Abstract
The pen shell, Pinna nobilis L., is a critically endangered bivalve threatened by mass mortality events throughout the Mediterranean, but the Alfacs Bay (Ebro Delta) still hosts many healthy individuals. Herein, we study the main factors controlling recruitment patterns in this locality, including gonadal development and abundance of critical life-stages, as well as the effect of environmental factors. Growth records from empty shells suggested a single major peak of recruitment during a period of 11 years, although many juveniles were found in two very shallow sand bars possibly acting as a barrier for water circulation and as a trap for larvae. Collectors deployed outside these sand bar areas showed zero settlers, and the availability of planktonic larvae was very low. Gonadal examination evidenced breeding throughout the summer period with successive hermaphroditism, but 20% of individuals were simultaneous hermaphrodites, a condition that has been associated with environmental stress and that could lead to in-breeding depression and potentially reduced fertility. Yet, given the large size of the population and the wide breeding period observed, planktonic processes causing larval mortality such as freshwater discharges from rice locally important rice agriculture are also proposed as possible impacts accounting for patterns of low larval availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pen shell Pinna nobilis L. is an endemic Mediterranean bivalve strictly protected by the European Union (Habitats Directive Annex IV EEC, 1992), the Barcelona convention (Protocol ASPIM Annex 2), and recently listed as “critically endangered” by the Spanish government (BOE 251-14181, 2018). Recent mortalities of 90–100% of the populations have been reported along the entire Mediterranean Spanish coast and other Mediterranean countries such as France, Italy, Greece, Tunisia, Cyprus, and Turkey (Cabanellas-Reboredo et al., 2019) have been associated with the presence of the parasite Haplosporidium pinnae (Catanese et al., 2018). Additionally, the virulence of other pathogens such as Mycobacteria spp. has also been proposed as a mortality agent (Carella et al., 2019) and the possible association with Haplosporidium outbreaks is currently being investigated. At present, only two Spanish populations located in Fangar Bay (North Ebro Delta, containing hundreds of individuals) and the Mar Menor (also hundreds of individuals by recent census) remain uninfected. A third population located in Alfacs Bay (South Ebro Delta) was infected in July 2018 in an area adjacent to the open sea, but the rest of the bay remains healthy (García-March et al., in review). Although the reasons driving these patterns are still uncertain, lower salinities associated to the rice cultivation period (Cerralbo et al., 2019) have been proposed to hamper the spread of the parasite (Cabanellas-Reboredo et al., 2019).
In healthy populations, natural mortality in P. nobilis has been indicated to be size-dependent, with young stages suffering much higher mortality rates than adults (García-March et al., 2007; Katsanevakis, 2007), which coupled with long life-expectancy (over 50 years age, and 120 cm length, Butler et al., 1993; Rouanet et al., 2015), typically leads to the dominance of large-sized individuals. Population sizes may vary from thousands to tens of individuals in densities that do not usually exceed one individual per 100 m2, although aggregated patterns with tens of individuals per 100 m2 are common in some places (review by Prado et al., 2014). Ultimately, the population structure is the result of a multiplicity of biotic and abiotic factors influencing spawning, larval production, pelagic dispersal and survival, settlement and post-settlement success, and recruitment at different spatial and temporal scales (Pineda et al. 2010).
The breeding cycle of P. nobilis has been described in detail (De Gaulejac, 1995), although little is known about mechanisms controlling sex determination, mass-spawning events, and fertilization rates. Individuals are not strictly gonocoric but feature a particular type of asynchronous and successive hermaphroditism, in which different degrees of sexual maturity are observed between male and female dominant phases (De Gaulejac, 1995; Deudero et al., 2017). Hence, each type of sexual gamete is sequentially released from June to September (De Gaulejac, 1995) at temperatures above 20°C (Deudero et al., 2017). Yet, many other environmental factors including temperature, phytoplankton blooms, tides, moonlight, chemicals, storms or high wave action may also have a role in mass-spawning events, as evidenced in many other marine invertebrates (Giese, 1959; Himmelman, 1975). Under some unclear factors captive individuals of P. nobilis may also exhibit simultaneous release of male and female gametes (38.7% of the individuals) leading to self-fertilization and full mortality of trocophora larvae (Trigos et al., 2018). Oocytes released to the water column (De Gaulejac et al., 1995a) appear to have a low buoyancy and a tendency to sink (Trigos et al., 2018), which may make dispersal difficult. Fertilization of oocytes occurs rapidly at 21°C and can be confirmed after 15–30 min by the appearance of the first polar body and the formation of a perivitelline membrane (Trigos et al., 2018). The exact duration of the larvae is not known, but incomplete studies of the life cycle until the pediveliger stage suggest a minimum of 10 days (Trigos et al., 2018; P. Prado pers. observ.) during which they may be exposed to large-scale factors such as currents, nutrient concentrations, temperature, and salinity that can strongly influence larval availability (Pineda et al., 2010). According to indirect genetic evidence from natural populations, larvae may occasionally travel distances in the order of hundreds of km, although self-recruitment seems to predominate (Wesselmann et al., 2018; Nebot et al., 2019). Given the strong similarity in larval bivalve morphology, identification to species level is very challenging (Malchus & Sartori, 2013), and no studies have been conducted to assess natural variability in the availability of P. nobilis larvae. Yet, recent methodological approaches using qPCR detection are now available to allow the estimation of larval abundances “per se” (Andree et al., 2018) and might provide a better understanding of early planktonic processes. Otherwise, the use of collector devices for P. nobilis settlers has been widely used across many populations as a proxy for larval supply (e.g., Cabanellas-Reboredo et al., 2009; Theodorou et al., 2015; Kersting & García-March, 2017), although they may be affected by differential factors such as preferential substrates for settlement, chemical cues, predation, and competition, among others (Pineda et al., 2010). Once in the benthos, reported differences in the age structure of populations (Richardson et al., 2004; Katsanevakis, 2005), as well as data obtained in situ, suggest that recruitment peaks in the Columbretes Islands (W Mediterranean) may occur every 3–4 years possibly associated to sea water temperature at the beginning of the reproductive period (Kersting & García-March, 2017).
