the amount of plasma membrane-bounded particles. Taken together, ROCKs may be required for the full activation of inflammasomes induced by fibrous particles. Our microscopic examination revealed that the ROCKs inhibitor did not affect the morphology of THP-1 cells, in contrast with the effects of cytochalasin D, which inhibited the extension of the plasma membranes (data not shown).
Macrophages can endocytose small or short fibrous materials, whereas macrophages attempt to phagocytose long fibrous materials. Macrophages often cannot enclose these long fibers due to their great length and high potential for aggregation, leading to “frustrated phagocytosis”. “Frustrated phagocytosis” was observed with silver nanowires ≥14 μm in length using backscatter scanning electron microscopy in an in vitro study (Schinwald and Donaldson 2012). It is therefore conceivable that the fibrous particles used in the present study also trigger both phagocytosis and “frustrated phagocytosis”. It was recently demonstrated that the actin cytoskeleton is necessary for both phagocytosis and “frustrated phagocytosis” (Dostert et al. 2008; Misawa et al. 2013), and both phagocytosis and “frustrated phagocytosis” of fibrous particles, such as DWCNTs, alum, asbestos, and silica, are involved in the NLRP3 inflammasome (Dostert et al. 2008; Meunier et al. 2012).
Notably, ROCKs inhibitor also suppressed IL-1β secretion induced by MSU as well as by MWCNTs and asbestos. Gout is triggered when crystals of MSU, a crystallized form of uric acid, nucleate in joints, the kidneys, and other tissues (Rock et al. 2013). MSU activates NLRP3 inflammasomes (Martinon et al. 2006). Currently, patients suffering from acute attacks of gout and pseudogout are treated with anti-inflammatory agents or colchicine, which is an inhibitor of microtubule assembly (Rock et al. 2013). It has been demonstrated that colchicine blocks IL-1β maturation by MSU (Martinon et al. 2006). Though further study is required to elucidate the function of ROCKs on NLRP3 inflammasomes activated by fibrous particles, present findings suggest that ROCKs inhibitor might directly or indirectly abrogate NLRP3 inflammasomes, leading to several autoinflammatory diseases triggered by fibrous particles.
In conclusion, we have demonstrated that ROCK inhibitor and siRNA designed for both ROCK1 and ROCK2 decreased asbestos- and MWCNT-induced IL-1β secretion in differentiated THP-1 cells, but had no effect on LPS-induced IL-1β secretion. These findings suggest that ROCKs are involved in fibrous particle-induced inflammasome responses. The present study provides new findings about the effect of ROCKs on the phagocytosis of fibrous particles-dependent NLRP3 inflammasomes leading to various inflammatory diseases.
References
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3:339–345
Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T (2011) Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 89(716–724):724A–724C
Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320:674–677
Freche B, Reig N, van der Goot FG (2007) The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin Immunopathol 29:249–260
Hirano S, Kanno S, Furuyama A (2008) Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharmacol 232:244–251
Hirano S, Fujitani Y, Furuyama A, Kanno S (2010) Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol Appl Pharmacol 249:8–15
Hirano S, Fujitani Y, Furuyama A, Kanno S (2012) Macrophage receptor with collagenous structure (MARCO) is a dynamic adhesive molecule that enhances uptake of carbon nanotubes by CHO-K1 cells. Toxicol Appl Pharmacol 259:96–103
Inoue K, Yanagisawa R, Koike E, Nishikawa M, Takano H (2010) Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: possible role of oxidative stress. Free Radic Biol Med 48:924–934
Ishizaki T, Maekawa M, Fujisawa K et al (1996) The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 15:1885–1893
Kahlenberg JM, Dubyak GR (2004) Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol 286:C1100–C1108
Kanno S, Furuyama A, Hirano S (2007) A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol Sci 97:398–406
Kanno S, Hirano S, Sagi M et al (2013) Sulfide induces apoptosis and Rho kinase-dependent cell blebbing in Jurkat cells. Arch Toxicol 87:1245–1256
Keka IS, Evans TJ et al (2014) A novel genotoxicity assay of carbon nanotubes using functional macrophage receptor with collagenous structure (MARCO)-expressing chicken B lymphocytes. Arch Toxicol 88:145–160
Kohyama N, Shinohara Y, Suzuki Y (1996) Mineral phases and some reexamined characteristics of the international union against cancer standard asbestos samples. Am J Ind Med 30:515–528
Leung T, Manser E, Tan L, Lim L (1995) A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem 270:29051–29054
