Abstract
Determining how, when, and why energy allocation occurs based on different life history traits, provides core knowledge for understanding evolution, ecology, and conservation of populations. We assumed that, in seasonal environments, Anodontites trapesialis, a common freshwater mussel in the Pantanal wetland, has to time its maturation, its larvae incubation time, and adjusts its breeding strategy seasonally. From histological analyses of gametes, larval count, and marginal increment of the shell rings, we present information about phenology and growth strategies to investigate the influence of environment and reproductive period on growth. We determined for the first time, asymptotic maximum size and longevity for this mussel. This species is a functional hermaphrodite, with maturation and spawning starting at the end of the flood period, when the water begins to recede and fishes return to the main river channel. The larvae, lasidium in this case, disperse on host fishes at this time. As we predicted, the flood pulse is the main regulatory factor to the growth patterns and reproductive period establishment. The species’ life history traits are discussed in the context of life history theory as adaptive responses to the dynamic balance imposed by the seasonality of the Pantanal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allocation of energy is a key point of discussion in life history theory and has been used to predict how investments in life history traits can co-vary in response to changing environment (Stearns, 1977; Roff, 1992). This concept proposes that an increased performance in one life history trait can increase fitness of individuals but at the expense of another trait(s), i.e., a “tradeoff” (Stearns, 1989). Allocation of energy can be explained by two strategies: (1) the ‘income breeder,’ when the energy assimilated by a breeder is directly and steadily redirected to reproduction and (2) ‘capital breeder,’ when energy is stored for reproduction (capital) and released during ideal or favored conditions. These two strategies were proposed by Stearns in the 1980’s, in order to illustrate that animal life cycles can vary in their dependence on energy storage for reproduction as part of their life history evolution (Varpe et al., 2009), where it is not obvious how traits are favored by evolution in a heterogeneous environment (Kozłowski, 1993).
In unpredictable or seasonal environments, the timing of resource allocation for reproduction has direct consequences for fitness and population dynamics (Sainmont et al., 2014). Seasonality also can drive plasticity in the energy allocation strategies, where individual organisms can shift along a gradient of life history strategies to accommodate a suite of environmental conditions (Baird et al., 1986). Generally, the basic tradeoff between survival and reproduction is one of the most studied, especially with regard to reaction to stress for the parental organism. Reproduction is energetically expensive and can be compromised under stressful conditions; therefore, energy resources devoted to reproduction must occasionally be re-allocated toward an organism’s immediate survival in order to reach higher lifetime fitness (Beukema et al., 2001; Bayne, 2004).
The life history traits of mussels, such as their shell growth, longevity, and fecundity renders them ideal organisms to understand how environmental factors can influence these traits and their energy allocation patterns (Kanasawa & Sato, 2008; Morris & Corkum, 1999; Peharda et al., 2002). Growth is marked by the annual or seasonal deposition of rings in the shells, similar to tree rings, and can provide a lengthy and detailed history of environmental dynamics. Most mussel species have long life spans that typically exceed 20 years and they are very fecund, with thousands of larvae per female (Bauer, 1987; Anthony et al., 2001). Not surprisingly, recent research in North America (Jones & Neves, 2011; Haag & Rypel, 2011; Haag, 2012) has shown that life history diversity of mussels is much greater than previously assumed, encompassing a range of very different strategies even in closely related groups.
Because of the high level of life history variation among mussel species (Haag, 2012), generalizations about their life history strategies are difficult to make. In South America especially, limited information has been produced about mussel life history strategies (Parada et al., 1989, 1990; Beasley et al., 2000, 2005; Callil & Mansur, 2007; Callil et al., 2012) and consequently, even less is known about their energy allocation strategies.
Of the 173 mussel species described from South America (169 native and 5 introduced), 117 species occur in Brazil (Pereira et al., 2014). However, only six species have been documented in the lakes of the Pantanal wetland (Colle & Callil, 2012). The Pantanal is an extremely hydrodynamic ecosystem, driven by a mono-modal flood-pulse caused by excessive precipitation during the rainy season, resulting in periodic sheet-flooding of large, flat inter-fluvial areas, periodic filling of depressions and oxbow lakes, and the lateral inundation of large areas along streams and rivers (Junk et al., 2014). Among the few freshwater mussels that can survive in this complex hydrological system, Anodontites trapesialis (Lamarck, 1829) is an abundant species (Colle & Callil, 2012). It is exclusively a simultaneous hermaphrodite (Callil & Mansur, 2007) with a lasidium larvae type (Bonetto & Ezcurra, 1962). The production of gametes in gonads, incubation of eggs, maturation of lasidium in gills, and release of lasidium can be influenced by this hydrological cycle (Callil et al., 2012).
Assuming that, in seasonal environments, mussels time maturation of their gametes and larvae to adjust their breeding strategy seasonally, we present information about phenology and growth strategies of Anodontites trapesialis to investigate the influence of environment and reproductive period on growth. Thus, the purpose of our study was to investigate how evolutionary factors such as fast growth, short life span and high reproductive effort drive population growth for rapid colonization of habitat to maximize chances of persistence in disturbed and unstable—but highly productive—ecosystems, like the Pantanal.