The population of P. nobilis within Alfacs Bay (Ebro Delta, Catalonia) was estimated in the summer of 2012, previous to the infection by Haplosporidium pinnae at over 90,000 individuals, with peak densities of up to 20 individuals per 100 m2, and mean densities of 1.61 individuals per 100 m2 (Prado et al., 2014). The population features an extremely shallow distribution of individuals (from 20 to 130 cm depth) growing over extensive seagrass meadows of Cymodocea nodosa (Prado et al., 2014), and is dominated by large adults of 45–55 cm length (Prado et al., 2014) although smaller individuals are also observed. The distribution of individuals in the bay is highly irregular but is inversely related to the influence of agricultural discharges (Cerralbo et al., 2019), with large unpopulated areas in the northern shore, low-density areas in the inner part of the southern shore, and high-density areas in the region closer to mouth of the bay (Prado et al., 2014).
In the context of extreme risk for the conservation of the species, the aim of this study was to identify the main factors controlling recruitment patterns and the age structure of the Alfacs Bay population, including gonadal development, larval availability, and juvenile densities in the benthos. To this end we conducted: (1) long-term (> 10 years) and mid-term (3 years, 2016 to 2018) assessment of recruitment events based on age determination from shells and on the abundance of juveniles (field densities) and settlers (artificial collectors), respectively. Further, we investigated the possible relationship between these data and long-term series of seawater temperature, salinity, and phytoplankton availability; (2) evaluations of larval availability in the water column using qPCR analysis (Andree et al., 2018); and (3) histological examination of the gonads during the fertility period were also conducted in 2016. Both larval availability and early benthic stages were investigated in three areas of the bay with distinctive adult densities in order to establish a potential relationship between the number of breeding individuals and reproductive outputs.
Materials and methods
Study area
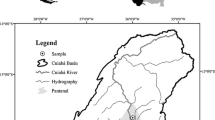
Alfacs Bay is a semi-enclosed estuarine water body (ca. 49 km2 and an average depth of 3.13 m) located at the south of the Ebro River delta (40°36′39″ N, 0°39′25″ E; Catalonia, NW Mediterranean), which has a great economic importance due to the presence of extensive shellfish aquaculture, particularly of oysters and mussels. The northern shore is bordered by rice fields and receives seasonal agricultural discharges (ca. 275 × 106 m3 year−1) high in nutrients and organic matter that causes a salinity gradient (from below 30 to 37; Camp & Delgado, 1987; Llebot et al., 2011; Cerralbo et al., 2019) and favors the development of epiphytes and fast growing macroalgae (Prado, 2018). Hence, the shallow submerged platform along the inner shore (< 2 m) is mostly composed of silty sediments and dominated by the seagrass Cymodocea nodosa along with patches of Zostera noltii in areas closer to the freshwater discharge (Prado, 2018). The southern shore (the Banya Sandspit) was included in the Ebro Delta Natural Park in 1986 and is also part of the Natura 2000 network of the European Union because of its importance for both C. nodosa and P. nobilis (Prado et al., 2014). Nevertheless, nearly half of the Banya Sandspit is occupied by extensive saltpans used for traditional salt extraction activities that are not subjected to the same regulations as the rest of the area. The Banya Sandspit stretches along a shallow platform of 18 km2 (ca. 700 m wide), which gently slopes from 0 to 1.5 m, with an average depth of 0.64 m. At a distance of ca. 500–600 m from the coast, the platform forms a large sand bar with several parts (two major areas of 13.79 and 12.56 Ha and several isolated areas) that emerge at low tides, at least during the winter period, when particularly low tides occur (see Fig. 1). By following the density of P. nobilis within these areas along the summer salinity gradient (Cerralbo et al., 2019), the Alfacs Bay can be divided into three zones with high (half outer region of the Banya Sandspit), intermediate (half inner region of the Banya Sandspit), and low abundance of individuals (inner northern shore; Prado et al., 2014). The high abundance zone was located in the outer half of the Banya Sandspit, adjacent to the mouth of the bay (ca. 2.5 km wide) and experienced major mortality by H. pinnae in July 2018; the intermediate abundance zone in the inner half of the bay (currently free of the parasite); and the low abundance zone (also free of the parasite) in the Northeast corner of the bay, facing a recovered salt marsh area (Fig. 1).
Map of the Alfacs Bay showing the location of sampling sites according to adult densities (Z1 zone of high abundance also sampled at the deep limit of the seagrass bed (Z1-deep), Z2 zone of intermediate abundance, Z3 zone of low abundance). The emerged parts of the Sand Spit sand bar are indicated in black. Juveniles from the 2017 recruitment were observed in the main area of 13.79 Ha adjacent to Z2, and in other minor area of ca. 434 m2 closer to Z1
Environmental variables including temperature, salinity, and phytoplankton availability (Chl a concentration) are measured weekly in a central point of the Alfacs Bay (40°36.54′ N, 0°39.36′ E) as a part of a monitoring program for shellfish safety of the Catalonian Government. Values from late May to October (potential reproductive period) were requested for study purposes and examined for possible associations with recruitment patterns of the population using multiple correlation analysis.
Gonadal development
Given the protected status of the species only 10 individuals were collected from the area with high adult densities (2 in June and 8 in August 2016) in order to minimize the impact on the population. Individuals were placed within iceboxes and transported to the lab for anatomical examination and description, measurement of valve length to the nearest mm, and dissection of organs for tissue sampling. From each animal, different transverse sections of each tissue, including gonad, digestive gland, kidney, mantle, and gills were fixed in Davidson’s solution and preserved for at least 48 h at room temperature. Subsequently, tissues were dehydrated in ascendant alcohol series and embedded in paraffin blocks, 3 µm thick sections were cut and stained with haematoxylin and eosin (H&E). The slides were examined under a light microscope (Nikon DS-Fi1 video camera mounted on a Nikon 50i microscope) for possible presence of pathogens and diseases. Estimation of gonadal status was evaluated following De Gaulejac (1995). In three selected individuals showing gonadal degeneration, the percentage of degenerated follicles over a total of 500 was estimated using a magnification of 10X. Captured images were examined using the cell counter plugin of ImageJ software (v1.50 h9).
Estimates of larval abundances by qPCR
Field sampling for P. nobilis larvae was conducted every 15 days between July and October 2016 in the three sites with high, intermediate, and very low density of individuals (Fig. 1) with a plankton net of 0.2 m diameter and 30 μm size mesh. Samples were collected (n = 3 replicates per site) by walking ca. 30 m over the seagrass bed of Cymodocea nodosa, equivalent to a filtration volume of 3.77 m3. All samples were preserved in 70% ethanol and transported to the lab for further processing. All bivalve larvae within each sample were carefully separated from the rest of the plankton under a dissecting microscope using a zooplankton micro-spoon with a loop of 100 µm in diameter.
