Abstract
The Borderlakes are a chain of ten shallow, largely artificial, interconnected lakes in the Netherlands. The ecological recovery of the central Borderlakes (viz. lake Veluwe and Wolderwijd) has been well documented. These lakes shifted from a eutrophic, Planktothrix dominated state in the 1970s to a clear state in 1996. Around 2010, the formerly hypertrophic, southern Borderlake Eem also reached a clear state, but at considerably higher nutrient levels. In this paper, monitoring data are used to compare these changes and identify the differences in driving processes and their consequences. The 1996 shift in Lake Veluwe was linked to increased fishery for benthivorous Bream, followed and stabilized by increase in Zebra Mussels and charophytes. Nutrients in Lake Eem decreased as well and Planktothrix disappeared here too in 1996, despite relatively high TP concentrations which remained stable over time. The start of the change into the clear state in this case also involved a decrease in the Bream population, but with a stronger additional role for dreissenids, particularly of Quagga Mussels. Remaining blooms of cyanobacteria almost disappeared, but the current situation in Lake Eem represents a different type of clear water state than in the central Borderlakes. This type is characterized by the combination of a relatively high phosphorus load, intense dreissenid filtration and filamentous macro-algae instead of either blooms of cyanobacteria or dominance of charophytes. With the dominant role of the River Eem, the relatively short residence time and increasing difficulty to bring down nutrient loading any further, the stability of this clear state depends on high densities (and filtration rates) of dreissenids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many lakes in Europe have suffered from eutrophication, causing shifts to a turbid state and a loss of biodiversity. Since then, many of these lakes have (partly) recovered as result of reduction in nutrient input, often in combination with biomanipulation (e.g. Hosper 1997; Ibelings et al. 2007; Jeppesen et al. 1990, 2007; Meijer 2000; Moss 1990). In a number of cases, such measures resulted in abrupt shifts back to a clear state in agreement with the theory of alternative equilibria (Scheffer 1998; Scheffer et al. 1993, 2001). Abrupt changes do, however, not prove the existence of alternative equilibria as they can also be caused by abrupt changes in an external driver or by nonlinear, non-catastrophic behavior.
The Borderlakes also have a history of eutrophication. Until the end of the 1960s, these lakes were dominated by charophytes, Potamogeton species and Zebra Mussels Dreissena polymorpha. Water quality deteriorated, however, and around 1970 eutrophication resulted in a shift to a turbid state with intense blooms of Planktothrix aghardii and a loss of macrophytes, Zebra Mussels and overall diversity. Several measures in the central Borderlakes (Lakes Veluwe and Wolderwijd) resulted in a decrease in nutrients and chlorophyll concentrations since 1979 (Hosper 1984; Hosper and Meijer 1986) and ultimately in overall ecological recovery (Hosper 1997; Meijer and Hosper 1996; Ibelings et al. 2007). Part of this recovery took place rather abruptly around 1996, when the central Borderlakes shifted back to the clear state (Meijer 2000; Scheffer et al. 2001; Ibelings et al. 2007). For Lake Veluwe, the 1996 shift was connected to increased commercial fishery for Bream Abramis brama (Lammens et al. 2004), working as unintended biomanipulation. The result was rather unexpected, because deliberate, large-scale Bream removal from Lake Wolderwijd in the winter of 1990/91 (Meijer and Hosper 1995, 1996) did only result in a temporary increase in water transparency. An attempt to reintroduce Zebra Mussels in Lake Veluwe in 1990, separate from the Bream removal in adjacent Lake Wolderwijd, was not successful. With the decrease in Bream in 1996, however, Zebra Mussels returned, and increased filtration capacity was suggested as an additional cause of the 1996 shift (Lammens et al. 2004).
More recently, transparency has also increased in the northern and southern Borderlakes. These improvements were also unexpected, because nutrient levels in those lakes are still relatively high and elevated dissolved fractions of phosphorus (PO4-P) and nitrogen (NO2/NO3-N + NH4-N) suggest phytoplankton growth is not limited by nutrients. It therefore appears that other factors, besides nutrient reduction, played an important role in causing the increase in transparency.
Developments of water quality in the central Borderlakes, particularly Lake Veluwe, have been analyzed and published before (e.g. Ibelings et al. 2007), but the recent developments in Lake Eem, as a representative of the Borderlakes with more direct riverine influence and relatively high loading, have not been reported yet. In this paper, the dataset is used to present these recent developments and to compare them to earlier developments in the central Borderlakes, highlighting differences between these lakes and re-evaluating the driving processes for the observed changes.
The Borderlakes
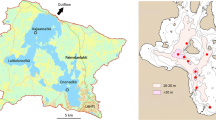
The Borderlakes are a series of about ten shallow, artificial lakes in the central part of the Netherlands (Fig. 1). These lakes remained after completion of the dike “Afsluitdijk” that separated the former Zuiderzee (Southern Sea) from the Wadden Sea in 1932. The newly formed lake turned fresh and was partly reclaimed. Most of the Borderlakes were formed in the 1950s and 1960s when land reclamation of the two Flevopolders left several smaller lakes at the southeast. They range in size from 600 to 3500 ha and in average depth from 1 to 3 m. They share their origin with two much larger and deeper lakes, Lake IJssel (114,000 ha, mean depth 4.6 m) and Lake Marken (70,000 ha, mean depth 3.8 m, including the southern part named “IJmeer”).
In this paper, two sets of Borderlakes are compared:
-
Central Borderlakes, particularly Lake Veluwe (3130 ha, mean depth 1.8 m) and Lake Wolderwijd (2540 ha, mean depth 2.1 m, including the narrow part named “Nuldernauw”, see Fig. 1). These lakes are relatively isolated and are fed by small, local brooks and by wastewater of the city of Harderwijk (currently some 45,000 inhabitants). The lakes used to have very long residence times (1.5 years) and water quality deteriorated in the 1960s as a result of local input of nutrients and organic waste, leading to a collapse of the ecosystems around 1970. Starting 1979, two measures were taken. Phosphorus removal in the local treatment plants reduced the external loading of phosphorus and flushing Lake Veluwe with nutrient-poor, but Ca2+- and HCO3 −-rich, water from the reclaimed polders (reducing residence time to a few months) resulted in dilution of nutrient concentrations as well as lowering of pH and reduction in internal loading from the sediment (Hosper 1984, 1997; Hosper and Meijer 1986). In Lake Wolderwijd nutrient concentrations decreased in 1982, when part of the flushwater from the polders was used to flush this lake as well. However, water transparency remained low and macrophytes failed to return at first. In 2002, the sluice between Lake Veluwe and Lake Wolderwijd was removed and they are now in open connection. Two attempts of biomanipulation carried out in 1990 were intended to stimulate recovery:
-
a.
Large-scale removal of Bream from Lake Wolderwijd in the winter of 1990/91 and additional fishery in the two succeeding winters, in order to reduce resuspension (Meijer and Hosper 1995, 1996).
-
b.
Reintroduction of Zebra Mussels in Lake Veluwe in 1990 to help increase transparency by filtration (Noordhuis 2010).
-
a.
-
Lakes with more direct riverine influence, for example Lake Eem (1520 ha, mean depth 2.1 m, including the narrow part named “Nijkerkernauw”, see Fig. 1), one of the southern Borderlakes. It mainly receives water from the River Eem (4–18 m3/s, two-thirds of total water load) and residence time is relatively short (1 month). The lake suffered from hypertrophic conditions with phosphorus concentrations twice as high as in Lake Veluwe during the 1970s, due to high concentrations in the river Eem. The high nutrient load of this river was harder to tackle than for the central Borderlakes because instead of loading from one dominant source, loading of Lake Eem was much more diffuse and many treatment plants in the river basin had to be adapted. No biomanipulation measures were carried out in the southern Borderlakes, but similar to the central Borderlakes, there has been substantial commercial fishery for Bream (and Roach Rutilus rutilus) each winter, at least since the early 1990s.