Materials and methods
Study area
The upper Paraguay River watershed area covers about 496,000 km2 and includes two hydrological systems” headwaters with elevations between 350 and 850 m and the Pantanal floodplain lowlands with less than 150 m elevation. The Pantanal extends around 160,000 km2, 140,000 km2 of which belonging to Brazil, 15,000 km2 to Bolivia, and 5000 km2 to Paraguay (Junk & Nunes da Cunha, 2005). The climate is characterized as tropical sub-humid with high temperatures that vary monthly between 27.4°C in December and 21.4°C in July, attaining an absolute maximum of 40°C. However, short-term ingressions of subpolar air masses can decrease the air temperature to 0°C. Annual rainfall is about 1,250 mm and 80% of precipitation falls between November and April and defines a mono-modal flooding dynamic, which is regionally divided into four distinct periods: wet season (“cheia”), ebb (“vazante”), dry season (“seca”), and flood (“enchente”) (Junk et al., 2014).
The flood pulse is the driving force of Pantanal and the system instability has a strong impact on the distribution, community structure, and population dynamics of many plant and animal species (da Junk et al., 2011). Due to the large impact of flood, biogenic processes and evapotranspiration further modify the chemical characteristics of the water bodies inside the Pantanal. It is one of the most productive ecosystems in the world (Junk et al., 2014), where energy for terrestrial communities is provided by the aquatic phase and in turn, most nutrients that support aquatic productivity come from the terrestrial phase. This seasonal energy exchange occurs because of the space–time dimension, called of ATTZ—aquatic terrestrial transition zone (da Junk et al., 2011). Because of the alternating inundations and harsh dryness, permanent rivers and lakes, and especially the connectivity between them, play important roles for the survival of aquatic biota. Benthic invertebrates show seasonal patterns and adaptations both to local changes (moving littoral) and hydraulic changes due to increased current and particulate organic and inorganic matter input (da Junk et al., 2011). Consequently, long-lived, low-mobility species face the hardest existence in the Pantanal, as they need to survive under severe conditions.
Among 31 species of mussels recorded from the Paraguay River Basin (Pereira et al., 2013), only six species of mussels can survive in this complex hydrological system (Colle & Callil, 2012) of lakes in the Pantanal: Anodontites elongatus (Swainson, 1823), Anodontites trapesialis (Lamarck, 1819), and Mycetopoda siliquosa (Spix, 1827) belonging to Mycetopodidae, Castalia inflata (d’Orbigny, 1835) to Hyriidae, one Sphaeridae—Pisidium sterkianum (Pilsbry, 1897) and the invasive alien species Corbicula fluminea (Müller, 1774) that was sampled once. The three most abundant species are A. trapesialis, A. elongatus, and C. inflata.
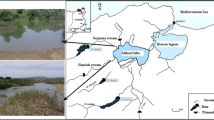
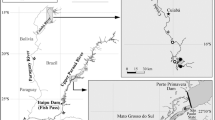
In this study, we investigated two periods separated by eleven years, the first in 1998–1999 and the second in 2010-2011. The samples were performed at Baía do Poço Lake (S 15°54′06,3″ W 56°01′17,0″), an oxbow lake at the border of the Cuiabá River’s alluvial flood plain, a main tributary of Paraguay River, about 45 km from Cuiabá in Mato Grosso State, Midwest of Brazil (Fig. 1). This site represents a shallow lake, where depth varied from 0.15 to 2.5 meters from November to February, and water temperature ranged from 22.1°C in July to 29.4°C in November (mean = 27.4°C). The mean value of pH was 7.2, becoming acidic at 5.8 during the dry season in August and electrical conductivity was stable at ~55 µS cm−3. The organic content of the lake bottom varied from 0.1 g in May to 1.0 g in December for each 10 g of sediment sampled.
Reproduction
In order to evaluate life history traits, approximately 10 mussels of Anodontites trapesialis (see Fig. 2 for the species appearance) were collected monthly throughout year, during both sampling periods. The gametogenic cycle and fecundity were used as attributes of reproduction. Histological analyses and criteria were used to classify of gametogenic stages because this method provides detailed information about the reproductive tissues and allows monitoring of the complete reproductive cycle, including the time of spawning. About 5 mm cubic of the visceral mass of each individual was fixed in Bouin’s solution for 48 h and then washed in 70% ethanol. The tissues samples were dehydrated through a series of graded ethanol baths and infiltrated with paraffin wax, and finally included into wax blocks to be sectioned at 5 µm with a Lieca RM 2125 microtome. The tissue thin-sections were mounted on glass slides and stained with Harris Hematoxylin with Eosin. Through microscopic analyses, individuals were characterized into five stages of gamete development: I—sexual inactivity; II—cell proliferation; III—cell differentiation; IV—maturation and spawning; and V—rest (Fig. 2). These stages (Fig. 3) were validated by quantitative analyses as performed in Callil & Mansur (2007).
The incubation period of A. trapesialis was determined by examining monthly (2010/2011) the frequency of individuals containing eggs and larvae inside of the inner demibranches. To estimate the fecundity, we used the gravimetric method (Hunter, 1985) adapted from Vazzoler (1996). The larvae were separated from tissues of the demibranches by Gilson solution (1:3) baths for 48 h and then transferred to 70% alcohol solution to decant into at graduated test tube. The number of larvae in a subsample of 0.1 ml was counted in a graduated blade under a light microscope and then estimated to total volume. The relationship between fecundity and mussel size was examined by simple linear regression where the number larvae was used as a response variable (dependent) and biometric measures—Lt, H, Wi, in millimeters; Wt, Ws, Wb in grams, and number of growth rings as a predictor variable (independent). We also used a regression analysis to assess the influence of the flood pulse on gametogenic cycle, using depth of water as a predictor and frequency of reproductively active mussels with incubated larvae as response variables.