Full methodological details of DNA extraction, qPCR amplification, calibration curves, and assay optimization can be obtained from Andree et al. (2018). Briefly, specific primers designed for the amplification of a small fragment (less than 200 bp) of the ribosomal DNA of P. nobilis were designed for assessing specific identity and abundance of target sequences in water samples, or for species identification among other bivalve larvae. Due to the methodology of qPCR a higher abundance of specific target is inversely correlated to Ct values; that is, a low Ct value correlates to high abundance (Andree et al., 2018).
Significant differences in qPCR Ct values for each Zone and Date were investigated with a 2-way factorial ANOVA (Zone and Date fixed factors with two and six levels). For all analyses, the critical level of significance was fixed at P < 0.05. SNK post hoc comparisons were used to identify significant differences between zones at each sampling date.
Abundance of settlers
Collector devices were constructed for observing new bivalve settling stages. Each collector consisted of one plastic mesh bag (ca. 5 mm opening) filled with nylon fishing thread or pieces of fishing net and a buoy, then each was anchored to a mooring on the seafloor with a rope. The efficiency of this type of collector for capturing P. nobilis settlers has been previously established by Kersting & García-March, (2017) in the Columbretes Islands Marine Reserve, located ca. 100 km south from Alfacs Bay. The devices remained immersed during 4 months, after which they were recovered and taken into the lab for separation and identification of juvenile bivalve species under the dissecting microscope.
These collectors were deployed between the first of June and the first of October of 2016 and 2017 at the same three sites used to estimate settlement rates, whereas in 2018 they were only installed along the main sand bar of 1100 m2 during the same period. In each site, five devices were randomly distributed within seagrass beds of C. nodosa (ten at the single site of 2018), close to living adults and at least 5 m apart from each other. Five additional devices were deployed beyond the seagrass limit of the site with high abundance of individuals at a depth of ca. 1.5 to 2 m, in order to assess possible habitat preferences.
Variability in the abundance of bivalve assemblages among study zones and between years was investigated with a 2-way Permanova available within the PRIMER v6 software package (Clarke & Gorley, 2006).
Short-term recruitment patterns
Juveniles from each summer recruitment period in 2016, 2017, and 2018 were sampled by snorkeling along 20 m long by 1 m wide transects (N = 20 per site and year). The three sites of Alfacs Bay with distinctive abundance of individuals were sampled at a depth of ca. 60–80 cm, at which adult densities are highest (Prado et al., 2014). Two additional areas of ca. 1100 m2 and 380 m2, that were exposed or with very shallow depth at low tide and within which were observed high numbers of juveniles in early 2018 (from 2017 recruitment), were also sampled that year and again in early 2019 to obtain further information of interannual patterns at the bay scale.
Long-term patterns of recruitment
Twenty shells between 42.8 and 62.1 cm long from Alfacs Bay—these being the most common sizes observed—were used to evaluate the age range of the population and to determine the availability of different cohorts indicative of successful long-term recruitment. Ten of them included the individuals used for histological analysis (i.e., the exact moment of death was known; see Table 1), and the other ten were found empty during the fieldwork in summer 2016. These consisted of recently dead individuals (i.e., in the order of a few days), which could be identified from the absence of encrusting epifauna and macroalgae in the inner surface of the valves, so its pearly sheen was still intact. A sample size of N = 20 has been shown as sufficient for accurate age estimation in population studies (García-March & Márquez-Aliaga, 2007; García-March et al., 2011). One valve was processed to study the record of the posterior adductor muscle scar (PAMS) from the interior of the shell according to García-March et al. (2011). The valve was radially cut through the PAMS and ca. 8 cm dorso-ventral sections of one side were mounted in slides. A thick section (ca. 200 μ) of the portion glued to the slide was cut using a low-speed Buehler Isomet saw. The free surface of the slide preparation was polished to improve observation of the growth record. From each polished section, the growth record was counted. Missing records were calculated using the width of the calcite layer in the three oldest records of all specimens (García-March et al., 2011).
The possible association between available environmental variables (temperature, salinity, and phytoplankton availability) and long-term recruitment patterns from the age study was investigated with multiple correlation analysis using the Statistica v. 12 software.
Results
Environmental variables
Seasonal patterns of water temperature in the Alfacs Bay showed lowest values in May and October (20.4 ± 0.2°C), intermediate in June and September (24.4 ± 0.2°C), and peaks in July–August (27 ± 0.2°C). Interannual differences (2001 to 2018) in temperature during the potential period of reproduction were small (Fig. 2a). For salinities, values fluctuated from 31.7 in May to 37 in October, depending on agricultural discharges but without any clear monthly trend (Fig. 2b). In contrast, chlorophyll-a levels tended to increase through the summer period but were highly variable among study years (May–June: 1.8 to 6.6 µg/l; Jul–Aug: 2.1 to 8.7 µg/l; and Sep–Oct: 2.6 to 9.6 µg/l) (Fig. 2c).
Variation in a temperature (°C), b salinity, and c chlorophyll-a content (µg l−1) during the reproductive period of P. nobilis from 2007 to 2018. In the X axis, years of larger recruitment (2008 and 2017) are indicated in bold, and years corresponding to the ± 1 year error in age estimations (i.e., 2007 and 2009) in dark gray
Gonadal development
Six developmental stages were identified for the 10 sacrificed individuals (2 in June and 8 in August) and indicated in Table 1 and Fig. 3. There was no apparent relationship between gonad development stage and valve length.