Connected to Lake Eem is Lake Gooi (2570 ha, 4.5 m), the other southern Borderlake. Developments in this lake were similar to those in Lake Eem, but nutrient concentrations have always been somewhat lower. For comparison, some information on Lake Gooi and on the northern Borderlakes, Zwarte Meer (1770 ha, 1.8 m) and Lake Ketel (3230 ha, 3.6 m) will be presented in this paper. The northern Borderlakes are also directly influenced by river water. They are fed by branches of the River IJssel and have even shorter residence times than Lake Eem (10 days–1 month and 3 days, respectively). In these lakes, water quality has improved due to international measures mainly.
Methods
This paper describes and compares changes in two different types of Borderlakes, based on data from monitoring of water quality and ecology, carried out by the Dutch Ministry of Infrastructure and Environment. Four sets of monitoring data are used:
-
Water quality data: recorded once a month at 1 m depth at one location per lake: variables used in this paper are total and dissolved phosphorus and nitrogen, transparency (Secchi depth), chlorophyll-a and species composition of phytoplankton. In this paper, summer average values (April–September) are used, unless stated otherwise.
-
Surveys of Zebra Mussels and Quagga Mussels Dreissena rostriformis bugensis were carried out once every 2 years between 1996 and 2008 and in 2013. Surveys were based on samples from 20 + 6 (Lake Eem + Nijkerkernauw), 22 + 10 (Wolderwijd + Nuldernauw) and 28 locations (Lake Veluwe) across the lakes. At shallow locations, five subsamples were taken with a 14.1 cm diameter core sampler. At deeper locations, three subsamples were taken with a 29.6 × 19.1 cm box corer or, from 2006 onwards, by a diver using a metal frame/shovel with the same dimensions. Mussels were frozen and biovolume, biomass and length distribution were determined in the laboratory. Filtration capacity was calculated based on length distribution (FC = 15.43/(0.293 + 52.38*e−0.367*L, FC = filtration capacity in ml per mussel per hour, L = shell length in mm) and corrected for summer average suspended matter concentration in the lake (FC = 187.1*e−0.037*TSM, TSM = total suspended matter in mg/l; Reeders et al. 1993; Noordhuis et al. 1994). Filtration capacity of Quagga Mussels was estimated with the above-mentioned formulas for Zebra Mussels, as no specific information was available for Quagga Mussels.
-
Surveys of fish were carried out every year on behalf of the Ministry of Agriculture, Directory Fisheries from 1966 until 1987, on behalf of the Ministry of Infrastructure and Environment every 3 years since 2003 and project-based in the central Borderlakes in most years in between as well.
-
Surveys of macrophytes were carried out since 1987 every three or 4 years, in the central Borderlakes every year until 2001, then every 3 years. These surveys are based on a grid of a large number of locations covering each lake, at a distance of 100 m, in later years 200 m apart. At each location, the vegetation is sampled with a double-sided rake on a rope, which is thrown overboard and dragged across the bottom for 5 m. Abundance is estimated per species in eight classes representing ranges of coverage percentage. For this paper, surveys from 1993 to 2011 were used, from which only locations were selected that were sampled during all surveys and that were <3 m deep (to exclude the shipping channels). From the selected data, the percentage of lake area (area <3 m deep) that was covered by a particular macrophyte species was determined by calculating the percentage of survey locations at which the species was present per lake, per survey year. This was done for all submerged macrophyte species, resulting in a dataset containing the percentage of area covered by each macrophyte species per lake, per survey year (in total 129 unique combinations of lake and survey year and 65 macrophyte species). A Principal Component Analysis (PCA), centered by species, was carried out using Canoco 5.
All institutional and national guidelines for the care and use of laboratory animals were followed.
Results
An overview of changes in relevant variables and a comparison between those in Lake Veluwe, Lake Wolderwijd and Lake Eem is given in Fig. 2. The box summarizes the five main moments of intervention or change, also indicated as vertical lines in Fig. 2. All of these events were preceded by a severe winter, which is indicated in Fig. 3. The least of these severe winters preceded (by coincidence) the reintroduction attempt of Zebra Mussels to Lake Veluwe and the Bream removal from Lake Wolderwijd: two measures with limited short-term success.
Comparison of developments in water quality and ecology in Lake Veluwe (open circles, water quality data 1970–2013), Lake Wolderwijd (open triangles, dashed lines, 1970–2010) and Lake Eem (closed circles, 1970–2014). Vertical lines represent the timing if important events (see text box): the start of measures in Lake Veluwe (1979/80), the first of the three severe winters of the mid-1980s (1984/85; dashed), biomanipulation measures 1990/91 (dashed), severe winter 1995/96 and relatively severe winter 2009/10. Bluegreens volume: no data on Lake Wolderwijd, x = Lake Veluwe, Planktothrix only. Macrophyte coverage: lines without symbols represent charophyte coverage only
Frost index per winter at De Bilt (central part of the Netherlands) with indications of the most important measures and shifts in the Borderlakes (dark colored bars) and the average NAO index for December–March. NB. The frost index is a commonly used Dutch index characterizing the severity of the winter at De Bilt, calculated from the number of frost days (min T below 0 °C), ice-days (min and max below 0 °C), and very cold days (min below −10 °C) per winter (IJnsen 1981). The NAO (North Atlantic Oscillation) index describes the difference in air pressure between the Icelandic low and the Azores high (data shown is from NOAA: National Oceanic and Atmospheric Administration, US). High values mean strong SW winds in the Netherlands, bringing warm Atlantic air in winter
Box: Overview of main changes
Following the decrease in nutrient input, the developments in ecology of the lakes are defined by five particular events with combinations of changes: |
1979: Start of measures in Lake Veluwe: TP drops, but little change in ecosystem behavior except for a chlorophyll decrease. Planktothrix remains present in high abundance, however, and large Bream keeps dominating the fish community until 1985 |
1985: First of three severe winters in succession: dominance of large Bream in Lake Veluwe/Wolderwijd finishes, crash of Planktothrix. No mussels yet, first signs of recovery of macrophytes. Some recovery of both Bream and Planktothrix in the following 10 years. TP in Lake Eem starts decreasing, but no response of chlorophyll until around 1994 |
1990/91: Biomanipulation measures in Lakes Veluwe and Wolderwijd: at least short-term effects on water quality. Start of return of charophytes in the shallowest parts of these lakes. Phosphorus in Lake Eem drops to the level in Lake Veluwe in the 1970s |
1996: Severe and extremely dry winter, in Lakes Veluwe and Wolderwijd increased commercial Bream fishery: Planktothrix becomes extinct, and Zebra Mussels return. Nutrients decrease to levels where macrophyte dominance becomes more likely, followed next year by strong increase in macrophytes (after five years of slow increase). Phosphorus concentrations in Lake Eem drop abruptly, the population of large Bream decreases, Planktothrix gets extinct and a Clear Water Phase develops in May |
2010: Bream biomass decreases in Lake Eem and the fish community shifts to a limnophylic community. Increase in mussel filtration in Lake Eem accelerates due to invasion of Quagga Mussels, coinciding with a strong increase in transparency and macrophyte abundance. Blooms of bluegreen algae decrease |
Lake Veluwe and Lake Wolderwijd
The Bream removal was followed by a short period of increased transparency, but in general, success seemed limited for the first few years. However, macrophytes did slowly increase, and the timing of the return of charophytes in Lake Wolderwijd may well have been set by the Bream removal (Hosper 1997; Meijer 2000; Meijer and Hosper 1996). In 1996, the lakes abruptly shifted to the clear state (Meijer 2000; Scheffer et al. 2001; Ibelings et al. 2007). For Lake Veluwe, this has been connected to increased commercial fishery for Bream (Lammens et al. 2004).