Biometry and growth parameters
To standardize measurements, the left shell valve was positioned inside a rectangle so that the umbo was aligned with the top edge of the rectangle and the ventral edge of shell was aligned with the base of the rectangle. To fit the growth model, we measured shell length (Lt) in millimeters (mm) as maximum distance from the anterior to posterior margin of the shell (the base of rectangle); height (H, mm) as maximum distance from the umbo to the ventral margin of the shell (the height of rectangle) (Fig. 2). To calculate marginal growth increment (MI), we used the height (mm) of the last and penultimate growth ring to apply the following model: MI = (H − Hi)/(Hi − (Hi−1)) × 100, where Hi is the distance from the umbo to the last ring and Hi−1 is the distance from the umbo to the penultimate ring. Only the continuous growth lines that formed a visibly elevated ring on the periostracum were considered true annuli. We tested if growth rings have a direct relationship with length-at-age using a simple linear regression analysis. A scatter plot was used to determine the formation period of the growth rings.
After the growth ring formation period was assessed to determine age of individuals, the Von Bertalanffy Growth Model (VBGM) was applied to estimate length-at-age using the mathematical model L t = L ∞ [1 − e−k(t−to)] (Bertalanffy, 1938). The L t is the estimated length-at-age t; L ∞ is the asymptotic length or maximum expected length; K is the rate at which maximum length is reached; t is the age, and t 0 is the theoretical age when length is zero. The L ∞ was estimated by the maximum length observed because the parameters of the VBGM are dependent on growth data for all age classes.
Based on the VBGM, we estimated growth parameters using nonlinear mixed effect models (Lindstrom & Bates, 1990). Because individuals were sampled in two different years, we compared two models. The model 1 was used to estimate the growth parameters regardless of the year. In the model 2, growth parameters were estimated considering year sampled using a hierarchical model, where individuals were nested in years. To compare the two models, we used Akaike Information Criterion (AIC) to select the best model based on the lowest AIC value, i.e., the best fit to the length-at-age data (Burnham & Anderson, 2002).
Environmental influences and reproduction on growth
To investigate the influence of environment and reproduction on growth, we tested the variation in marginal increment (MI) as a response variable and season and reproductive activity as predictor variables using a Factorial Analysis of Variance (ANOVA) with permutation tests (Anderson & Ter Braak, 2003). Seasons were defined as Wet (January–March), Ebb (April–June), Dry (July–September), and Flood (October–December). The reproductive activity was categorized by ‘yes’ or ‘no.’ Were qualified ‘yes’, all mussels on cell differentiation (Stage III) and maturation-spawning (Stages IV). We evaluated significant differences in the MI among seasons (wet, ebb, dry, and flood) and reproductive activity. This procedure was applied because outliers were present. We also performed the permutational post hoc Tukey’s test (Higgins, 2004) for pairwise comparisons when significant differences were found. Each test was performed with 10,000 permutations. All analyses and model estimates were performed using program R version 3.0.3 (R Core Team, 2014).
The slides and shells were deposited in the Zoology Collection—Mollusks Section of Federal University of Mato Grosso under the records: Anodontites trapesialis. Brasil, Mato Grosso: (Santo Antônio de Leverger), Cuiabá River, Baia do Poço, 7 ex. V/98, 5 ex. VI/98, 11 ex. VII/98, 9 ex. 31/VIII/98, 11 ex. 25/IX/98, 11 ex. 30/X/1998, 11 ex. XII/98, 19 ex. 24/I/99, 13 ex. II/99, 11 ex. IV/99 Callil, C. T. (Zoological Collection-MOLMT 0075 to 0100).
Results
Reproduction
Gametogenesis was examined for 203 mussels during both sampling periods, 78 from 1998 to 1999 and 125 from 2010 to 2011. The production of gametes occurred exclusively in acinar structures distributed by the median and posterior portion of the visceral mass. Anodontites trapesialis at the Baía do Poço Lake is an exclusively and functional hermaphrodite; all individuals analyzed produce male and female gametes simultaneously (Fig. 3). The development of gametes showed a similar pattern during both periods and therefore was computed in the same dataset. In our sample, 9.85% were sexually inactive (Stage I n = 20); 18.23% in cell proliferation (Stage II n = 37); 13.79% in cell differentiation (Stage III n = 28); 30.54% in mature gametes and spawning (Stage IV n = 62); and 27.54% in rest (Stage V n = 56) (Fig. 4a).
The first signal of gametogenesis occurred slowly, only in a few mussels, when primordial germ cells (oogonia and spermatogonia) started cell proliferation (Stage II) during the Flood season (October to December), after the onset of the rains. When the water level was high during the Wet season (January to March), it was possible to assess the differentiation of spermatogonia to the spermatocytes and the oocytes were typically attached to the inner wall of the acinus, attached at an early stage (Stage III). This initial process occurred in residual gametogenic tissues, since most individuals were still at Rest (Stage V) during the Wet season. The increase of acini and maturation of gametes, occurred when the oocytes became noticeably larger and the radial spermatic series showed evident free sperm on the center or aggregated as spermballs (Stage IV), coinciding with the receding waters of the Ebb season (April to June). Spawning (Stage IV) and hatching of larvae occurred during the Dry season (July to September). The higher frequency of individuals in reproductive activity (Stadium III and IV n = 90) was during the Ebb (n = 51) and Dry (n = 29) seasons, with a smaller number of reproductively active individuals during the Flood period (n = 10), but at a lower frequency (Figs. 4–8).