Histological feature of gonadal developmental stages of P. nobilis during the summer season. a Simultaneous maturation: presence of visible male germinal epithelium represented by spermatogonia (Sg) and female previtellogenic oocytes (PVO) and residual spermatozoa (Spz); an early male germ line is visible in a few follicles (insert); b mature female: female gonad with vitellogenic (VO) and mature oocytes (MO); phagocytes in the lumen (*); male early germ line are present in some follicle (insert); c male degeneration-female formation: previtellogenic oocytes (PVO) border the follicle and spermatozoa (Spz) are visible in the lumen; d female/male line degeneration: regressive phenomena at both male and female germ cells with apoptotic oocytes (AO) and residual male germ line in the lumen (e). Spent: empty follicles with visible phagocytes (*) and residual germ cells; f Male Spawning: spermatozoa (Spz) fill the follicles with phagocytes in the center (*). Few early developing oocytes against the follicle wall are also visible
Microscopic examination of gonads showed that some individuals had overlapping stages of male and female development within individual follicles (i.e., successive and asynchronous hermaphroditism; De Gaulejac, 1995; Deudero et al., 2017). There was one spawning male releasing spermatozoa from the central part of the follicle; one male in a degenerating state with spermatozoa in the lumen of the acini and oocytes developing on the wall of the follicle; and one active female with mature oocytes in the center of the follicle (see Fig. 3; Table 1). It was possible to determine that gamete production is continuous during the summer period whereas spawning is partial, particularly in females, with remains of residual oocytes in most examined individuals. Two of the individuals collected in August already presented a disorganized gonad structure with no evidence of any type of male or female germ lines, suggesting the end of the reproductive period. However, in two of the examined individuals (one from June and one from August), the development of both types of sexual gametes occurred simultaneously from the germinal epithelium of the same follicle (Fig. 3; Table 1), a gonadal stage not described in previous works.
Further, individuals 1 and 7 collected in August showed 100% degenerating gonad, whereas individual 8 presented some residual final oocytes in some follicles (10%) (Fig. 3). Such significant degeneration coincided with the presence of multiple nematode eggs (gonad of individual number 7) and with the occurrence of oocysts of a possible coccidian and of some unknown crustacean (individuals 1, 7 and 8) (Fig. 4a–c). Extensive damage represented by necrosis of digestive tissue was also observed in individual 1 of June with no evident aetiological agent (Fig. 4d). Nevertheless, microscopic evaluation of the different tissues (gonad, digestive gland, kidney, mantle and gills) showed no evidence of haplosporidian infections.
Pathogens and disease observed in individuals of Pinna nobilis during the summer season. a–c Pathogens detected in the gonad: Nematode eggs (arrowheads) (a) in a degenerated gonad, oocyst of a possible coccidian (arrowheads) (b) and a crustacean (arrowheads) (c); d Extended colliquative necrosis (*) of digestive tubules (DT) of the digestive gland
Larval abundances
qPCR results for the three study zones and six study dates (from 7-15-2016 to 1-10-2016) only detected 15 positives for the presence of P. nobilis DNA (total N = 54 samples). In addition, Ct values were rather high (between 31 and 39, with a global average of 36.4), which indicates very low larval densities ranging from 0.2 to 32 larvae per sample (see Fig. 5) equivalent to 0.05 to 8.5 larvae per m3. In addition, qPCR for water samples was in agreement with these patterns, since no positives were detected, suggesting low availability of gametes and/or tissue remains in the water column.
Abundance of bivalve larvae (all species) within plankton samples from July to October 2016. Estimate numbers of P. nobilis larvae within positive samples extrapolated from the calibration curve (Andree et al., 2018) are indicated per zone below each sampling date
The high Ct values observed in Zone 2 and 1 (31.9 and 32.1, respectively), analyzed by ANOVA showed no differences among zones (df = 2, MS = 166.7, P = 0.433). In contrast, there were significant effects related to time of sample collection (df = 5, MS = 517.9, P = 0.038), and a Zone × Date interaction (df = 10, MS = 509.69, P = 0.0166). Further SNK post hoc analyses indicated that significant effects were only due to differences between two dates: the first of September 2016 (positive samples recorded in the three zones, but particularly at Zone 2 with an estimate of ca. 47 individuals) and the first of August 2016 (no positive samples recorded at any site).
Fluctuations in the abundance of P. nobilis larvae from qPCR were found to be unrelated to overall abundances for all bivalve larvae, which tended to be higher in Zone 3 (df = 2, F = 2.038, P = 0.1449), particularly on 9-15-2016 with a peak of over 5,000 bivalve larvae per sample (Fig. 5), although neither temporal effects (df = 5, F = 1.235, P = 0.3129) nor the Zone × Date interaction were significant (df = 10, F = 1.2247, P = 0.3088).
Abundance of settlers
No P. nobilis settlers were observed within collectors deployed from 2016 to 2018 at different bay locations (in 2018 only adjacent to the main sand bar). Yet, collectors deployed in 2016 and 2017 showed the presence of ten different bivalve species, thus demonstrating trapping efficiency. Community results from PERMANOVA showed significantly higher settling rates in 2016 than in 2017 (MS = 10,968; Pseudo-F = 20.04; P = 0.001), particularly in zones 1 and 2; whereas zone 3 showed similar numbers between years (MS = 6842; Pseudo-F = 12.5; P = 0.001). Additionally, differences among zones were also significant (MS = 14,351; Pseudo-F = 26.2; P = 0.001, see Fig. 5), with zone 1 (closer to the open sea) displaying ca. 2 and 15 times higher number of settlers than zone 2 and 3, respectively. The most abundant species in collectors from all zones were juveniles of Parvicardium spp., especially in Zones 1 and 2 during 2016 (Fig. 6). Limaria hians was also locally abundant, especially at the deeper site (Zone 1), as well as Ruditapes spp. and Mytilus galloprovincialis in Zone 1. Other species present in low numbers in the collectors were: Anomia ephippium, Arca noae, Barbatia barbata, Mimachlamys varia, Modiolula phaseolina, and Musculus subpictus.
Abundance of bivalve species within collector devices deployed at each study zone and at the deep site in zone 1 (2016 only, those at the deep site were lost in 2017). Other species present in very low abundances and not visible in bars are indicated in the results section. a Summer 2016; and b summer 2017
Short-term recruitment patterns
Only one juvenile was observed within transects in 2016, two in 2017 (N = 20 transects per site, each of 20 m2), and none in 2018 in the study area of high adult densities and close to the collectors deployed for evaluation of benthic settlement outside the main sand area. This resulted in densities ranging from 0 (Zones 2 and 3) and 0.25 to 0.5 ind./100 m2 (Zone 1). Nevertheless, in two of the emerged areas along the main bay sand bar (9271.8 m2) and (434.5 m2), densities of 2.5 and 20.7 indiv./100 m2 from the 2017 recruitment were observed, respectively, many of them fully emerged at low tide and with compromised survival (Fig. 7). Considering only the dimensions of the largest sandbar area but with lowest density, the estimated abundance of juveniles is over 3,000 individuals. In contrast, juvenile densities from 2016 and 2018 recruitments were comparably low (0.1 to 2 and 0 to 0.01 indiv./100 m2, respectively). The higher recruitment observed in 2017, could not be explained by the environmental variables measured (see Fig. 2a–c).
