Abstract
The age of the Mediterranean endemic Bivalve mollusc Pinna nobilis is estimated using the total size and the number and position of posterior adductor muscle scars (PAMS): a series of straight lines and rings observed in the dorsal nacre-lobe. Nevertheless, the position of PAMS of adult individuals inhabiting the same population and depth is highly variable. To assess the source of this variability, the shells of nine individuals were cut into radial sections along PAMS and the inner register was studied. The cuts showed clear marks of the inner register formed around the PAMS composed by a short nacre tongue introduced within the calcitic layer and two calcitic strips of differing colour (bright and dark). The three components are directed towards the posterior of the shell. The marks of the inner register coincide only with the straight lines of PAMS, but are observed in an anterior position further than the straight lines and rings, and in a posterior position further than the rings. Also, some of the most posterior marks can be observed one under the other in the oldest individuals. The existence of the inner register’s marks in a position further anterior than the straight lines and rings implies that a variable number of PAMS are obscured beneath nacreous deposits. Eventually, we advise against the utilization of PAMS to study P. nobilis age. We instead recommend the use of the inner register as it records more accurately the life history of the individuals, and data indicates that each mark is formed annually.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The determination of a species’ growth rate requires the establishment of a relationship between a measurable dimension of the individual and its age. The knowledge of the temporal change of that dimension permits the development of growth curves. The most delicate step is the identification of a suitable structure and the accurate establishment of its relationship with the age of the animal (Margaleff 1998). Depending on the species, growing individuals in controlled conditions can be expensive and slow. Also, the generalization of results from manipulative experiments may be constrained by their artificial nature (Quinn and Keough 2004). Sclerochronology has proven to be a very useful technique to assess individual age and population growth rates (Francillon-Vieillot et al. 1990) when generalization of in vitro growth studies is not straightforward.
The fan mussel Pinna nobilis L., 1758 is a Mediterranean endemic included in Annex II of Habitats Directive and Annex IV of Barcelona Convention. P. nobilis may live more than 37 years (http://www.pinnanobilis.free.fr) and can measure more than 100 cm (Zavodnik et al. 1991). Typical densities of individuals are around 1–10 individual/100 m2 (Vicente 1990; Zavodnik 1991; García-March 2006) and large underwater areas have to be explored to find enough specimens to study their growth in situ. Owing to the fact that the shell of P. nobilis has no external bands or marks to correlate with age, the best approximation is the relationship between total size and age of the individuals. However, different approximations may be necessary in the same population, for growth rates change markedly according to the depth of the habitat (García-March et al. 2006). This multiplies the number of surveys required to gather the data for the application of modal class progression analysis [e.g. Bhattacharya’s (1967) method]. The use of the unbiased repeated-mark-recapture methods (Wang 2004) reduces the number of individuals required to study the growth rates of the population, but the specimens have to be marked and inventoried in situ and long periods of research are necessary.
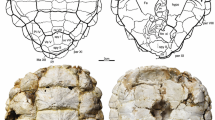
In order to ease the study of growth rates in P. nobilis, previous authors focused their efforts on the search for one feature of empty shells to be correlated with age. This key feature was the posterior adductor muscle scars (PAMS): a series of straight lines (SL) and rings (R) were observed in the dorsal nacre-lobe, in the inner side of the valves (Fig. 1). Important advances were achieved with the establishment of a linear relationship between the position of PAMS and total shell-size (Ht) (Moreteau and Vicente 1980). Recently, the seasonal temperature changes of occidental Mediterranean were correlated with regular periods in the proportions of stable oxygen isotopes recorded in the external calcitic shell-layer of P. nobilis (Richardson et al. 1999). The minimum temperatures recorded from the δ18O: δ16O proportions of the shell usually occur around one internal PAMS and the authors concluded that each PAMS was deposited annually. Having the relationships of PAMS with total size and PAMS with age, growth curves could be calculated using length-at-age data (but see Richardson et al. 1999, 2004; Kennedy et al. 2001).