The frequency of incubated individuals also was observed during the second sampling period (2010/2011). Larvae development and incubation were most frequent during May, when 90% of mussels contained demibranches full of larvae, indicating that after the gamete maturation and spawning period, incubation of larvae begins at the end of the Wet season (Fig. 4b). The smallest individual with larvae incubated was 64.2 mm, and the largest 147.6 mm in length, with individuals between 80 and 100 mm long the most frequently observed with incubated larvae. A. trapesialis is a high fecundity species, with a monthly average (±SD) of 281,634 (±214,916) larvae per individual, with fecundity ranging from 101,468 (±52,290) in May to 341,801 (±166,851) larvae in September. Mussels with high number of larvae had a mean length of 80 mm and the minimum and maximum number of larvae was 2142 larvae in April and 1,092,037 larvae in July.
The linear regression relationship between fecundity to biometrical variables showed a positive trend but a low coefficient of determination (0.33 < r 2 < 0.57) when analyzed for all mussels with larvae incubated. However, this coefficient improves when analyzed for those individuals sampled only in May when 90% were incubated (0.85 < r 2 < 0.92) (Fig. 5).
Relationship between biometrics variables and number of larvae of Anodontites trapesialis in the Baía do Poço Lake, in the Santo Antônio de Leverger, Mato Grosso State, Brazil. The graphs on the right column correspond to the total individuals sampled and those on the left column to the individuals sampled on the peak of reproductive activity
Growth
We measured 312 mussels, 108 from the 1998 to 1999 sampling period and 174 from the 2010 to 2011 sampling period. Length varied from 24.6 to 90 mm during the first period and from 3.8 to 168.1 mm during the second period. The more frequent modal range was recorded for individuals of 100 mm length class in both sampled periods, where histograms showed a bimodal pattern with two frequency peaks. As expected, the number of growth rings and length were highly related (r 2 = 0.873), proving to be good indicators of age and useful in the estimated VBGM parameters (Fig. 6).
Two models were fitted using nonlinear mixed effect models to test growth pattern. Both models yielded very similar estimates for the VBGM growth parameters (model 1: L ∞ = 274.11; K = 0.072; t 0 = −0.67; model 2: L ∞ = 273.07; K = 0.072; t 0 = −0.67). Model 1, which did not consider the effect of sample period (year), had the lowest AIC value (model 1: AIC = 2534; model 2: AIC = 2540) and therefore, model 1 was considered the best model. Due to no difference in growth between the sampling periods, the best growth curve to describe the data (Fig. 7) was the expression: L t = 274.11(1 − e−0.072(t−0.67)).
Environmental influences and tradeoff
The monthly values of marginal increment and reproduction showed a similar tendency related to flood dynamic. The decreasing water depth was associated with an increase in the marginal increment, observed in March and from July to October, in the Dry season. During this period, mussels also were reproductively active and exhibited a lower growth rate during the months of higher frequency of individuals in reproduction, especially from April to June in the Ebb Season (Figs. 8a, 9). The Factorial ANOVA detected differences in the marginal increment data in each season, but this was reproductive activity-independent (season x reproductive activity interaction: F3; 166 = 1.675; P value = 0.174).
Absolute frequency of mussels in Reproductive Activity (Stages III and IV) during seasons of Baia do Poço Lake, Pantanal—a and b Boxplot of seasonal variation in marginal increment (MI). Horizontal line within the box = median; boundaries of the box = first and third quartiles (interquartile range IQR); bars denote the lower (first quartile − 1.5 × IQR) and upper (third quartile + 1.5 × IQR) inner fences; circles outliers
When tested pair to pair, the relationship between reproduction and growth despite being visually perceptible, there was no significant difference in MI found between reproductive activity (F; 166 = 2.90; P value = 0.091). However, MI was different among season (F3; 166 = 3.287; P value = 0.022). Specifically, during the Dry season, when there was a higher marginal growth increment and it was statistically different from the Wet season (P value = 0.027) and from the Ebb season (P value = 0.013). No difference was found between Dry and Flood seasons (P value = 0.842) (Figs. 8b, 9).
Discussion
Reproduction
The reproduction of Anodontites trapesialis was previously described using gametogenic processes (Callil & Mansur, 2007) and incubation period (Callil et al., 2012). Since those studies, we suspected that the reproductive dynamic was related to the depth of the water. However, only now, from this study it was possible to demonstrate that gamete production, incubation of larvae, and fecundity were linked and that the flood pulse acted as a trigger for reproduction.
During the cell proliferation and differentiation stages (Stages II and III), we frequently observed sperm morulae developing into sperms balls, characterizing this species as a spermatozeugmata (Coe, 1943; Callil & Mansur, 2007). Several functions are attributed to spermatozeugmata. They are bigger and more resistant than isolated sperm, they concentrate lipids inside the ball as an energetic resource, and the flagella moves synchronously to improve locomotion—all of these traits make spermatozeugmata an important adaptation to increasing fertilization success (Haag, 2012).
The lasidium of A. trapesialis is a small larvae (<100 microns), typical of the Mycetopodidae. It is incubated in the central part of the inner demibranch, characterized by whitish colored intumescences of the gill filaments. In this study, fully gravid females of A. trapesialis contained >300,000 lasidium. Defined as fecundity, the number of larvae inside of gills also can reflect a species life history strategy (Bauer, 1998; Haag, 2012). This level of fecundity agrees with the general finding that freshwater mussels, as a group, have been shown to have uniformly high fecundity (>200,000) to compensate for the low chances of larvae encountering hosts (McMahon & Bogan, 2001). However, these values can range from 725 (e.g., Castalia ambigua, Beasley et al., 2005) to more than 500,000 (e.g., Actinonaias, Haag, 2012). Although fecundity differs among phylogenetic groups, it is clear that large differences occur among species, within species and between sites, suggesting that this trait is responsive to both evolutionary and environmental factors.