Long-term patterns of recruitment
The analysis of the growth record (posterior adductor muscle scars) of the empty shells showed a population dominated by the 8 ± 1 year-old class (Fig. 8). Nine of the 20 shells cut had this age when they died. The remaining shells were 5, 6, 7, 9, and 15 ± 1 years of age (two shells for each size class except for the 7-year-old age class, which occurred for three shells). Counting back from the year of collection for empty shells (shells N = 10) and living adults (sacrificed N = 10), approximate years of recruitment could be estimated, ranging from 2001 to 2011, with a recruitment peak in 2008 and very little or no recruitment between 2002 and 2006.
Multiple correlation analysis between available shell records of recruitment and environmental factors (temperature, salinity and chlorophyll-a levels) measured in the middle point of the bay, showed no evident association with values recorded at any summer month (May to October) during the 2001 to 2011 period (R = 0.618; F5,5 = 37.304; P = 0.5884). The 2008 peak (± 1 year methodological error) alone could also not be explained by the presence of any maximum or minimum in the environmental variables (Fig. 2a–c).
Discussion
Populations of P. nobilis in the Ebro Delta (Alfacs and Fangar Bays) constitute a critical genetic resource for the long-term conservation of the species, currently threatened by massive large-scale mortalities (Cabanellas-Reboredo et al., 2019). Examined individuals from the Alfacs Bay showed no evidence of abnormal gametes at any gonadal development stage. Eight of them were considered to be within some stage of the asynchronous and successive hermaphroditic sexual cycle described for P. nobilis (De Gaulejac, 1995; De Gaulejac et al., 1995a, b; Deudero et al., 2017). However, the other two presented simultaneous sexual maturation which has not been described in previous studies (De Gaulejac, 1995; Deudero et al., 2017), and may result in self-fertilization and low larval viability (Trigos et al., 2018). In other bivalves, the incidence of simultaneous hermaphrodites has been reported to range from ca. 0.4 to 2.5% (Syasina et al., 1996). However, unfavorable ecological situations (including pollution) may have the potential to trigger the development of simultaneous hermaphrodites in bivalve populations (Pekkarinen, 1991). For instance, Syasina et al., (1996) found that up to 6% of scallops (Mizuhopecten yessoensis) in a bay polluted with hydrocarbons were simultaneous hermaphrodites, along with reduced percentage of fertilized eggs and early veligers. Although rates observed in our study (20%) must be interpreted with care (low N of 10 individuals), this stage has not been observed in other pristine locations from the Cabrera National Park (Balearic Islands, Spain) where a total of 120 individuals were examined during a seasonal study previous to the Haplosporidium outbreaks (Deudero et al., 2017). The application of pesticides for rice cultivation is suspected to be associated to recurrent episodes of shellfish mortality in the Ebro Delta (Köck-Schulmeyer et al., 2010). In fact, up to 26 endocrine disrupting chemicals (EDCs) (pesticides, phthalates, alkylphenols, and natural and synthetic hormones) have been identified in water samples from the Ebro River, irrigation canals, and bays (Brossa et al., 2005). The possible stress induced by agricultural pollution is also consistent with the low age of adult individuals in the Alfacs Bay (7 to 9 years old) compared with the theoretical life-expectancy of the species (> 50 years; Rouanet et al., 2015). Among other abnormal reproductive conditions, the presence of a degenerating gonad in the presence of parasites (a possible coccidian and an unknown crustacean) in three individuals collected in August could also reduce the reproductive output. Although identification of observed parasites to species level was not attempted, copepods have been shown to account for intense haemocytic reactions and metaplasia of infected epithelia (Carballal et al., 2001). Furthermore, coccidian parasites are commonly reported across different tissues of marine bivalves, including gonads, with responses varying from no apparent effect to light to moderate lesions and hypertrophy of infected tissues (Whyte et al., 1994; Carballal et al., 2001).
In spite of these deviations in gonadal development, the availability of breeding individuals (ripe female and female in developing stages) throughout the summer period, and the large number of adult pen shells in the bay (> 90,000 individuals; Prado et al., 2014) suggest that there are factors occurring later in the water column that interfere with fertilization success and/or cause larval mortality. Given the great environmental gradients occurring within the Alfacs Bay (Llebot et al., 2011; Cerralbo et al., 2019) and the potential transport of larvae across different water masses, a single central monitoring point is insufficient to capture all the variability to which they might be exposed. Elevated temperatures and inadequate food supply have been associated with the mortality of bivalve larvae (Rumrill, 1990), but interannual differences in these variables at the monitoring point were small or uninformative. In contrast, the salinity gradient associated to agricultural discharges (along with agrochemicals and/or siltation) could be structuring the species distribution within the bay (see Prado et al., 2014; Cerralbo et al., 2019) and variable inputs and exposure times might be affecting the viability of planktonic stages. Although the effects of salinity have not been yet investigated for P. nobilis embryos and larvae, evidence in other species such as the marine snail Ilyanassa obsoleta suggest that short-term fluctuations in salinity could influence planktonic growth rates, size, and the overall larval viability with direct ecological effects on recruitment success (Richmond & Woodin, 1996). Similarly, Lough & Gonor (1971) found that low salinities retards the development of the boring bivalve Adula californiensis, and it becomes abnormal below 26.3‰, which is comparable to lowest salinities occurring in the northern shore of the Alfacs Bay during the summer period (Camp & Delgado, 1987; Llebot et al., 2011). In this scenario, the dominant local winds and summer currents acting along the NW-SW axis of the Alfacs Bay (Llebot et al., 2014; Cerralbo et al., 2019) may also contribute to the random transport of larvae from the Banya Sandspit to the North coast of the bay with lower salinity and vice versa.