The recovery of Lake Veluwe started with phosphorus removal in the local treatment plant from early 1979 and flushing, starting November 1979. Due to the flushing, Ca2+ and HCO3 − concentrations increased from average values of 45 and 86 mg/l, respectively (1971–1972) to 96 and 142 mg/l (1982–1988). TP and TN concentrations in the lake dropped abruptly and permanently during the winter of 1979/1980 (Fig. 2). In Lake Wolderwijd TP and TN dropped in 1982, after a substantial part of the water from the polders was used to flush this lake as well. During the 1980s, average TP concentrations decreased from 0.2–0.3 mg/l in Lake Wolderwijd and 0.4–0.6 mg/l in Lake Veluwe to around 0.1 mg/l in both lakes. TN decreased from 3 (Lake Wolderwijd) and 5 mg/l (Lake Veluwe) to around 2 mg/l in both lakes. Especially in Lake Veluwe, the dissolved fraction of phosphorus also decreased in 1980, as well as chlorophyll concentrations. Chlorophyll concentrations dropped almost as much as TP. Abundance of Planktothrix aghardii decreased only slightly at first, but showed a strong decrease a few years later in 1985. Bream abundance had decreased substantially (without increased human interference) during the severe winter of 1984/85. Transparency only showed limited increase until 1996. In the second half of the 1980s and the first half of the 1990s, there was little further change in water quality and Bream and Planktothrix even slightly increased again, but from 1990 onwards macrophyte abundance (particularly charophytes) did slowly increase. After the winter of 1995/96, which was as severe as 1985/86, chlorophyll concentrations abruptly decreased, and summer average in both lakes permanently decreased below 15 μg/l. TP also decreased again, but not as strongly as chlorophyll concentrations. Planktothrix virtually disappeared and has not been recorded at all after 2000. Transparency went up abruptly from summer average values of 0.4 m until 1995 to 0.8–1.0 m afterward. This change coincided with a strong decrease in Bream biomass from 96 kg/ha >25 cm (benthivorous Bream) in 1993 to 25 kg/ha in 1996 and 18 kg/ha in 1997. During this decrease, Zebra Mussels returned to the lake bottom. While Zebra Mussels introduced in 1990 did not survive or reproduce, densities increased without further human intervention from absence in 1994 to 231 mussels per m2 in 1996, and av. 297/m2 in 1998, 2000, 2002 and 2004. Calculated filtration capacity was 10 l/h/m2 on average, corresponding to 19 % of lake volume per day (1998–2004). The increase in 1996 was followed by a strong increase in macrophyte abundance a year later (44 % of lake bottom covered in 1996, 45 % in 1997, but with 43 and 82 % coverage within that area, respectively). Charophyte abundance had been increasing gradually, but doubled from 1996 to 1997.
In Lake Wolderwijd, there was an increase in transparency following the fish removal of winter 1990/91 (summer average increased from 0.31 m in 1990 to 0.57 m in 1991, maximum of 1.3 m in June). However, summer average transparencies were lower again in the following years, in spite of additional fish removal in the winters of 1991/92 and 1992/93, due to which Bream biomass remained low (19–26 kg/ha >25 cm in 1991–1994). The first chlorophyll level measured after Bream removal was also unusually low, but afterward concentrations returned to the level of the years before removal and no difference with Lake Veluwe (no fish removal) was recorded anymore. On the other hand, charophytes, never before recorded in this lake, were first found in 1991, after the initial Bream removal. This marked the beginning of a large-scale colonization. Like in Lake Veluwe, there was an abrupt change in water quality from 1995 to 1996, marked by a strong, abrupt increase in transparency and a decrease in TP and chlorophyll. Bream biomass was even lower than during the first years after removal (15 kg/ha >25 cm in 1996, 12 kg/ha in 1997). Zebra Mussels were present on the lake bottom in 1996, which may represent a return, but there is no information on the previous years. Like in Lake Veluwe, charophyte abundance doubled the next year, the percentage of the lake bottom covered by macrophytes increased from 47 % in 1996 to 70 % in 1997 (Fig. 2; internal coverage shown, which is the product of area and density).
Lake Eem
In Lake Eem, TP concentrations were twice as high as in Lake Veluwe by the end of the 1970s (Fig. 2). TN, on the other hand, was similar, and chlorophyll concentrations were slightly lower. Phosphorus started to decrease in 1985, but the decrease was more gradual than in Lake Veluwe. By 1991, TP concentrations had been reduced by more than half and were about as high as the concentrations in Lake Veluwe in the 1970s (TP around 0.5 mg/l, dissolved P 0.2 mg/l). At the time of the 1996 shift in Lake Veluwe and Lake Wolderwijd, TP and dissolved phosphorus in Lake Eem dropped again abruptly by 30 and 50 %, respectively (to 0.35 and 0.1 mg/l, respectively). This drop was at least partly caused by improvements at one of the 10 treatment plants in 1995 and closure of another (TP concentrations in the River Eem decreased in 1995), as well as by low discharge of the river and decrease in the input from Lake Wolderwijd in 1996. Nevertheless, the concentrations of phosphorus remained considerably higher than in the other two lakes, but had still dropped well below the concentrations of Lake Veluwe in the 1970s. Phytoplankton composition changed drastically. Like in the other lakes, Planktothrix disappeared (timing unclear, no records between 1993 and 1996). Chlorophyll concentrations decreased gradually and from 1995 on a Clear Water Phase occurred every year in May.
Similar to Lake Veluwe and Lake Wolderwijd, Bream abundance may have decreased in this period as large Bream was almost absent in the 1995/96 survey (only 3 % of the fish were larger than 25 cm in Lake Eem, 2.5 % in Lake Veluwe) as compared to 1993/94 (34.5 and 28.4 %, respectively). Estimated Bream biomass was relatively high in the southern Borderlakes (including lake Eem) in 2002 (39–55 kg/ha >25 cm; Fig. 4), 2005 (30–57 kg/ha) and 2009 (40–103 kg/ha). Biomass in 1996 in comparison is difficult to determine, due to differences in fishing methods, but was probably considerably lower, and may have been as low as in the central Borderlakes. Zebra Mussel densities have not been recorded until 1998. Densities were relatively low at that time, but strongly increased during the following years, in spite of relatively high Bream biomass (Fig. 2). In 2004, the total filtration capacity of mussels per day was equal to the water volume of Lake Eem. Numbers of benthivorous Tufted Ducks Aythya fuligula, which may be used as an indicator of dreissenid population changes, had slowly been increasing since 1990 and suddenly doubled or tripled in all three lakes from winter 1995/96 to 1996/97. Macrophyte abundance in Lake Eem was and remained low at that time.