Dorsal nacre lobe of the right valve of Pinna nobilis showing posterior adductor muscle scars (PAMS). Black arrows mark the position of the straight lines (SL), white arrows mark the position of the rings (R). Note that in the most posterior part of the dorsal nacre lobe it is difficult to distinguish between the R and the SL. Scale bar 20 mm
However, during the study of growth rates of a western Mediterranean population of P. nobilis located in Moraira (Spain), the existence of important variability in the position of PAMS among individuals was observed. Especially striking was the observation that young and adult individuals’ PAMS positions were uncorrelated. For this reason we have undertaken research to validate the utility of PAMS to approximate P. nobilis age. In this work we describe how to count the obscured PAMS and we explain the causes of the mismatch between young and adult individuals’ PAMS positions. Also, a new method to study P. nobilis age from the internal register (IR) is presented and compared with the age estimations obtained using previous methodologies.
Materials and methods
The distances of the PAMS to the umbo of 24 empty valves gathered during the surveys performed by García-March et al. (2006) in the same population and depth (12–14 m) were compared (Table 1). These shells were also used to relate the positions of the PAMS to Ht using linear regression analysis. Coefficient of determination between these two variables was also calculated. Two of these specimens, and another seven empty shells or anterior parts of shells from other populations, representing a wide-ranging of sizes (Table 2), were imbibed in polyester resin (STRATIL AL-100), cut into radial section along the PAMS with a power saw, and polished to 1,200 μ with carborundum. The whole surface of the radial cut of one specimen (identified as 1M in Table 2) was mounted in thin sections to carry out microstructural analysis. The polished surfaces of radial cuts as well as thin sheets were studied using magnifying binocular lens and optical microscopy. The positions of the PAMS were compared with those of the IR observed in the cuts.
Results
The positions of the oldest and newest PAMS of individuals in Table 1 are highly variable. For the oldest PAMS, positions fluctuate between 12 and 19.1 cm (\( \overline {\text{Y}} \) = 15.9, SD=2.1) showing neither a linear trend nor a constant value between Ht and mark position (P value ≫ 0.05; Table 1, Fig. 2). On the other hand, for the newest PAMS, positions fluctuate between 19.9 and 28.8 cm (\( \overline {\text{Y}} \) = 24.5, SD = 2.5) showing a linear relationship with Ht. Both variables follow a Gaussian distribution, so no data transformation is necessary to improve normality. Regressing Ht as dependent variable and the positions of the newest PAMS as predictor variable, it is possible to obtain a predictive equation:
Scatterplot of the position of the oldest posterior adductor muscle scars (PAMS) and total size (Ht) of individuals of Table 2. There is no linear relationship between these two variables (P value ≫ 0.05). Note that the positions of the oldest PAMS neither show a constant value, indicating that a variable number of PAMS might be unregistered in each individual
The coefficient of determination (R2) indicates that there is an important part of the variability in Ht unexplained by its linear relationship with the PAMS. Understanding the causes of this low correlation, as well as the causes of the variability in the position of the oldest PAMS, is key to accurately estimating the age of P. nobilis from empty shells.
Age estimation from posterior adductor muscle scars
Using Eq. 1, Ht corresponding to the oldest observable PAMS for each individual of Table 1 was calculated. Hitherto, it was considered that this Ht would correspond to the size after the first or second year of life (Richardson et al. 1999, 2004; Kennedy et al. 2001). Calculated Ht corresponding to the maximum and minimum values of the oldest PAMS in Table 1 (belonging to individuals 17M and 29) are 41.5 and 28.3 cm, respectively. Therefore, these sizes would correspond to the extreme values, smallest and largest Ht, when the first or second PAMS were deposited. Also, the Ht corresponding to the mean position of the oldest PAMS is 35.6 cm. Ages for these three Ht can be found using the Von Bertalanffy growth equation calculated from real growth data for the same population and depth [Ht = 56.0345(1−e−0.1652t)] (García-March et al. 2006). After finding t in the latter equation, these three values of Ht would correspond to ages around 4.3, 6.1 and 8.2 years (in ascending order of Ht). This new data does not agree with previous ideas, and indicates that more than one or two PAMS might be obscured or not registered in the anterior portion of the shell.