Although the plankton survey was only conducted in 2016, our results for that particular year evidenced very low larval availability (only 15 positive samples for P. nobilis DNA, ca. 0.05 to 8.5 larvae per m−3), despite that gonadal examination showed the presence of breeding individuals throughout most of the summer (June to August). To our knowledge, this is the first time that larval stages of P. nobilis are quantified using qPCR analysis, so no previous information of natural larval supply is available. Nevertheless, compared to other bivalve species such as the brown mussel Perna perna or the clam Ruditapes decussatus showing larval peaks of hundreds to thousands of larvae per m3 and high rates of recruitment (Chícharo & Chícharo, 2001; Porri et al., 2008), numbers of P. nobilis larvae identified by qPCR were too low. In fact, the number of total bivalve larvae that were visually counted ranged from 0 to a peak of 15,272 individuals per sample (ca. 4,051 per m−3), most of which were other species, as also occurred with settlers found in collectors, including oysters, mussels, and/or clams, some of which are cultivated in the bay. Zone 3 recorded the highest overall numbers of bivalve larvae (ca. 4 and 8-fold those of Zone 1 and 2, respectively) but those peaks did not include P. nobilis larvae, which was more frequent in Zone 1 with high abundance of adults. Temporally, the highest number of positive samples (n = 9) for P. nobilis were detected in late summer when bay temperatures range from ca. 20 to 24°C (similar to early summer), and therefore must have been originated in late August or early September, when breeding individuals were still present. In contrast, deeper populations in the Balearic Islands (> 5 m) have only been observed to spawn from May to July, at water temperatures ranging from approx. 20 to 26°C (Deudero et al., 2017). During the study, larval availability was not estimated in May–June 2016 since the qPCR technique was still under development (Andree et al., 2018), and therefore the presence of an earlier peak could have been overlooked.
Unfortunately, collector devices deployed from beginning of June to the end of September (2016–2018) in different zones outside the sand bar area showed no evidence of P. nobilis settlers. They were, however, effective for other common Mediterranean bivalves, particularly Parvicardium spp. that reached values of from 15 to ca. 500 individuals per collector depending on zone and year. Settlers of P. nobilis are commonly observed in other Mediterranean locations, although with great variability. For instance, an average of 29 and 46 settlers were found in two different types of collectors deployed in the Marine Protected Area of Palma Bay, Balearic Islands (Cabanellas-Reboredo et al., 2009). In the nearby location of Columbretes Islands, settlers have been detected during nine consecutive years, with interannual differences ranging from zero to > 50 individuals per collector (Kersting & García-March, 2017). In particular, a peak of 200 settlers was also found in the collectors deployed at that locality in 2017, even though most adult individuals had presumably died due to infection by H. pinnae (Diego Kurt, pers. comm.). Given that the Columbretes Islands are located only 100 km south from the Ebro Delta in the direction of the main North to South current (Millot & Taupier-Letage, 2005) the Alfacs Bay population could have been a possible source, as also proposed for the Balearic Islands (Wesselmann et al., 2018). However, this long-distance connectivity is possibly occasional, since oocytes appear to have a tendency to sink and the greatest swimming activity occurs during the trochophore stage lasting only 24 h of the overall planktonic period (a minimum of 10 days; Trigos et al., 2018; Prado pers. observ.). Recent research conducted on the family structure of P. nobilis in the Cabrera National Park (Balearic Islands), featuring a comparable size to the Alfacs Bay, demonstrated that individuals (N = 771) formed a single panmictic population, and that the number of connections with other individuals from the same exact location can reach values close to 60% (Nebot et al., 2019). Although no similar research has been conducted in the Alfacs Bay, the more restricted patterns of water circulation compared to open sea sites suggest that self-recruitment could be even higher.
According to the age distribution obtained from the growth record of empty shells, the Alfacs Bay population seems to undergo major recruitment events followed by periods of slow population growth. A first recruitment event would have occurred around 2001, with subsequent slow or no population growth between 2002 and 2006, a moderate recruitment around 2007, and a major recruitment event in 2008. The time lapse between 2002 and 2006, when little or no recruitment was observed, cannot be solely explained by the time necessary for the 2001 recruits to attain maturity (ca. 2 years, Richardson et al., 1999; PPrado pers. observ.) and points to some ongoing unfavorable local factors. This is coherent with patterns observed in Fangar Bay (North Ebro Delta), where the population (not affected by mass mortality) is entirely comprised by individuals of ca. 37–38 cm total length (Prado, pers. observ.), suggesting that they were originated during a short window period of favorable local conditions by larvae arriving from elsewhere. In fact, the salinity regime of the Fangar Bay is considerably lower than that in the Alfacs Bay (below 20‰; Camp & Delgado, 1987; Llebot et al., 2011), which may also account for the lack of reproductive success. In the particular case of the Alfacs Bay, given that the population is located south from the Ebro River acting as a main geographical barrier from possible larvae transported by the main North to South circulation along the coast (Millot & Taupier-Letage, 2005), recruitment patterns seem to be due to factors affecting individuals already dwelling within the bay.
Large numbers of juveniles (2.5 to 20.7 indiv. · 100 m−2) from the 2017 recruitment were found in two very shallow areas of a large sand bar running parallel to the Banya Sandspit, suggesting that the geomorphology of the site may act as a physical barrier for local tidal and wind currents possibly involved in the dispersal of larvae. Recruitment in the largest sand bar area (ca. 13 Ha), resulted on juveniles’ exposure to desiccation at low tide, which can shatter survival rates, and account for enhanced abundance of individuals at greater depths (60 to 80 cm of water; Prado et al., 2014). In contrast, in Lake Vouliagmeni (Greece), a location also featuring restricted patterns of water circulation and a large population size of ca. 8,500 individuals, the abundance of juvenile sizes has been reported to be equivalent to that of adults. Interestingly, a clear preference for shallower areas has been indicated, although at greater depth (< 7 m vs. > 7 m, respectively; Katsanevakis, 2005) than in Ebro Delta sites. Yet, the presence of large numbers of adult individuals at greater depths demonstrates that recruitment also occurs outside the sand bar, possibly during exceptional major peaks.
To conclude, temporal recruitment patterns in the P. nobilis population of Alfacs Bay appear to be the result of factors acting on the fecundity of the adult stock and during the phases in the water column. At the individual level, although the total number of samples examined was low, the numerous reproductive anomalies presented, collectively give a negative overall view for the population, which also features reduced life-expectancy (Rouanet et al., 2015). Additionally, simultaneous hermaphroditism is a condition associated to environmental stress (Pekkarinen 1991; Syasina et al., 1996) which might result from local agricultural pollution (Brossa et al., 2005). Nevertheless, given the large population of the bay (Prado et al., 2014) and the extended breeding period observed, detrimental effects of low salinities on larval stages (Lough & Gonor, 1971; Richmond & Woodin, 1996) are proposed as a the most plausible factor accounting for patterns of low larval availability. Since agricultural discharges are extremely variable depending on management by the farmers, this might cause stochastic temporal effects on the survival of larval pools and later recruitment. The remaining larvae may be entrapped and forced to settle in the shallow sand bar areas, where juveniles and adults have a limited chance of survival. These constrictions contrast with predatory bottlenecks reported for benthic stages in other nearby areas such as the Columbretes Islands (Kersting & García-March, 2017) suggesting factors controlling recruitment patterns may also be dependent on local habitat conditions.