Recent changes
Bream biomass in the southern Borderlakes (including Lake Eem) was high in 2009, with biomass of fish >25 cm estimated at 40 kg/h in Lake Eem, but 57 kg/ha in Lake Gooi and as much as 103 kg/ha in Lake Nijkerkernauw (Fig. 4), which is not separated in any way from Lake Eem. Around this year, there were conspicuous dips in transparency and mussel filtration capacity, (less conspicuous) peaks in chlorophyll and nutrients (Fig. 2) and blooms of Anabaena and Microcystis in late summer (Fig. 5). When the next fish survey took place in 2012, Bream biomass had decreased toward the level of the other lakes (39, 23 and 5 kg/ha >25 cm in Lake Nijkerkernauw, Eem and Gooi, respectively). The rest of the fish community had also changed: Ruffe Gymnocephalus cernuus, which used to be dominant in numbers, had been replaced by Roach and abundance of three-spined stickleback Gasterosteus aculeatus had increased as well (Fig. 4).
Around 2010, the ecosystem of Lake Eem showed changes reminiscent of those in Lake Veluwe and Lake Wolderwijd in 1996, though less abrupt. After 2000, Zebra Mussels increased faster in Lake Eem than in Lake Veluwe and Wolderwijd. Quagga Mussels were already present in low densities in most of the Borderlakes during the survey of 2008. In 2013, their densities in Lake Eem had increased to an average of 2274/m2, in addition to 284 Zebra Mussels per m2, a decrease from 1121, 1058 and 1225/m2 in 2004, 2006 and 2008, respectively. Average dreissenid biovolume per m2 in 2013 was twice as high as the maximum value previously recorded (2006). In Lake Veluwe and Lake Wolderwijd, total densities were higher (3426 and 7185/m2), but mussels were much smaller here (Zebra Mussel average shell length 7, 6 and 12 mm in Lake Veluwe, Wolderwijd and Eem, respectively, Quagga Mussel 8, 7 and 18 mm on average in 2013), causing the increase in filtration capacity to be limited. Large average size in Lake Eem in 2013 is partly caused by low recruitment, only 10 % of Quagga Mussels and 19 % of Zebra Mussels were smaller than 10 mm (N = 244 and 177, respectively). Data on densities is not available for 2014, but a length-frequency sample showed underrepresentation of yearclass from 2013 and heavy recruitment in both species, with 91 % smaller than 10 mm in Quagga Mussels and 77 % in Zebra Mussels (N = 530 and 184, respectively). In Lake Eem, filtration capacity in 2013 was equal to the lake’s volume being filtered five times a day. Since 2012, average summer transparency in Lake Eem is around a meter, higher than in the central Borderlakes. Nutrient concentrations were still relatively high, however, particularly the dissolved fractions (Fig. 2). Chlorophyll was already low since 2004 but decreased even more in 2010, following the first relatively severe winter since 1995/96. Average summer chlorophyll values dropped under 15 μg/l, resembling the values that have been recorded in the central Borderlakes since 1996. Blooms and local scums of bluegreen algae have been reported in Lake Eem every year until a few years ago, but the responsible species, Aphanizomenon, Anabaena and Microcystis have hardly been present in phytoplankton samples since 2010 (Fig. 5). A short August/September phytoplankton peak is still present, but now species like Aphanothece, Aphanocapsa and other Chroococcales dominate. In 2014, however, Microcystis reappeared in the August bloom along with Aphanothece (Fig. 5), albeit with little change in chlorophyll concentration and transparency (Fig. 2).
In Lake Eem, macrophyte abundance has been increasing fast, particularly from 2009 to 2010. Several species of Potamogeton (P. crispus, P. pectinatus) as well as Zannichellia palustris have become abundant. Charophytes have been present for a number of years now, but their share in total cover remains low (<0.1 % of total cover in 2012). Other macro-algae, on the other hand, filamentous species (not identified in recent years, but mainly Cladophora spp in the 1990s; Koeman 1997), but also Enteromorpha spp. and Hydrodictyon reticulatum, have recently been very abundant in Lake Eem. They are largely responsible for the recent increase in macrophytes here (45 % of total cover in 2012). The abundant growth of such algae characterizes macrophyte development in Lake Eem and other relatively nutrient rich lakes in the area. In the mid-1990s, these algae were relatively abundant in the central Borderlakes as well, but only for a limited number of years as a stage in succession, and maximum abundance was much lower than in Lake Eem in recent years. Succession in those lakes shows a short route toward a charophyte dominated community (Fig. 6).
Development of the submerged vegetation, based on the first two PCA components. Lines connect successive surveys, usually carried out once every 3 years (1992–2011). The first symbol of each series, representing data from the early 1990s, is located on the left side of the y-axis. Top: central Borderlakes. Center: southern and northern Borderlakes. Bottom: general species plot (dashed lines are macro-algae, P. = Potamogeton)
PCA analysis of macrophyte data
Differences in development of the macrophyte community between Lake Eem and the central Borderlakes are clearly shown by the PCA analysis of the macrophyte data. A plot of the first two principal components, containing 65 % of the variation, shows how succession of macrophytes followed a different path in the central Borderlakes, with relatively low phosphorus levels (Fig. 6a), as compared to southern and northern Borderlakes, with high phosphorus levels (Fig. 6b). In these graphs, lines connect successive surveys in these lakes. Other Borderlakes as well as several parts of Lake IJssel and Lake Marken are included for comparison (unconnected dots). Lakes with relatively low phosphorus levels developed from turbid waters with practically no macrophytes on the left of the graph, to a community with high abundance of charophytes on the lower right of the graph (Fig. 6a). In lakes with high phosphorus levels, on the other hand, a community characterized by filamentous macro-algae developed, as these lakes moved toward the upper right of the graph (Fig. 6b). Apart from Lake Eem, three other lakes with relatively high (non-limiting) phosphorus concentrations are shown. Lake Gooi is connected to Lake Eem and has intermediate concentrations (dissolved summer average 0.055 mg/l, Eemmeer 0.096 mg/l). Lake Ketel is one of the northern Borderlakes, directly fed by the River IJssel and with a residence time of only 3 days. Dissolved phosphorus summer average since 2004 is 0.066 mg/l. Lake Zwarte Meer is fed by one of the branches of the River IJssel and has an average summer concentration of 0.026 mg/l.
Differences between Lake Veluwe/Wolderwijd and Lake Eem
After comparing developments between the central and southern Borderlakes three main differences come to light:
-
The southern Borderlakes (Lake Eem) reached a clear state at considerably higher phosphorus levels (TP and dissolved P) than the central Borderlakes did. The process was less abrupt, but the timing was similar in the sense that both in the central Borderlakes and in Lake Eem, water quality and ecology has improved in two major steps with around 15 years in between. The first step, sufficient decrease in nutrients for a phytoplankton response (here with dissolved phosphorus at 0.1 mg/l or less) in Lake Eem coincided with the second step (final shift to the clear state) in the central Borderlakes in 1996. The recent changes in Lake Eem resulted in transparency and chlorophyll values similar to those in the other two lakes after the 1996 shift. However, the TN/TP ratio has always been much lower in Lake Eem, and there was less delay between the moment when TP decreased below 0.2 mg/l and the change to the clear state (Fig. 2). So far, both TP and TN have shown little change since the clear state has been reached.
-
Dreissenid densities and filtration capacity are much higher in Lake Eem. This is not only a result of greater impact of the invasion by Quagga Mussels, but started with a stronger increase in Zebra Mussel abundance since 2000.
-
Macrophyte species composition developed differently in Lake Eem. The macrophyte communities of the central Borderlakes and of Lake Eem show several differences. Most conspicuous is the low abundance of charophytes and high abundance of other macro-algae in Lake Eem. In Lake Veluwe and Wolderwijd, the macrophyte community has become heavily dominated by charophytes (over 80 % of total cover since 1996). Filamentous algae have never been so abundant here. Even at their maximum abundance in 1998 and 1999, filamentous algae only covered around 10 % of Lake Veluwe.