Posterior adductor muscle scars and internal register
The radial cuts along the PAMS showed clear marks of the IR (Fig. 3). These are formed in the limit between the external calcitic and the internal nacreous layer and consist of (1) a short tongue of nacre situated within the calcite prisms and directed towards the posterior of the shell, and (2) two strips of calcite prisms, one starting from the tongue and the other from the top of the tongue, both of them directed towards the posterior. The latter two are distinguished under normal light by their different colour (bright and dark). The nacre tongue is progressively less conspicuous in the oldest signals (anterior in position). At the most anterior part of the shell, the marks of the IR are only shown by the calcitic strips (Fig. 4). As observed by J. R. García-March et al. (in press), the myostracum is buried beneath nacreous deposits in the anterior portion of the shell, confirming that the nacre covers progressively the old PAMS (Fig. 5).
Thin sheet of the inner register (IR) of Pinna nobilis at the level of posterior adductor muscle scars (PAMS). Arrows signal the nacre tongues. c calcite. n nacre. s dark and bright calcite stripes. The question marks signal the position of doubtful nacre tongues or stripes. Note how at the most posterior part (right of the figure) some nacre tongues are deposited one under the other. The two last nacre tongues are behind the previous one, which means that a slight retreat of the nacre layer has occurred. Scale bar 1 mm
We have observed that the IR marks and the R do not correlate either in number or position. Only a variable number of the oldest R (those in more anterior portion of the shell) are observed at small regular distances to the marks of the IR, but this becomes an irregular pattern in the posterior part of adult individuals. Also in adult individuals, the marks are extended ahead of the R in both anterior and posterior directions. At the most posterior part of the oldest individuals some marks are sometimes deposited one under the other (Table 3 and Fig. 3). On the other hand, the marks of the IR correlate with all the observable SL because these SL are the only visible traces of the IR. As a consequence, the SL are not part of PAMS, but are formed independently before the posterior adductor muscle arrives to their position. In Table 3 the mismatch in number and positions of the R and the IR as well as the considerable number of obscured marks in adult individuals, shown by the existence of IR anterior to the last R, can be observed. There is also a high number of IR covered by nacre in individual BLT39M. This is probably due to the accelerated migration of the tissues during umbo reconstruction.
The IR marks of both individual 24M and 22M are only formed by the two calcite strips. They match accurately with the SL and are a little displaced with respect to the R, as shown in Table 3. This observation confirms that the two calcite strips without nacre tongue also represent the existence of a SL. Comparing IR marks and the external observable SL of specimens 24M and 22M with those of adult individuals (Table 3), it is noticeable that the oldest observable SL of adult individuals usually have a marked nacre tongue in their IR. What is more, the adults usually have several (3–4) pairs of calcite strips further anterior to the oldest nacre tongue. The myostracum of these strips is buried beneath nacreous deposits and they are unnoticeable in the inner side of the valves (see individuals 1M, 11M, BLT39M, etc. of Table 3). Considering these pairs of calcite stripes homologous to those of individuals 24M and 22M, this means that at least the first 3–4 SL of an adult are completely obscured by nacre, as well as their adjacent R.
Discussion and conclusions
Formation and periodicity of internal register
The structure of the nacre tongue in the IR indicates that it must be generated after a switch in the deposition rate between both calcite and aragonite. Many molluscs change the rate of deposition of calcium carbonate polymorphs seasonally, depending on water temperature or food availability (Taylor et al. 1969; Carter 1980; Cohen and Branch 1992). Dodd (1964) observed nacre tongues directed towards the anterior part of the shell in the IR of Mytilus californianus, and demonstrated that they were generated annually by a seasonal switch in the formation of the polymorphs. Richardson et al. (2004) have indicated that the Adriatic P. nobilis deposit nacre predominantly in winter, whereas Richardson et al. (1999) indicated that the nacre would be formed in spring and early summer. The calcite is layered predominantly between spring and early autumn, in the western Mediterranean (Garcia-March 2006). Assuming a similar trend of deposition of the two polymorphs in both the Adriatic and the western Mediterranean, we suggest that each mark of the IR shows a switch in the mineral deposition, predominantly nacre between winter and early summer and calcite between summer and early autumn. Therefore, it would indicate 1 year of age. Also, we suppose that the patterns found by Richardson et al. (1999) based in the proportions of δ18O: δ16O isotopes of the shell calcite layer might be signalling the position of our proposed SL instead of the position of the rings referred by the previous authors; they worked with young and middle-aged individuals, where the positions of the R and the SL are still correlated.