References
Andree, K. B., S. Trigos, N. Vicente, N. Carrasco, F. Carella & P. Prado, 2018. Identification of potential recruitment bottlenecks in larval stages of the giant fan mussel Pinna nobilis using specific quantitative PCR. Hydrobiolgia 818: 235–247.
Brossa, L., R. M. Marcé, F. Borrull & E. Pocurull, 2005. Occurrence of twenty-six endocrine-disrupting compounds in environmental water samples from Catalonia, Spain. Environmental Toxicology and Chemistry 24: 261–267.
Butler, A., N. Vicente & B. De Gaulejac, 1993. Ecology of de pterioid bivalves Pinna bicolor Gmelin and P. nobilis L. Marine Life 3: 37–45.
Cabanellas-Reboredo, M., S. Deudero, J. Alós, J. M. Valencia, D. March, I. E. Hendriks & E. Álvarez, 2009. Recruitment of Pinna nobilis (Mollusca: Bivalvia) on artificial structures. Marine Biodiversity Records 2: e126.
Cabanellas-Reboredo, M., M. Vázquez-Luis, B. Mourre, et al., 2019. Tracking the dispersion of a pathogen causing mass mortality in the pen shell Pinna nobilis: a collaborative effort of scientists and citizens. Scientific Repports 9: 13355.
Camp, J. & M. Delgado, 1987. Hidrografía de las bahías del delta del Ebro. Investigaciones Pesqueras 51(3): 351–369.
Carballal, M. J., D. Iglesias, J. Santamarina, B. Ferro-Soto & A. Villalba, 2001. Parasites and pathologic conditions of the cockle Cerastoderma edule populations of the coast of Galicia. Journal of Invertebrate Pathology 78: 87–97.
Carella, F., S. Aceto, G. Pollaro, A. Micccio, N. Carrasco, P. Prado & G. De Vico, 2019. An emerging mycobacterial disease is associated with the silent mass mortality of the Pen shell Pinna nobilis along Tyrrhenian coastline of Italy. Scientific Reports 9: 2725.
Catanese, G., Grau, A., Valencia, J.M., Garcia-March, J.R., Vázquez-Luis, M., Alvarez, E., Deudero, S., Darriba, S., Carballal, M.J., Villalba, A., 2018. Haplosporidium pinnae sp. nov., a haplosporidan parasite associated with mass mortalities of the fan mussel, Pinna nobilis, in the Western Mediterranean Sea. Journal of Invertebrate Pathology 157: 9–24. https://doi.org/10.1016/j.jip.2018.07.006.
Cerralbo, P., M. Espino, M. Grifoll & A. Valle-Levinson, 2019. Subtidal circulation in a microtidal Mediterranean bay. Scientia Marina 82(4): 231–243.
Chícharo, L. & M. A. Chícharo, 2001. A juvenile recruitment prediction model for Ruditapes decussatus (L.) (Bivalvia: Mollusca). Fisheries Research 53: 219–233.
Clarke, K. R. & R. N. Gorley, 2006. PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth.
De Gaulejac, B., 1995. Mise en évidence de l’hermaphrodisme successif à maturation asynchrone de Pinna nobilis (L.) (Bivalia: Pterioidea). Comptes rendus de l’Académie des Sciences, Sèrie 3, Sciences de la Vie 318: 99–103.
De Gaulejac, B., M. Henry & N. Vicente, 1995a. An ultrastructural study of gametogenesis of the marine bivalve Pinna nobilis (Linnaeus 1758) I. Oogenesis. Journal of Molluscan Studies 61: 375–392.
De Gaulejac, B., M. Henry & N. Vicente, 1995b. An ultrastructural study of gametogenesis of the marine bivalve Pinna nobilis (Linnaeus 1758) II. Spermatogenesis. Journal of Molluscan Studies 61: 393–403.
Deudero, S., A. Grau, M. Vázquez-Luis, E. Álvarez, C. Alomar & I. E. Hendriks, 2017. Reproductive investment of the pen shell Pinna nobilis Linnaeus, 1758 in Cabrera National Park (Spain). Mediterranean Marine Science 18: 271–284.
García-March, J. R. & A. Márquez-Aliaga, 2007. Pinna nobilis L., 1758 age determination by internal shell register. Marine Biology 151: 1077–1085.
García-March, J. R., A. M. García-Carrascosa, A. L. Peña Cantero & Y. G. Wang, 2007. Population structure, mortality and growth of Pinna nobilis Linnaeus, 1758 (Mollusca, Bivalvia) at different depths in Moraira bay (Alicante, Western Mediterranean). Marine Biology 150: 861–871.
García-March, J. R., A. Marquez-Aliaga, Y. G. Wang, D. Surge & D. K. Kersting, 2011. Study of Pinna nobilis growth from inner record: how biased are posterior adductor muscle scars estimates? Journal of Experimental Marine Biology and Ecology 407: 337–344.
García-March, J. R., J. Tena-Medialdea, S. Henandis-Caballero, et al., in review. Can we save a marine species affected by a highly-infective-highly-lethal-waterborne disease from extinction? Biological Conservation.
Giese, A. C., 1959. Comparative physiology: annual reproductive cycles of marine invertebrates. Annual Review of Physiology 21(1): 547–576.
Himmelman, J. H., 1975. Phytoplankton as a stimulus for spawning in three marine invertebrates. Journal of Experimental Marine Biology and Ecology 20(2): 199–214.
Katsanevakis, S., 2005. Population ecology of the endangered fan mussel Pinna nobilis, in a marine lake. Endangered Species Research 1: 1–9.
Katsanevakis, S., 2007. Growth and mortality rates of the fan mussel Pinna nobilis in Lake Vouliagmeni (Korinthiakos Gulf, Greece): a generalized additive modelling approach. Marine Biology 152: 1319–1331.
Kersting, D. K. & J. R. García-March, 2017. Long-term assessment of recruitment, early stages and population dynamics of the endangered Mediterranean fan mussel Pinna nobilis in the Columbretes Islands (NW Mediterranean). Marine Environmental Research 130: 282–292.