Discussion
This study compares two (interconnected) sets of lakes with similar changes into a clear water state, where in both cases decrease in Bream as well as increase in dreissenids was involved. Details of water quality before the shift or change as well as of ecological response, however, were different. To understand processes that have taken place in the central Borderlakes, additional modeling has been used in other studies (Ibelings et al. 2007; Lammens et al. 2004). As an alternative, the developments in connected lakes with similar dimensions and history but partly different characteristics like in loading and residence time are compared in this manuscript.
The 1996 shift in the central Borderlakes and earlier biomanipulation attempts
In 1996, the central Borderlakes abruptly shifted to the clear state (Meijer 2000; Scheffer et al. 2001; Ibelings et al. 2007). The lakes showed hysteresis in their response to the decrease in phosphorus concentrations; the macrophyte dominated state disappeared at TP concentrations of over 0.2 mg/l, and returned at 0.1 mg/l (Meijer 2000; Scheffer et al. 2001; Ibelings et al. 2007). The shift took place 16 years after the first substantial decrease in nutrient concentrations. Earlier, the three severe winters of 1985–87 resulted in strong decrease in Bream, but not in the return of dreissenids. Five years later, biomanipulation attempts had only limited success. However, macrophytes did slowly increase, and the timing of the return of charophytes in Lake Wolderwijd may well have been set by the Bream removal (Hosper 1997; Meijer 2000; Meijer and Hosper 1996). In 1984, before the three severe winters of 1985–1987, and particularly just before the biomanipulation attempts of 1990, TP had decreased well into the range where macrophyte dominance becomes more likely according to multilake studies, but TN had not (Kosten et al. 2009; Fig. 7). The decrease in large Bream in 1985 and the Bream removal from Lake Wolderwijd in 1990 were followed by only a temporary further decrease in TP and TN, and for TN, this did not result in values much below 1.5 mg/l. At such values, macrophytes tend to be abundant only when TP is very low (Kosten et al. 2009).
Changes in summer average values of TP and TN in three of the Borderlakes, 1975–2013. Circles before the change into the clear state, triangles after the shift (Lake Veluwe and Wolderwijd from 1996, Lake Eem from 2010), connected, solid triangles represent the change from 1995 to 1996. The years 1985 and 1991, after the first of three severe winters (TP > 1 mg/l in Lake Eem) and after Bream removal from Lake Wolderwijd, are shown separately. Shaded areas indicate the range of TP with the strongest change in the share of lakes with macrophyte dominance (0.05–0.2 mg/l), and the range of TN at or above which macrophyte dominance is only likely with very low phosphorus concentrations (1–2 mg/l; after Kosten et al. 2009)
The changes in 1996, however, resulted in decrease in average TP and TN concentrations to values where dominance by macrophytes becomes more likely, and in 1997 charophytes, after a few years of relatively slow increase, strongly responded. Along with high abundance of Bream, high TN values (high N/P ratio) may have contributed to the delayed ecological response after TP input was reduced following measures in 1979/80. This suggests that successful manipulation of such large, shallow lakes requires reduction in both P and N loading, and if biomanipulation is considered, proper timing is crucial and a multiple approach is advisable.
The 1996 shift in Lake Veluwe was also shown to be connected to increased commercial fishery for Bream between 1993 and 1997 (Lammens et al. 2004). This worked out as unintended biomanipulation with perhaps a more favorable timing than the measure of Bream removal from Lake Wolderwijd. Estimated biomass of larger Bream in Lake Wolderwijd had been just as low or lower than the 1996 biomass in Lake Veluwe, ever since the first removal of winter 1990/1991. But like in Lake Veluwe, the final shift did not occur until 1996, when Bream biomass was even lower. At that time, increased fishery coincided with the effects of a severe winter (Fig. 3), and charophytes and mussels, which had returned after the Bream removal, both showed an abrupt response after a few years of slow increase.
For Lake Veluwe, Lammens et al. (2004) suggested that reduced disturbance of sediment by benthivorous bream induced the increase in Zebra Mussels (cf. Martel et al. 1994; Bially and MacIsaac 2000) and that their filtration resulted in the clearing of the deeper parts of the lake (up to then unvegetated). Their increase was probably also connected to the final disappearance of Planktothrix. Zebra Mussels were shown to filter and trap Planktothrix in pseudofeces (Dionisio et al. 2005a) and were repeatedly suggested to be used as a biofilter to break dominance of cyanobacteria in shallow lakes (Reeders and Bij de Vaate 1990; Dioniso et al. 2005b; Gulati et al. 2008). The effect of filtration on phytoplankton is also indicated by a clear decrease in the chlorophyll/P ratio in summer 1996, a season earlier than the biggest increase in coverage of charophytes. Exclusion of Bream may also result directly in increase in charophyte cover due to decreased light attenuation and sediment disturbance (Ten Winkel and Meulemans 1984), but in this case, mussel filtration will have contributed. This may have happened in the other lakes as well, as suggested by the patterns of increase in benthivorous ducks (Fig. 2).
Changes in Lake Eem
In addition to local measures to reduce P loading, increased commercial fishery for Bream in combination with the effects of a severe winter could be an explanation why the marked changes in the plankton community occurred in Lake Eem at the same time as the final shift of 1996 in the central Borderlakes, in spite of much higher nutrient levels. Reproductive potential of mussels and macrophytes was not large enough yet for a substantial response and macrophyte dominance was still very unlikely based on TN and TP values (Fig. 7). However, the decrease in TP, TN and Bream biomass and the response of the plankton community are enough to consider this the first step of ecological recovery in Lake Eem, comparable to the 1979 and 1985 changes in Lake Veluwe. Similar processes also took place around 1996 in the other Borderlakes with relatively high P concentrations (Zwarte Meer, Lake Gooi; Noordhuis 2010). In some cases, these changes started earlier than winter 1995/96, favoring the theory of decrease in Bream biomass as a main driver. The effects of this winter, however, may have caused additional mortality and may have synchronized changes in different lakes (cf. Alheit et al. 2005). The winter of 1995/96 was not only severe (ice coverage for more than 3 months) but also extremely dry, and the number of days with high windspeeds (maximum hourly average >12.5 m/s) was unusually low. Climatic events have been connected to ecosystem shifts before (Scheffer and Carpenter 2003; Mayer and Rietkerk 2004; Jentsch and Beierkuhnlein 2008). However, effects of severe winters are not visible in developments of average or median values of water quality data in a multilake study concerning 300 lakes in the Netherlands (Pot 2010). This suggests that like Bream removal, a severe winter by itself does not guarantee a shift in a lake under re-oligotrophication (as long as nutrients are not low enough yet). Part of the effects of severe winters may be compensated by opposite effects of climate warming (Mooij et al. 2005; Jeppesen et al. 2012). Generally, climate warming seems to result in increase in eurythermal fish species like Bream in spite of oligotrophication (Jeppesen et al. 2012), with increasing winter survival due to reduced ice cover as part of the mechanism (Jackson et al. 2007). But even with lower frequency, severe winters still occur, and in combination with increased Bream fishery may still be able to cause a shift in a lake under pressure of oligotrophication, if the timing is right.
The most recent developments in Lake Eem represent a change into a clear state with dominance of macrophytes, filter feeders and limnophylic fish and are in that sense similar to the 1996 changes in the central Borderlakes. Fourteen years after the first major change in 1996, around 2010 (between 2009 and 2012), Bream abundance decreased again and dreissenid abundance increased (between 2008 and 2013). And again, a severe winter (2010) may have contributed. Most of the changes in transparency, chlorophyll concentrations and macrophyte, however, took place gradually within this 14-year period rather than as a regime shift at the end.