We agree with the observation by Richardson et al. (1999) that the first year of life is not registered. In all of our specimens there is a considerable part of calcite before the IR last mark. This calcite portion is usually lost in the most anterior portion of the adult individuals shell. Consequently, we propose that 1 year should be added to the estimated individual age using the IR marks.
Age estimation and fitting of growth curves
The study of the IR marks gives older ages than the estimations from the R of all individuals, except for those no more than 4–5 years old. There is little relationship between the IR and the R positions. The IR is generated at the boundary between the dorsal nacre lobe and calcite, an active shell-growing zone. The R is formed around the posterior adductor muscle, in relation to posterior migration of the muscle (Kennedy et al. 2001). This explains why some marks of the IR can be observed in a more posterior position than the R. The reason why the IR and the R correlate only in the oldest shell area is unknown. According to the stacking increments model by Ubukata (2001), the growth of the shell is due to three parameters: (1) the ratio of accretion of shell material at the shell margin to growth of the mantle by cell division, (2) the ratio of shell accretion at the pallial line to mantle growth, and (3) the ratio of the amount of pallial muscle translation. Whereas R generation would be only affected by (3), the IR would be also affected by (1), which might be partially responsible of the gradual disynchronization between R and IR positions. Punctual differences in the rates of posterior migration of the PAM and shell accretion in the dorsal nacre lobe could also be a reason for the irregular pattern between the R and the IR in the posterior marks of adult individuals (see Table 3).
Therefore, the utilization of PAMS to calculate P. nobilis age and growth rates can produce strong biases. The age can neither be accurately estimated from the external register of the SL, since (1) the number of obscured marks is variable among individuals depending on the age and the degree of posterior migration of the tissues, (2) some of the newest SL can be layered one under the other in the most posterior boundary of the dorsal nacre-lobe in old specimens (Table 3), and (3) the newest R and SL are easily confused in mature individuals (Fig. 3). The number of PAMS obscured by nacre among adult individuals would also produce the high variability in the position of the oldest PAMS with respect to Ht. (Table 2 and Fig. 2). On the other hand, the hiding of the oldest external marks of adult specimens would explain the mismatch between young and adults PAMS positions.
Apex erosion is variable among individuals of the same population and depth depending on seabed microtopography (García-March 2006). The erosion shortens the shell changing the relationship between all the marks and Ht and this would be one cause of the variability in the relationship between Ht and the position of the newest PAMS found in Eq. 1. Furthermore, anterior erosion leads to the calculation of smaller growth rates than the real ones. For an accurate estimation of P. nobilis growth rates, it would be useful to find an accurate method for calculating the level of anterior erosion of each individual, as this is not straightforward. P. nobilis is capable of some kind of shell reshaping to counteract anterior erosion and maintain the acute form of the umbo (García-March 2006). Therefore, prolongation of dorsal and ventral edges of the shell (Butler and Brewster 1979; Butler 1987) would introduce greater bias in Ht estimation. However, IR could be useful to estimate the anterior erosion of the shell. The correlation among the IR of the individuals of the same population and depth could highlight those specimens where anterior erosion is more important and as a result some models could be developed from them. Until then, any growth model using the IR and Ht would be biased by the shell shortening due to anterior shell erosion.