Fecundity is strongly correlated with body size and age, generally increasing with both traits (Haag, 2012). The relationship among biometric variables, such as length, mass or shell volume is typically best described by a power function where r 2 typically is >0.95 (Haag, 2013). In our study, the relationships among these variables were lower than 0.95, where r2 varied on two scales: (1) the first scale was when we considered all incubated individuals sampled throughout 2010 and 2011 (0.32 ≤ r 2 ≤ 0.57), and (2) the other scale of variation was only for May, when all of sampled mussels were incubated (0.85 ≤ r 2 ≤ 0.92). These variations can be related to the time gradient that the samples were collected, or even by differences in energetic status among females and may be substantial in some populations (Bauer, 1998). The most fecund individuals were represented by the 80 mm to 100 mm length class, but mean fecundity in this length class was lower when compared with the longer individuals. Despite the higher frequency of smaller and younger individuals (3–5 growth rings) in reproductive activity and fecundity, the absolute number of eggs and larvae produced were higher in larger individuals (6–8 growth rings). The relationship between fecundity and age appeared to be more variable than fecundity and length, especially once fecundity decreases with age due to reproductive senescence (Bauer, 1987; Haag & Stanton, 2003).
Growth
The growth rings, formed ring by ring from the mantle edge in mussels shells (Callil & Mansur, 2005), can provide lengthy and detailed growth histories. Shell growth rings are formed in part by inherent differences in the metabolism and growth rate of species and also by environmental influences such as temperature or food availability. For many aquatic species, growth is highly seasonal and does not necessarily proceed at the same rate throughout the year (Haag, 2012). The growth parameter K (0.072) and the maximum asymptotic length (274.11 mm) suggest that individuals are growing faster when they are smaller and gradually their growth slows, when length reaches its maximum. Because of the intense productivity and higher availability of food resources in the Pantanal, the annual growth rings are more evenly spaced and relatively difficult to detect. This is one reason why age-related techniques can be less useful in tropical regions than in temperate or boreal systems (Haddon, 2001).
Patterns of energy investment in growth differ greatly among mussel species. Similar to how A. trapesialis can reach 10 cm in just 5 years, an ecological equivalent North American species Leptodea fragilis (Rafinesque, 1820) (papershell) and Pyganodon grandis (Say, 1829) (giant floater) share the same growth pattern in that they can grow fast and to a large size in just a few years (Haag, 2012). On the other hand, the relationship between life span and the rate at which species approach their growth asymptote is strong. As observed in A. trapesialis, life span (10 years) seems to show a negative correlation with K = 0.072. The literature for North hemisphere species reports a range of K from 0.02 to 1.01 (Bauer, 1992; Haag & Rypel, 2011; Jones et al., 2012), generally indicating a rapid attainment to the growth asymptote. This higher growth rate can be interpreted as representing earlier and greater investment in growth (Haag & Rypel, 2011). As highlighted by Jones et al. (2012), mussel species expressing life history traits with short lifespan and high somatic growth may warrant special conservation consideration because they are likely to have higher natural mortality and a reduced capacity to withstand long-term impacts, despite the possible advantages of having higher somatic and population growth rates.
Influence of flood pulse on reproduction and growth
The Pantanal is a system where the energy flow and nutrient cycling depend on a permanent interchange (Junk et al., 1989). During the Ebb season (April to June), the water recedes and the autochthonous processes increase nutrients leading to higher densities of algae (Marçal & Loverde-Oliveira, 2015). In this season, individuals of A. trapesialis in populations are maturing their gametes, the oocytes become larger, and in the radial spermatic series, sperm begins differentiation (Stage III), precisely when a large amount of energy is needed for vitellogenesis. The higher phytoplankton concentrations occur in the Dry season (July to September), with an average of 5.5 mg fresh weight l−1 (Loverde-Oliveira & Huszar, 2007), when mussels were spawning (Stage IV) and incubating their larvae in the demibranches. On the other hand, during the Flood season (October–December) and Wet Season (January–March), the concentration of nutrients and organic matter is diluted and consequently, the density of phytoplankton is lower (Loverde-Oliveira et al., 2009). At this time, the acini were flaccid and showed only a few remaining gametes (Stage V), the visceral mass was empty and there was no reproductive activity (Stage I). Hence, the flood period in the Pantanal seems to be a stressful time for A. trapesialis. However, during the entire flood pulse (flood season), fish enter the floodplain, occupy the shallow lakes to feed, and reproduce, and when the floodwaters begin to recede (Ebb), the wetlands start to dry and the fish migrate back to main river channel (Fernandes et al., 2014). This synchronicity is necessary to ensure that mussel larvae interact effectively with host fishes to assure their dispersion and to maintain viable populations over a broad geographical scale (Fig. 10).
In the Pantanal, the flood pulse acts on reproduction as main environmental factor. This situation differs from several studies conducted in temperate or subtropical regions (Bauer, 1998; Vaughn & Taylor, 2000; Haag, 2012) which attribute temperature as the primary factor controlling reproduction (Bayne, 2004; Beukema & Dekker, 2005; Beukema et al., 2009). However, in tropical regions, temperature does not fluctuate greatly; therefore, it is unlikely that it serves as the primary factor influencing reproduction. In the Northern Pantanal, a slight variation of temperature matches the flood and ebb period, suggesting that the extended time of gamete maturation of A. trapesialis is related to a higher reproductive investment as an adaptive response. To facilitate better recruitment, mussels would ideally reproduce when temperature (Parada et al., 1989; Avelar & Mendonça, 1998), water level (Beasley et al., 2000), and availability of the host fishes (Callil et al., 2012; Haag, 2012) are working together in synchrony.