Li M, Gunter ME, Fukagawa NK (2012) Differential activation of the inflammasome in THP-1 cells exposed to chrysotile asbestos and Libby “six-mix” amphiboles and subsequent activation of BEAS-2B cells. Cytokine 60:718–730
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
McCarty DJ Jr, Faires JS (1963) A comparison of the duration of local anti-inflammatory effect of several adrenocorticosteroid esters—a bioassay technique. Curr Ther Res Clin Exp 5:284–290
Meunier E, Coste A, Olagnier D et al (2012) Double-walled carbon nanotubes trigger IL-1beta release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine 8:987–995
Misawa T, Takahama M, Kozaki T et al (2013) Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 14:454–460
Mitchell JA, Paul-Clark MJ, Clarke GW, McMaster SK, Cartwright N (2007) Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J Endocrinol 193:323–330
Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC (2011) Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev 14:76–121
Nagai H, Toyokuni S (2010) Biopersistent fiber-induced inflammation and carcinogenesis: lessons learned from asbestos toward safety of fibrous nanomaterials. Arch Biochem Biophys 502:1–7
Niemi K, Teirila L, Lappalainen J et al (2011) Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol 186:6119–6128
Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol 12:1413–1418
Osuka A, Hanschen M, Stoecklein V, Lederer JA (2012) A protective role for inflammasome activation following injury. Shock 37:47–55
Pacurari M, Castranova V, Vallyathan V (2010) Single- and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A 73:378–395
Palomaki J, Valimaki E, Sund J et al (2011) Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 5:6861–6870
Perregaux D, Gabel CA (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269:15195–15203
Qu Y, Franchi L, Nunez G, Dubyak GR (2007) Nonclassical IL-1β secretion stimulated by P2×7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179:1913–1925
Rajamaki K, Lappalainen J, Oorni K et al (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5:e11765
Rock KL, Kataoka H, Lai JJ (2013) Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol 9:13–23
Sakamaki I, Inai K, Tsutani Y, Ueda T, Tsutani H (2008) Binding of monosodium urate crystals with idiotype protein efficiently promote dendritic cells to induce cytotoxic T cells. Cancer Sci 99:2268–2273
Sandberg WJ, Lag M, Holme JA et al (2012) Comparison of non-crystalline silica nanoparticles in IL-1beta release from macrophages. Part Fibre Toxicol 9:32
Schinwald A, Donaldson K (2012) Use of back-scatter electron signals to visualise cell/nanowires interactions in vitro and in vivo; frustrated phagocytosis of long fibres in macrophages and compartmentalisation in mesothelial cells in vivo. Part Fibre Toxicol 9:34
Schinwald A, Murphy FA, Prina-Mello A et al (2012) The threshold length for fiber-induced acute pleural inflammation: shedding light on the early events in asbestos-induced mesothelioma. Toxicol Sci 128:461–470
Shi J, Wei L (2007) Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 55:61–75
Shukla A, MacPherson MB, Hillegass J et al (2009) Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. Am J Respir Cell Mol Biol 41:114–123
Shvedova AA, Kisin E, Murray AR et al (2008) Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol 295:L552–L565
Soldatos AN, Metheniti A, Mamali I, Lambropoulou M, Marmaras VJ (2003) Distinct LPS-induced signals regulate LPS uptake and morphological changes in medfly hemocytes. Insect Biochem Mol Biol 33:1075–1084
Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ (2010) Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS One 5:e8774
Tsuchiya S, Kobayashi Y, Goto Y et al (1982) Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res 42:1530–1536
Yazdi AS, Guarda G, Riteau N et al (2010) Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc Natl Acad Sci USA 107:19449–19454
Yoneda A, Multhaupt HA, Couchman JR (2005) The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 170:443–453
Zalma R, Bonneau L, Guignard J, Pezerat H (1987) Formation of oxy radicals by oxygen reduction arising from the surface activity of asbestos. Can J Chem 65:2338–2341
Acknowledgments
We would like to thank Mr. T. Ikawa for the technical support. This work was supported by JSPS KAKENHI (Grant No. 24590868).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanno, S., Hirano, S., Chiba, S. et al. The role of Rho-kinases in IL-1β release through phagocytosis of fibrous particles in human monocytes. Arch Toxicol 89, 73–85 (2015). https://doi.org/10.1007/s00204-014-1238-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1238-2