Differences between central Borderlakes and Lake Eem: nutrients and mussel grazing
Most important difference with the central Borderlakes is that in Lake Eem TP and especially dissolved P is considerably higher and the N/P ratio is lower. Biomass of large Bream in Lake Eem was low around 1996, but in 2002 and 2005, it was about twice as high as in the central Borderlakes. In spite of that, Zebra Mussel filtration capacity showed stronger increase here, until Bream biomass temporarily increased again in 2009. In the following years, Bream biomass strongly decreased (collapsed), probably in relation to fishery again (unpublished data catch registration). Subsequently, Quagga Mussels increased and changes toward a clear state accelerated.
Quagga Mussels were first recorded in the Netherlands in 2006 (A. Bij de Vaate, pers. com.) and started to colonize the Borderlakes the next year. Since 2009, high densities are accompanied by strong increase in transparency in parts of Lake IJssel and Marken (Noordhuis in prep.). Colonization of lakes by dreissenids has often led to increase in transparency (Barbiero and Tuchman 2004; Higgins 2014) and even regime shifts (Nicholls et al. 2011). Like in our cases, chlorophyll concentrations generally decrease following dreissenid invasion, as well as zooplankton abundance (Higgins 2014) and pelagic productivity (Fahnenstiel et al. 2010a, b). Within the framework of the theory of alternative stable states (Scheffer et al. 1993), invading dreissenids can provide the extrinsic disturbance required to shift a system from a turbid to a clear state (Mayer et al. 2014) at relatively high nutrient levels. In the case of Lake Eem, the invasion of Quagga Mussels accelerated a process that had already started with an increase in Zebra Mussels. Both species reached considerably larger shell lengths than in the central Borderlakes, which is probably related to higher nutrient concentrations and proximity of the mouth of the River Eem (Noordhuis et al. in prep., cf. Stancykowska 1964; Kolodziejczyk 1989). This difference is the main reason for filtration capacity to increase faster in this lake, despite higher Bream biomass. However, both the initial increase or return of Zebra Mussels and the acceleration of the increase in filtration capacity by Quagga Mussels from 2010 were facilitated by a marked decrease in biomass of benthivorous Bream. Shifts induced by (extrinsic) changes in biomass of benthivorous fish also tend to occur at higher nutrient levels in more eutrophic systems (Zambrano et al. 2001). In this regard, the strength of the Quagga Mussel increase can be seen as part of a positive feedback process in response to changes in water quality in a river-fed lowland lake with relatively high (natural) nutrient loading and a short residence time. Like in the central Borderlakes, Bream fishery may have been the original extrinsic disturbance or perturbation (Scheffer 2009).
Macrophytes and mussel grazing at high nutrient levels
Unlike the situation in Lake Veluwe and Wolderwijd, strong increase in transparency was reached in Lake Eem without depletion of dissolved nutrients. With chlorophyll concentrations just as low as in Lake Veluwe, chlorophyll/TP ratio was several times lower and phytoplankton in Lake Eem is likely to be limited by grazing. This will have promoted increase in macrophytes with a strong representation of filamentous benthic macro-algae like Cladophora, which take their nutrients only from the water column during most of the growing season. Charophytes are also present in Lake Eem, but densities remain very low, in spite of efforts to accelerate this process by introducing sediment with spores from other parts of the area (preliminary results of running experiment). Increase in Cladophora is reported in many North American studies in association with dreissenid invasions (Ozersky et al. 2009; Bootsma and Liao 2014; Skubinna et al. 1995; Higgins et al. 2008), including locations where it had declined earlier following reduction in nutrient levels (Canale and Auer 1982; Auer et al. 2010). Macrophytes, periphyton and zoobenthos other than dreissenids generally increased after dreissenid invasion (Haynes et al. 1999; Higgins 2014). Bivalves in both marine and freshwater situations seem to promote production of macrophytes and macro-algae through enrichment of the sediment with soluble nutrients from their excrements (Dreissena and Cladophora: Ozersky et al. 2009; Modiolus and seagrass: Peterson and Heck 2001; Mytilus and marine algae: Pfister 2007). As a result of these processes, there is often a general shift in energy production from the open pelagic to benthic region, or a “benthification” after colonization with dreissenids. This process may be driven more by these dreissenids than by nutrient reduction (Mayer et al. 2014). After introduction, the benthic community seems to be capable of controlling processes and dynamics in the entire ecosystem (Karatayev et al. 2002).
Some authors also state that increase in dreissenids promotes blooms of cyanobacteria (Makarewicz et al. 1999; Raikow et al. 2004), particularly Microcystis, which is negatively selected by the mussels (Vanderploeg et al. 2001; Fishman et al. 2010). But in the end, findings from different studies in this matter are inconsistent, and selectivity seems to depend on the specific strain of Microcystis and the relative grazing pressure of the mussel population (Vanderploeg et al. 2014; Dionisio Pires et al. 2005a). In Lake Eem, concentrations of Microcystis as well as other blooming cyanobacteria (Anabaena and Aphanizomenon) were relatively low in the years after D. rostriformis invasion (Fig. 5).
Two types of clear states
By comparing the different Borderlakes, it might be concluded that, while the turbid state in the 1970s was rather similar, in the present situation, there are considerable differences in the characteristics of the clearwater states in the central and southern Borderlakes. The two different clear states, one characterized by dominance of charophytes and limited dreissenid filtration at low nutrient levels, the other by high mussel abundance and dominance of filamentous algae at higher nutrient levels, may represent two types, or even two alternative stable clear states. Scheffer (2009) presented a relatively clear, angiosperm dominated state at intermediate nutrient levels as an alternative to states characterized by mixed phytoplankton or cyanobacteria at higher nutrient levels, or to a second clear state dominated by charophytes at low nutrient levels. Meijer (2000), Scheffer et al. (2001) and Ibelings et al. (2007) illustrated how in Lake Veluwe hysteresis occurred in a number of variables with decreasing nutrient concentrations and also identified three alternative stable states. Hysteresis was mainly attributed to non-algal light attenuation due to sediment resuspension caused by benthivorous Bream, while the resilience of the present clear state depends on sufficient abundance of charophytes and dreissenids. Comparison of the central Borderlakes and Lake Eem shows that the transparency to TP ratios during recovery of water quality in Lake Eem was similar to the ratios in the central Borderlakes during deterioration in the 1970s, though they were changing in opposite direction. Recovery in the central Borderlakes since 1979 took a path of much lower ratios (Fig. 8).
Mean TP concentrations and transparency (April–September) in Lake Veluwe (LV), Wolderwijd (WW) and Eem (LE), with indications of the direction of change. Values from successive years connected by lines. The earliest values for Lake Veluwe from Ibelings et al. (2007)
Hysteresis is not only caused by positive feedbacks and self-stabilizing mechanisms present in the turbid state, but also by increased positive feedbacks and self-stabilizing mechanisms of the clear state before a shift into a turbid state, caused by increased abundance and filtration capacity of dreissenids during the earlier stages of eutrophication. At the low nutrient levels of recent years, dreissenid abundance in the central Borderlakes after recovery remained moderate and mean shell length is low (Noordhuis et al. in prep.). Transparency in these lakes never returned to the high ratio with TP that they had around 1970. In Lake Eem, on the other hand, where nutrient levels as well as dreissenid abundance and mean shell length are considerably higher, this (type of) clear state persists.