Timing of reproduction to a seasonal cycle is a life history adaptation with important consequences for growth. Furthermore, resource allocation also is affected by environmental factors, such as changes in the availability of food resources (Heino & Kaitala, 1999). During this study, A. trapesialis exhibited continuous growth but it was possible to observe MI mean values varying with abundance of food resources The sudden increase of MI in March (Fig. 8) probably was associated with the water level decrease and phytoplankton density increases, reaching absolute values of 11.6 mg l−1 (Loverde-Oliveira & Huszar 2007).
Until now, we have only emphasized the reproductive behavior and growth pattern of A. trapesialis but this was reproductive activity-independent, leading us to accept that the tradeoff between growth and reproduction was not demonstrated in our study. However, subtle differences may exist that are worth noting. The level of significance (P value = 0.091) between reproduction and growth (pair to pair) was close to the 95% confidence limit. Joining reproduction and growth events, we can see that in March, the start of gamete maturation was marked by the highest mean value of marginal growth increase, when both events were induced by the increase of phytoplankton density. From April to July when the water recedes in the Pantanal, the phytoplankton becomes more concentrated (Loverde-Oliveira et al., 2009), overlapping with an increase in frequency of mussels in the maturation and spawning stages. May was the critical point of growth, when the lowest value of marginal growth increment was recorded, this is when most individuals were mature, spawning, and 90% of mussels had incubated larvae (Fig. 8).
While it is evident that adjustment of energy allocation to reproduction and growth is an integrated response to variation in resource availability in the Pantanal, how much energy allocation can vary to balance these demands with environmental oscillations remains unclear. Assessing the two strategies of allocation: (1) storing energy—”capital breeders,” and (2) use of concurrent intake for a reproduction attempt—”income breeding” (Stearns, 1992; Stephens et al., 2009) is in need of continued testing. However, we believe that A. trapesialis fits better as an “income breeder.” Although as with many dichotomous typologies in ecology, no sharp distinction exists between capital and income breeding; these strategies represent end-points on a continuum (Stephens et al., 2009).
Summary and conclusions
Importantly, although the effect of reproduction on growth was not statistically significant in our study, likely due to high variation in the data, we observed a decrease in MI during the most intense time of reproduction (maturation and spawning). This finding leads us to a basic and important question: are energy resources better spent on reproduction or growth? Some studies suggest that reproduction may take priority at the expense of somatic resources and growth (Jokela & Mutikainen, 1995). The costs of reproduction and the optimal allocation of energy are central issues in life history and evolutionary theory (Stearns, 1992), but also for understanding how populations respond to environmental conditions. As previously stated, A. trapesialis has fast growth and high fecundity, traits likely adapted to a dynamic tropical wetland system. Generally, the emergence of these traits has been viewed as a result of an adaptive process in the evolutionary history of the species; but we must also consider that on a short temporal scale that food resource availability coupled to environmental conditions (i.e., the flood pulse) drive responses of the population toward allocating energy between maintenance, growth, and reproduction.
References
Anderson, M. & C. Ter Braak, 2003. Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation 73: 85–113.
Anthony, J. L., D. H. Kesler, W. L. Downing & J. A. Downing, 2001. Length-specific growth rates in freshwater mussels (Bivalvia: Unionidae): extreme longevity or generalized growth cessation? Freshwater Biology 46: 1349–1359.
Avelar, W. & S. Mendonça, 1998. Aspects of gametogenesis of Diplodon rotundus gratus (Wagner 1827) (Bivalvia: Hyriidae) in Brazil. American Malacological Bulletin 14: 157–163.
Baird, D. G., L. R. Linton & R. W. Davies, 1986. Life-history evolution and post-reproductive mortality risk. Journal of Animal Ecology 55: 295–302.
Bauer, G., 1987. Reproductive strategy of the freshwater pearl mussel Margaritifera margaritifera. Journal of Animal Ecology 56: 691–704.
Bauer, G., 1992. Variation in the life span and size of the freshwater pearl mussel. Journal of Animal Ecology 61: 425–436.
Bauer, G., 1998. Allocation policy of female freshwater pearl mussels. Oecologia 117: 90–94.
Bayne, B. L., 2004. Phenotypic flexibility and physiological tradeoffs in the feeding and growth of marine bivalve molluscs. Integrative and Comparative Biology 44: 425–432.
Beasley, C. R., E. Tùry, W. G. Vale & C. H. Tagliaro, 2000. Reproductive cycle, management and conservation of Paxyodon syrmatophorus (Bivalvia: Hyriidae) from Tocantins River, Brazil. Journal of Molluscan Studies 66: 396–402.
Beasley, C. R., L. de Quadros Miranda, S. T. Alves, A. G. Melo, J. O. Souza & C. H. Tagliaro, 2005. Brood size and larval length of Paxyodon syrmatophorus (Bivalvia, Hyriidae) from the Tocantins River, Brazil. Amazoniana 18: 173–184.
Bertalanffy, L. Von, 1938. A quantitative theory of organic growth (inquiries on growth laws II). Human Biology 10: 181–213.