Köck-Schulmeyer, M., M. L. de Alda, E. Martínez, M. Farré, A. Navarro, A. Ginebreda & D. Barceló, 2010. Pesticides at the Ebro River delta: occurrence and toxicity in water and biota. In Barceló, D. & M. Petrovis (eds), The Ebro River Basin. Springer, Berlin: 259–274.
Llebot, C., J. Solé, M. Delgado, M. Fernández-Tejedor, J. Camp & M. Estrada, 2011. Hydrographical forcing and phytoplankton variability in two semi-enclosed estuarine bays. Journal of Marine Systems 86(3–4): 69–86.
Llebot, C., F. J. Rueda, J. Solé, M. L. Artigas & M. Estrada, 2014. Hydrodynamic states in a wind-driven microtidal estuary (Alfacs Bay). Journal of Sea Research 85: 263–276.
Lough, R. G. & J. J. Gonor, 1971. Early embryonic stages of Adula californiensis (Pelecypoda: Mytilidae) and the effect of temperature and salinity on developmental rate. Marine Biology 8(2): 118–125.
Malchus, N., Sartori, A.F., 2013. The early shell: ontogeny, features, and evolution. In: Treatise Online, Part N, Revised, Vol 1, Chapter 4, pp 1–114.
Millot, C. & I. Taupier-Letage, 2005. Circulation in the Mediterranean sea. The Mediterranean Sea. Springer, Berlin: 29–66.
Nebot, E., M., Vázquez, E., Boissin, C., Peyran, S., Deudero & S. Planes, 2019. Inferred family structure of an endangered species, Pinna nobilis, using molecular analyses: implications of connectivity for conservation. Book of Abstracts, XX Iberian Symposium of Marine Biology (SIEBM). Braga, Portugal, 9–12 September.
Pekkarinen, M., 1991. Notes on the general condition of Mytilus edulis L. of the Southwestern coast of Finland. Bivalve Studies in Finland 1: 20–40.
Pineda, J., F. Porri, V. Starczak & J. Blythe, 2010. Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. Journal of Experimental Marine Bioloy and Ecology 392: 9–21.
Porri, F., T. Jordaan & C. D. McQuaid, 2008. Does cannibalism of larvae by adults affect settlement and connectivity of mussel populations? Estuarine Coastal and Shelf Science 79: 687–693.
Prado, P., 2018. Seagrass epiphytic assemblages are strong indicators of agricultural discharge but weak indicators of host features. Estuarine Coastal and Shelf Science 204: 140–148.
Prado, P., N. Caiola & C. Ibáñez, 2014. Habitat use by a large population of Pinna nobilis in shallow waters. Scientia Marina 78: 555–565.
Richardson, C. A., H. Kennedy, C. M. Duarte, D. P. Kennedy & S. V. Proud, 1999. Age and growth of the fan mussel Pinna nobilis from south-east Spanish Mediterranean seagrass (Posidonia oceanica) meadows. Marine Biology 133: 205–212.
Richardson, C. A., M. Peharda, H. Kennedy, P. Kennedy & V. Onofri, 2004. Age, growth rate and season of recruitment of Pinna nobilis (L) in the Croatian Adriatic determined from Mg: Ca and Sr: Ca shell profiles. Journal of Experimental Marine Biology and Ecology 299(1): 1–16.
Richmond, C. E. & S. A. Woodin, 1996. Short-term fluctuations in salinity: effects on planktonic invertebrate larvae. Marine Ecology Progress Series 133: 167–177.
Rouanet, E., S. Trigos & N. Vicente, 2015. From youth to death of old age: the 50-year story of a Pinna nobilis fan mussel population at Port-Cros Island (Port-Cros National Park, Provence, Mediterranean Sea). Scientific Reports of the Port-Cros National Park 29: 209–222.
Rumrill, S. S., 1990. Natural mortality of marine invertebrate larvae. Ophelia 32(1–2): 163–198.
Syasina, I. G., M. A. Vashchenco, P. M. Zhadan & E. M. Kareseva, 1996. State of the gonads and development of offspring of the scallop Mizuhopecten yessoensis from polluted areas in Peter the Great Bay, the Sea of Japan. Russian Journal of Marine Biology 22: 233–240.
Theodorou, J. A., R. James, I. Tzovenis & C. Hellio, 2015. The recruitment of the endangered fan mussel Pinna nobilis (Linnaeus, 1758) on the ropes of a Mediterranean mussel long line farm. Journal of Shellfish Research 4: 409–414.
Trigos, S., N. Vicente, P. Prado & F. J. Espinós, 2018. Adult spawning and early larval development of the endangered bivalve Pinna nobilis. Aquaculture 483: 102–110.
Wesselmann, M., M. González-Wangüemert, E. A. Serrão, A. H. Engelen, L. Renault, J. R. García-March, C. M. Duarte & I. E. Hendriks, 2018. Genetic and oceanographic tools reveal high population connectivity and diversity in the endangered pen shell Pinna nobilis. Scientific Reports 8(1): 4770.
Whyte, S. K., R. J. Cawthorn & S. E. McGladdery, 1994. Co-infection of bay scallops Argopecten irradians with Perkinsus karlssoni (Apicomplexa, Perkinsea) and an unidentified coccidian parasite. Diseases of Aquatic Organisms 18: 53–62.
Acknowledgements
The authors thank the Zoo Barcelona Foundation for an Antoni Jonch grant 2015. JR García-March and J Tena were funded by the Albert II of Monaco Foundation to develop the age study within the project “The study, protection and possible breeding of the pen shell (P. nobilis) in the Boka Kotorska Bay”. Additional funding was obtained from a 2017 INIA Grant for project EMERGER (E-RTA2015-00004-00-00). We thank Department of Territory and Sustainability for granting us a permit for the collection of P. nobilis (Ref.: SF/030). Environmental factors were obtained from the project “Programa de seguiment de la qualitat de les aigües, mol·luscs i fitoplancton toxic les zones de producció de marics del litoral català de la DGPAM”. IRTA acknowledges support from the CERCA Program from the Catalonian Government. Authors thank Dr. Miguel Alonso García-Amilivia for help with the separation of bivalve larvae, and Pep Cabanes, Lluis Jornet, and Jose Luis Costa for fieldwork assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Manuel Lopes Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prado, P., Andree, K.B., Trigos, S. et al. Breeding, planktonic and settlement factors shape recruitment patterns of one of the last remaining major population of Pinna nobilis within Spanish waters. Hydrobiologia 847, 771–786 (2020). https://doi.org/10.1007/s10750-019-04137-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-04137-5