Future developments
As the two types of clear states observed in the Borderlakes are either in different lakes or separated in time by a turbid state, we cannot confirm that they are truly alternative stable states, even though ecosystems dominated by filamentous macro-algae can also be stable (Folke et al. 2004). If this is the case, with relatively high nutrient loading from the River Eem and permanent establishment of Quagga Mussels, Lake Eem is likely to stay on this path for the coming years.
Future development of ecology in Lake Eem will primarily depend on nutrient levels and developments of the dreissenid population. The latter is illustrated by the reappearance of Microcystis in August 2014 in association with low recruitment of mussels in 2013. Due to strong recruitment in 2014, such a bloom may not occur in 2015, but return in some of the future years with fluctuations in the mussel population and in total filtration capacity. Quagga Mussels are better adapted to low nutrient concentrations than Zebra Mussels (Baldwin et al. 2002; Mandemakers 2013). Due to relatively high nutrient concentrations in the proximity of the River Eem, the mussels in Lake Eem are among the largest in the area (Noordhuis et al. in prep.). While sometimes invaders are expected to establish at modest levels after an initial population explosion, Higgins (2014) concluded the effects of dreissenid invasions in US waters are persistent and could not find evidence of diminished size of effects within two decades after establishment. Phosphorus concentrations in Lake Eem have not changed much since 2000 and after a period of strong decrease due to measures like phosphorus removal in treatment plants, further decrease in loading will probably depend on ecological response within the River Eem basin.
References
Alheit J, Möllman C, Dutz J, Kornilovs G, Loewe P, Mohrholz V, Wasmund N (2005) Synchronous ecological regime shifts in the central Baltic and the North Sea in the late 1980s. J Mar Sci 62:1205–1215
Auer MT, Tomlinson LM, Higgins SN, Malkin SY, Howell ET, Bootsma HA (2010) Great Lakes Cladophora in the 21st century: same algae, different ecosystem. J Great Lakes Res 36:248–255
Baldwin BS, Mayer MS, Dayton J, Pau N, Mendilla J, Sullivan M, Moore A, Ma A, Mills EL (2002) Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Can J Fish Aquat Sci 59:680–694
Barbiero RP, Tuchman ML (2004) Long-term dreissenid impacts on water clarity in Lake Erie. J Great Lakes Res 30:557–565
Bially A, MacIsaac HJ (2000) Fouling mussels (Dreissena spp.) colonize soft sediments in Lake Erie and facilitate benthic invertebrates. Freshw Biol 43:85–97
Bootsma HA, Liao Q (2014) Nutrient cycling by Dreissenid mussels. Controlling factors and ecosystem response. In: Nalepa TF, Schloesser DW (eds) Quagga and Zebra. Mussels biology, impacts and control. CRC Press, Boca Raton, pp 555–574
Canale RP, Auer MT (1982) Ecological studies and mathematical modelling of Cladophora in Lake Huron: 5. Model development and calibration. J Great Lakes Res 8:112–125
Dionisio Pires LM, Bontes BM, Van Donk E, Ibelings BW (2005a) Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha. J Plankton Res 27:331–339
Dionisio Pires LM, Ibelings BW, Brehm M, Van Donk E (2005b) Comparing grazing on lake seston by Dreissena and Daphnia: lessons from biomanipulation. Microb Ecol 50:242–252
Fahnenstiel G, Nalepa T, Pothoven S, Carrick H, Scavia D (2010a) Lake Michigan lower food web: long-term observations and Dreissena impact. J Great Lakes Res 36(suppl. 3):1–4
Fahnenstiel G, Pothoven S, Vanderploeg H, Klarer D, Nalepa T, Scavia D (2010b) Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. J Great Lakes Res 36:20
Fishman DB, Adlerstein SA, Vanderploeg HA, Fahnenstiel GL, Scavia D (2010) Phytoplankton community composition of Saginaw Bay, Lake Huron, during the zebra mussel (Dreissena polymorpha) invasion: a multivariate analysis. J Great Lakes Res 36:9–19
Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS (2004) Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst 35:557–581
Gulati RD, Dionisio Pires LM, van Donk E (2008) Lake restoration studies: failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 38:233–247
Haynes JM, Stewart TW, Cook GE (1999) Benthic macroinvertebrate communities in south western Lake Ontario following invasion of Dreissena: continuing change. J Great Lakes Res 25(25):828–838
Higgins SN (2014) Meta-analysis of dreissenid effects on freshwater ecosystems. In: Nalepa TF, Schloesser DW (eds) Quagga and Zebra. Mussels biology, impacts and control. CRC Press, Boca Raton, pp 487–494
Higgins SN, Malkin SY, Howel ET et al (2008) An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. J Phycol 44:839–854
Hosper SH (1984) Restoration of Lake Veluwe, The Netherlands, by reduction of phosphorus loading and flushing. Water Sci Technol 17:757–768
Hosper SH (1997) Clearing lakes: an ecosystem approach to the restoration and management of shallow lakes in the Netherlands. Dissertation, Wageningen University, the Netherlands
Hosper SH, Meijer M-L (1986) Control of phosphorus loading and flushing as restoration methods for lake Veluwe, the Netherlands. Hydrobiol Bull 20:183–194
Ibelings BW, Portielje R, Lammens EHRR, Noordhuis R, Van den Berg MS, Joosse W, Meijer M-L (2007) Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: lake Veluwe as a case study. Ecosystems 10:4–16
IJnsen F (1981) Onderzoek naar het optreden van winterweer in Nederland. KNMI, De Bilt
Jackson LJ, Søndergaard M, Lauridsen TL, Jeppesen E (2007) A comparison of shallow Danish and Canadian lakes and implications of climate change. Freshw Biol 52:1782–1792
Jentsch A, Beierkuhnlein C (2008) Research frontiers in climate change: effects of extreme meteorological events on ecosystems. Comptes Rendus Geosci 340:621–628
Jeppesen E, Jensen JP, Kristensen P, Søndergaard M, Mortensen E, Sortkjær O, Olrik K (1990) Fish manipulation as a lake restoration tool in shallow, eutrophic, temperate lakes 2: threshold levels, long-term stability and conclusions. Hydrobiologia 200(201):219–228
Jeppesen E, Meerhoff M, Jacobsen B et al (2007) Restoration of shallow lakes by nutrient control and biomanipulation—the successful strategy varies with lake size and climate. Hydrobiologia 581:252–269
Jeppesen E, Hehner T, Winvield IJ et al (2012) Impacts of climate warming on the long-term dynamics of key fish species in 24 European lakes. Hydrobiologia 694:1–39
Karatayev AY, Burlakova LW, Padilla DK (2002) Impacts of zebra mussels on aquatic communities and their roll as ecosystem engineers. In: Leppakoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe. Distribution, impacts and management. Kluwer Academic, Dordrecht, pp 433–446
Koeman RPT (1997) Soortensamenstelling van draadalgen in zoete rijkswateren, 1996. Koeman en Bijkerk bv, Haren
Kolodziejczyk A (1989) Malacofauna in isolated and interconnected lakes. Arch Hydrobiol 114:431–441