Beukema, J. J. & R. Dekker, 2005. Decline of recruitment success in cockles and other bivalves in the Wadden Sea: possible role of climate change, predation on postlarvae and fisheries. Marine Ecology Progress Series 287: 149–167.
Beukema, J. J., R. Dekker, K. Essink, & H. Michaelis, 2001. Synchronized reproductive success of the main bivalve species in the Wadden Sea: causes and consequences. Marine Ecology Progress Series 211: 143–155.
Beukema, J. J., R. Dekker & J. M. Jansen, 2009. Some like it cold: populations of the tellinid bivalve Macoma balthica (L.) suffer in various ways from a warming climate. Marine Ecology 384: 135–145.
Burnham, K. P. & D. R. Anderson, 2002. Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York: 488.
Bonetto, A. A. & I. D. Ezcurra, 1962. El desarrollo del lasidium of Anodontites trapesialis forbesianus (Lea) (Mollusca, Lamellibranchiata). Physis 23: 195–220.
Callil, C. T. & M. C. D. Mansur, 2005. Ultrastructural analysis of the shells of Anodontites trapesialis (Lamarck) and Anodontites elongatus (Swainson) (Mollusca, Bivalvia, Etherioidea) from the Mato Grosso Pantanal Region, Brazil. Revista Brasileira de Zoologia 22: 724–734.
Callil, C. T. & M. C. D. Mansur, 2007. Gametogênese e dinâmica da reprodução de Anodontites trapesialis (Lamack) (Unionoida, Mycetopodidae) no lago Baia do Poço, planície de inundação do rio Cuiabá, Mato Grosso, Brasil. Revista Brasileira de Zoologia 24: 825–840.
Callil, C. T., D. Krinski & F. A. Silva, 2012. Variations on the larval incubation of Anodontites trapesialis (Unionoida, Mycetopodidae): Synergistic effect of the environmental factors and host availability. Brazilian Journal of Biology 72: 1–8.
Coe, W. R., 1943. Sexual differentiation in mollusks. I. Pelecypods. The Quarterly Review of Biology 194: 154–164.
Colle, A. C. & C. T. Callil, 2012. Environmental influences on the composition and structure of the freshwater mussels in shallow lakes in the Cuiabá River floodplain. Brazilian Journal of Biology 72: 249–256.
da Silva Junk, W. J. C. J., C. Nunes da Cunha & K. M. Wantzen, 2011. The Pantanal: Ecology, biodiversity and sustainable management of a large neotropical seasonal wetland, 1st ed. Pensoft, Sofia: 857.
Fernandes, I. M., R. Henriques-Silva, J. Penha, J. Zuanon & P. R. Peres-Neto, 2014. Spatiotemporal dynamics in a seasonal metacommunity structure is predictable: the case of floodplain-fish communities. Ecography 37: 464–475.
Haag, W. R., 2012. North American freshwater mussels: Natural history. Ecology and Conservation, Cambridge.
Haag, W. R., 2013. The role of fecundity and reproductive effort in defining life-history strategies of North American freshwater mussels. Biological Reviews 88: 745–766.
Haag, W. R. & J. Stanton, 2003. Variation in fecundity and other reproductive traits in freshwater mussels. Freshwater Biology 48: 2118–2130.
Haag, W. R. & A. L. Rypel, 2011. Growth and longevity in freshwater mussels: Evolutionary and conservation implications. Biological Reviews 86: 225–247.
Haddon, M., 2001. Modelling and Quantitative Methods in Fisheries. Chapman and Hall, Boca Raton.
Heino, M. & V. Kaitala, 1999. Evolution of resource allocation between growth and reproduction in animals with indeterminate growth. Journal of Evolutionary Biology 12: 423–429.
Higgins, J., 2004. Introduction to Modern Nonparametric Statistics. Brooks Cole Cengage Learning, Belmont: 384.
Hunter, J. R., 1985. Preservation on Northern anchovy in formaldehyde solution. In: Lasker, R. (ed.) An egg production method for estimating spawning biomass of pelagic fish: Application to the Northernanchovy, Engraulis mordax. U.S. Dep. Comm., NOAA Tech Rep., 36: 63–66.
Jokela, J. & P. Mutikainen, 1995. Phenotypic plasticity and priority rules for energy allocation in a freshwater clam: A field experiment. Oecologia 104: 122–132.
Jones, J. W. & R. J. Neves, 2011. Influence of life-history variation on demographic responses of three freshwater mussel species (Bivalvia: Unionidae) in the Clinch River, USA. Aquatic Conservation Marine and Freshwater Ecosystems 21: 57–73.
Jones, J. W., R. J. Neves & E. M. Hallerman, 2012. Population performance criteria to evaluate reintroduction and recovery of two endangered mussel species, Epioblasma brevidens and Epioblasma capsaeformis (Bivalvia: Unionidae). Walkerana, Journal of the Freshwater Mollusk Conservation Society 35: 27–44.
Junk, W. J. & C. Nunes da Cunha, 2005. Pantanal: A large South American wetland at a crossroads. Ecological Engineering 24: 391–401.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplain-systems. Canadian Special Publications for Fisheries and Aquatic Sciences 106: 110–127.
Junk, W. J., M. T. F. Piedade, R. Lourival, F. Wittmann, P. Kandus, L. D. Lacerda, R. L. Bozelli, F. A. Esteves, C. Nunes da Cunha, L. Maltchik, J. Schöngart, Y. Schaeffer-Novelli & A. A. Agostinho, 2014. Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquatic Conservation 24: 5–22.