References
Bhattacharya CG (1967) A simple method of resolution of a distribution into Gaussian components. Biometrics 23:115–135
Butler AJ (1987) Ecology of Pinna bicolor Gmelin (Mollusca: Bivalvia) in Gulf St Vincent, South Australia: density, reproductive cycle, recruitment, growth and mortality at three sites. Aust J Mar Freshwat Res 38:743–769
Butler AJ, Brewster FJ 1979 Size distributions and growth of the Fan-shell Pinna bicolor Gmelin (mollusca: Eulamelibranchia) in South Australia. Aust J Mar Freshwat Res 30:25–39
Carter JG (1980) Environmental and biological controls of Bivalve shell mineralogy and microstructure. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms biological records of environmental change. New York, London, pp 69–114
Cohen AL, Branch GM (1992) Environmentally controlled variation in the structure and mineralogy of Patella granularis shells from the coast of southern Africa: implications for paleotemperature assessments. Palaeogeogr Palaeoclimatol Palaeoecol 9:49–57
Dodd JR (1964) Environmentally controlled variation in the shell structure of a pelecypod species. J Paleontol 38:1065–1071
Francillon-Vieillot H, de Bufférnil V, Castanet J, Géraudie J, Meunier FJ, Sire JY, Zylberberg L, de Ricqlès A (1990) Microstructure and mineralization of vertebrate skeletal tissues. In: Carter JG (ed) Skeletal biomineralization: patterns, processes and evolutionary trends, vol I. Van Nostrand Reinhold, New York, pp 471–530
García-March JR (2006) Aportaciones al conocimiento de la Biología de Pinna nobilis Linneo, 1758 (Mollusca: Bivalvia) en el litoral mediterráneo Ibérico. Publication Service of the University of Valencia, Valencia
García-March JR, García-Carrascosa AM, Peña AL, Wang YG (2006) Population structure, mortality and growth of two populations of Pinna nobilis Linnaeus, 1758 located at different depths in Moraira bay (Alicante, western Mediterranean). Mar Biol. DOI 10.1007/s00227-006-0386-1
García-March JR, Márquez-Aliaga A, Carter JC. The duplivincular ligament of recent Pinna nobilis L., 1758: further evidence for Pterineid ancestry of the Pinnoidea. J Paleontol. http://www.pinnanobilis.free.fr (in press)
Kennedy H, Richardson CA, Duarte CM, Kennedy DP (2001) Oxygen and carbon stable isotopic profiles of the fan mussel, Pinna nobilis, and reconstruction of sea surface temperatures in the Mediterranean. Mar Biol 139(6):1115–1124
Margaleff, R (ed) (1998) Ecología. Ediciones Omega S.A., Barcelona
Moreteau JC, Vicente N (1980) Etude morphologique et croissance de Pinna nobilis L. (Mollusque Eulamelibranche) dans le parc national sous-marin de Port-Cros (Var-France). Vie Marine 2:52–58
Quinn G, Keough M (eds) (2004) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Richardson CA, Kennedy H, Duarte CM, Kennedy DP, Proud SV (1999) Age and growth of the fan mussel Pinna nobilis from south-east Spanish Mediterranean seagrass (Posidonia oceanica) meadows. Mar Biol 133:205–212
Richardson CA, Peharda M, Kennedy H, Kennedy P, Onofri V (2004) Age, growth rate and season of recruitment of Pinna nobilis (L) in the Croatian Adriatic determined from Mg:Ca and Sr:Ca shell profiles. J Exp Mar Biol Ecol 299:1–16
Taylor JD, Kennedy WJ, Hall A (1969) The shell structure and mineralogy of the Bivalvia. Introduction. Nuculacea-Trigonacea. Bulletin of the British Museum (Natural History) Zoology. Supplement 3. London
Ubukata T (2001) Stacking increments: a new model and morphoespace for the analysis of Bivalve shell growth. Hist Biol 15:303–321
Vicente N (1990) Estudio ecológico y protección del molusco lamelibranquio Pinna nobilis L. 1758 en la costa mediterránea. Iberus 9(1–2):269–279
Wang YG (2004) Estimation of growth parameters from multiple-recapture data. Biometrics 60:670–675
Zavodnik D, Hrs-Brenko M, Legac M (1991) Synopsis on the fan shell Pinna nobilis L. in the eastern Adriatic sea. In: Boudouresque CF, Avon M, Gravez V (eds) Les Espèces Marines à Protéger en Méditerranée. Gis Posidonie publ. Marseille pp 169–178
Acknowledgments
We are grateful to J.G. Carter, N. Vicente, A.M. García-Carrascosa, A.L. Peña Cantero and M. Goodstadt for their support and constructive criticism of the manuscript and Araceli Terrones, for her help with the processing of the shells. The research was partially funded by the project KGL2005-015201BTE of the Spanish Science Education Ministry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Poulet, Roscoff.
Rights and permissions
About this article
Cite this article
Garcia-March, J.R., Márquez-Aliaga, A. Pinna nobilis L., 1758 age determination by internal shell register. Mar Biol 151, 1077–1085 (2007). https://doi.org/10.1007/s00227-006-0547-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0547-2