Kosten S, Kamarainen A, Jeppesen E, Van Nes EH, Peeters EHM, Mazzeo N, Sass L, Hauxwell J, Hansel-Welch N, Lauridsen TL, Søndergaard M, Bachmann RW, Lacerot G, Scheffer M (2009) Climate-related differences in the dominance of submerged macrophytes in shallow lakes. Glob Change Biol 15:2503–2517
Lammens EHRR, Van Nes EH, Meijer ML, Van den Berg MS (2004) Effects of commercial fishery on the bream population and the expansion of Chara aspera in Lake Veluwe. Ecol Model 177:233–244
Makarewicz JC, Lewis TW, Bertram P (1999) Phytoplankton composition and biomass in the offshore waters of Lake Erie: pre and post-Dreissena introduction (1983–1993). J Great Lakes Res 25:135–148
Mandemakers J (2013) The impact of suspended sediments and phosphorus scarcity on zebra mussel and quagga mussel growth. Master’s thesis, Utrecht University, the Netherlands
Martel A, Mathieu AF, Findlay CS, Nepszy SJ, Leach JH (1994) Daily settlement rates of the zebra mussel, Dreissena polymorpha, on an artificial substrate correlate with veliger abundance. Can J Fish Aquat Sci 51:851–861
Mayer AL, Rietkerk M (2004) The dynamic regime concept for ecosystem management and restoration. Bioscience 54:1013–1020
Mayer CM, Burlakova LE, Eklöv P, Fitzgerald D, Karatayev AY, Ludsin SA, Millard S, Mills EL, Ostapenya AP, Rudstam LG, Zhu B, Zhukova TV (2014) Benthification of freshwater lakes. Exotic mussels turning ecosystems upside down. In: Nalepa TF, Schloesser DW (eds) Quagga and Zebra. Mussels biology, impacts and control. CRC Press, Boca Raton, pp 575–585
Meijer M-L (2000) Biomanipulation in the Netherlands—15 years of experience. Dissertation, Wageningen University, the Netherlands
Meijer M-L, Hosper H (1995) Actief Biologisch beheer in het Wolderwijd-Nuldernauw: evaluatie en aanbevelingen voor het beheer. Rijkswaterstaat, Lelystad
Meijer M-L, Hosper H (1996) Actief Biologisch beheer in het Wolderwijd-Nuldernauw leidt tot een toename van kranswieren. H2O 18:536–538
Mooij WM, Hülsmann S, de Senerpont Domis LN, Nolet BA, Bodelier PLE, Boers PCM, Dionisio Pires ML, Gons HJ, Ibelings BW, Noordhuis R, Portielje R, Wolfstein K, Lammens EHRR (2005) The impact of climate change on lakes in the Netherlands: a review. Aquat Ecol 39:381–400
Moss B (1990) Engineering and biological approaches to the restoration from eutrophication of shallow lakes in which aquatic plant communities are important components. Hydrobiologia 200(201):367–378
Nicholls KH, Hoyle JA, Jonansson OE, Dermott R (2011) A biological regime shift in the Bay of Quinte ecosystem (Lake Ontario) associated with the establishment of invasive dreissenid mussels. J Great Lakes Res 37(2):310–317
Noordhuis R (2010) Ecosysteem IJsselmeergebied: nog altijd in ontwikkeling. Trends en ontwikkelingen in water en natuur van het Natte Hart van Nederland. Rijkswaterstaat, Lelystad
Noordhuis R, Reeders HH, Marteijn ECL (1994) Inzet van driehoeksmosselen bij biologisch waterbeheer; resultaten van veldexperimenten. H2O 27(6):150–160
Ozersky T, Malkin SY, Barton DR, Hecky RE (2009) Dreissenid phosphorus excretion can sustain C. glomerata growth along a portion of Lake Ontario shoreline. J Great Lakes Res 35:321–328
Peterson BJ, Heck KL Jr (2001) Positive interactions between suspension-feeding bivalves and seagrass—a facultative mutualism. Mar Ecol Prog Ser 213:143–155
Pfister CA (2007) Intertidal invertebrates locally enhance primary production. Ecology 88:1647–1653
Pot R (2010) Toestand en trends in de waterkwaliteit van Nederlandse meren en plassen. Resultaten van de vijfde eutrofiëringsenquête in opdracht vande werkgroep Routekaart Heldere Meren. Roelf Pot Onderzoek- en Adviesbureau voor Water- en Oevervegetatiebeheer, Oosterhesselen, the Netherlands
Raikow DF, Sarnelle O, Wilson WE, Hamilton SK (2004) Dominance of the noxious cyanobacterium Microcystis aeruginosa in low-nutrient lakes is associated with exotic zebra mussels. Limnol Oceanogr 49:482–487
Reeders HH, Bij de Vaate A (1990) Zebra mussels (Dreissena polymorpha): a new perspective for water quality management. Hydrobiologia 200(201):437–450
Reeders HH, Bij de Vaate A, Noordhuis R (1993) Potential of the Zebra Mussel (Dreissena polymorpha) for water quality management. In: Nalepa TF, Schloesser DW (eds) Zebra Mussels, biology, impacts, and control. Lewis, Boca Raton, pp 439–452
Scheffer M (1998) Ecology of Shallow Lakes. Chapman and Hall, London
Scheffer M (2009) Critical transitions in nature and society. Princeton University Press, Oxford
Scheffer M, Carpenter S (2003) Catastrophic regime shifts in ecosystem: linking theory to observation. Trends Ecol Evol 18:648–656
Scheffer M, Hosper SH, Meijer ML, Moss B, Jeppesen E (1993) Alternative equilibria in shallow lakes. Trends Ecol Evol 8:275–279
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596
Skubinna JP, Coon TG, Batterson TR (1995) Increased abundance and depth of submersed macrophytes in response to decreased turbidity in Saginaw Bay, Lake Huron. J Great Lakes Res 21:476–488
Stancykowska A (1964) On the relationship between abundance aggregations and condition of Dreissena polymorpha (Pall.) in 36 Masurian lakes. Ekol Pol A 12:653–690
Ten Winkel EH, Meulemans JT (1984) Effects of cyprinid fish on submerged vegetation. Hydrobiol Bul 18:157–158
Vanderploeg HA, Liebig JR, Carmichael WW, Agy MA, Johengen TH, Fahnenstiel GL, Nalepa TF (2001) Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Can J Fish Aquat Sci 58:1208–1221
Vanderploeg HA, Wilson AE, Johengen TH, Dyble Bressie J, Sarnelle O, Liebig JR, Robinson SD, Horst GP (2014) Role of selective grazing by dreissenid mussels in promoting toxic Microcystis blooms and other changes in phytoplankton composition in the Great Lakes. In: Nalepa TF, Schloesser DW (eds) Quagga and Zebra. Mussels biology, impacts and control. CRC Press, Boca Raton, pp 509–523
Zambrano L, Scheffer M, Martinez-Ramos M (2001) Catastrophic response of lakes to benthivorous fish introduction. Oikos 94:344–350
Acknowledgments
Most of the data used in the paper were derived from the monitoring programmes of Rijkswaterstaat, the agency of the Dutch Ministry of Infrastructure and the Environment responsible for the main water systems, which also supported much of the research financially. Surveys of dreissenids were largely carried out and reported by Bureau Waardenburg bv, Culemborg. We thank Dr. Harry Hosper, Dr. Eddy Lammens, Ir. Simon Groot and Dr. Ellis Penning for their comments on earlier versions of the manuscript.
Conflict of interest
Ruurd Noordhuis, Bastiaan van Zuidam, Edwin Peeters and Gerben van Geest declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Petra M. Visser, Bas W. Ibelings, Jutta Fastner & Myriam Bormans / Cyanobacterial blooms. Ecology, prevention, mitigation and control.
Rights and permissions
About this article
Cite this article
Noordhuis, R., van Zuidam, B.G., Peeters, E.T.H.M. et al. Further improvements in water quality of the Dutch Borderlakes: two types of clear states at different nutrient levels. Aquat Ecol 50, 521–539 (2016). https://doi.org/10.1007/s10452-015-9521-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-015-9521-8