Kanasawa, T. & S. Sato, 2008. Environmental and physiological controls on shell microgrowth pattern of Ruditapes philippinarum (Bivalvia, Veneroidae) from Japan. Journal of Molluscan Studies 74: 89–95.
Kozłowski, J., 1993. Measuring fitness in life-history studies. Trends in Ecological and Evolution 8: 84–85.
Lindstrom, M., & D. Bates, 1990. Nonlinear mixed effects models for repeated measures data. Biometrics: 673–687.
Loverde-Oliveira, S. M. & V. L. M. Huszar, 2007. Phytoplankton ecological responses to the flood pulse in a Pantanal lake, central Brazil. Acta Limnologica Brasiliensia 19: 117–130.
Loverde-Oliveira, S., V. L. M. Huszar, N. Mazzeo & M. Scheffer, 2009. Hydrology-driven regime shifts in a shallow tropical lake. Ecosystems 12: 807–819.
McMahon, R. F. & A. E. Bogan, 2001. Mollusca Bivalvia. In Thorp, J. H. & A. P. Covich (eds.), Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego.
Marçal, S. F. & S. Loverde-Oliveira, 2015. Phytoplankton in Coqueiro Lake (Pantanal de Poconé, Mato Grosso, Brazil). Biotemas 28: 9–25.
Morris, T. & L. D. Corkum, 1999. Unionid growth patterns in rivers of differing riparian vegetation. Freshwater Biology 42: 59–68.
Parada, E., S. Peredo, G. Lara & I. Valdebenito, 1989. Growth, age and life span of the freshwater mussel Diplodon chilensis (Gray, 1828). Archiv fur Hidrobiologie 115: 563–573.
Parada, E., S. Peredo & C. Gallardo, 1990. Tácticas reproductivas y dinámica poblacional de Diplodon chilensis (Gray, 1828) (Bivalvia: Hyriidae). Revista Chilena de História Natural 63: 23–35.
Peharda, M., C. A. Richardson, V. Onofri, A. Bratos & M. Crncevic, 2002. Age and growth of the bivalve Arca noae L. in the Croatian Adriatic Sea. Journal of Molluscan Studies 68: 307–310.
Penha, J., L. A. F. Mateus & J. Lobón-Cerviá, 2015. Population regulation in a neotropical seasonal wetland fish. Environmental Biology of Fishes 98: 1023–1034.
Pereira, D., M. C. D. Mansur, L. D. S. Duarte, A. S. Oliveira, D. M. Pimpão, C. T. Callil, C. Ituarte, E. Parada, S. Paredo, G. Darrigran, F. Sacarabino, C. Clavijo, L. Gladys, I. C. Miyahira, M. T. R. Rodriguez & C. Lasso, 2014. Bivalve distribution in hydrographic regions in South America: historical overview and conservation. Hydrobiologia 735: 15–44.
Roff, D. A., 1992. The Evolution of Life Histories: Theory and Analysis. Chapman and Hall, New York.
Sainmont, J., K. H. Andersen, Ø. Varpe & A. W. Visser, 2014. Capital versus income breeding in a seasonal environment. The American Naturalist 184: 466–476.
Stearns, S. C., 1977. The evolution of life history traits: A critique of the theory and a review of the data. Annual Reviews in Ecology and Systematics 8: 141–171.
Stearns, S. C., 1989. Trade-offs in life-history evolution. Functional Ecology 3: 259–268.
Stearns, S. C., 1992. The evolution of life histories. Oxford: Oxford University Press.
Stephens, P. A., I. L. Boyd, J. M. McNamara & A. I. Houston, 2009. Capital breeding and income breeding: Their meaning, measurement, and worth. Ecology 90: 2057–2067.
Varpe, Ø., C. Jørgensen, G. A. Tarling & Ø. Fiksen, 2009. The adaptive value of energy storage and capital breeding in seasonal environments. Oikos 118: 363–370.
Vaughn, C. C. & C. M. Taylor, 2000. Macroecology of a host-parasite relationship. Ecography 23: 11–20.
Vazzoler, A. E. A. M., 1996. Biologia da Reprodução de Peixes Teleósteos: Teoria e Prática. Editora da Universidade Estadual do Maringá.
Acknowledgements
We are grateful to CNPq Process No. 246223/2012-0 for the support provided during the post-doctoral and sabbatical period of C. Callil. Our thanks are also directed to the Institute of Biosciences and to the Graduate Program in Water Resources of UFMT for the financial support and logistics. We also are indebted to Manuel Lopes Lima, who invited and encouraged us to present these results at the II International Meeting of Biology and Freshwater Conservation of Bivalves, Buffalo, New York, U.S.A. Our many thanks to Professor Felipe Franco Curcio, from PPG of Zoology - UFMT, who reviewed the manuscript and made suggestions to improve it. The views expressed in this article are of the authors and do not necessarily represent those of the United States Fish and Wildlife.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Manuel P. M. Lopes-Lima, Ronaldo G. Sousa, Lyuba E. Burlakova, Alexander Y. Karatayev & Knut Mehler / Ecology and Conservation of Freshwater Bivalves
Rights and permissions
About this article
Cite this article
Callil, C.T., Leite, M.C.S., Mateus, L.A.F. et al. Influence of the flood pulse on reproduction and growth of Anodontites trapesialis (Lamarck, 1819) (Bivalvia: Mycetopodidae) in the Pantanal wetland, Brazil. Hydrobiologia 810, 433–448 (2018). https://doi.org/10.1007/s10750-017-3097-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3097-3