Abstract
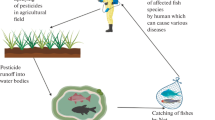
Pesticide use has increased worldwide to protect the food supply of the swelling global population. Although it is undisputed that pesticides are essential in modern agriculture, there is a growing concern about environmental contamination from agrochemicals. For example, application of pesticides in the Ebro River delta (NE, Spain) during the rice-growing season is suspected to be one of the major causes behind the shellfish mortality episodes that occur yearly in this area at springtime. In an attempt to disclose the causes of these seafood mortality episodes, this chapter presents the results obtained from a monitoring study carried out in April–June 2008 in the Ebro River delta (NE, Spain), where surface water and seafood samples were analyzed for both toxicity and pesticides. The main conclusion of this study was that pesticides are likely responsible, together with other not investigated parameters, such as metals, for the observed mortality. The results obtained are discussed also in relation to others previously published for the same or other similar areas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Almost half of the plants and animals listed in the Habitats Directive [1] are present in the Mediterranean region. This large number reflects not only the variety of threats in the region, but also its remarkable abundance of species. There are more plants here than in other European biogeographic regions together [2].
Among these regions is the Ebro River, the Spain’s most voluminous and important river in the country. With 910 km, the river accounts for approximately 6% of all riverine waters entering the Mediterranean and drains approximately one-sixth of the Iberian Peninsula, including important urban and industrial areas [3]. But because it is highly exploited by industrial and agricultural activities, the Ebro River basin is the focus of several scientific studies [4–10].
For example, 95 km upstream from the river mouth there is the Flix reservoir, where there are around 200,000–360,000 tons of industrial waste with a high concentration of heavy metals and organochlorines due to the activity of an chloro-alkali industry for more than half a century [11].
Downstream, in the mouth, there is the Ebro River delta, considered one of the most important natural wetland areas in the Mediterranean [12], and one of the river areas most affected by the presence of human activity.
1.1 Ebro River Delta
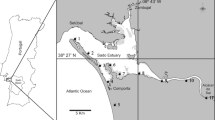
In this delta, we can contemplate orchards, vegetables, fruit-bearing trees, and mainly rice fields. The delta, which occupies 35,000 ha and goes some 20 km into the sea, is a dynamic and complex structure originated from the alluvial sediments transported by the river, which have given rise, among other physical features, to two coastal shallow bays that extend along both sides of the river mouth [13] (Fig. 1). The Ebro delta is considered one of the most important natural wetland areas in the western Mediterranean also for birdlife preservation. Just to have an idea, this delta also hosts numerous beaches, marshes, and salt pans that provide habitat for more than 300 species of birds. The main economic activity of this area is agriculture, which is mostly dominated by rice (about 80% of the land is dedicated to its production) [14]. A network of channels and irrigation ditches constructed by both agricultural and conservation groups help to maintain the ecologic and economic resources of the Ebro delta.
1.2 Shellfish Culture
However, the fertile land is not the only star of the delta. The bays also play an important role in the economy of this delta. Shellfish culture has been well developed in the shallow waters of the two aforementioned bays, and has become the second economic activity of the area after agriculture. In the Ebro delta, there are currently around 164 platforms for the production of mussels and oysters, 74 in Fangar bay (northern), and 90 in Alfacs bay (southern). Annual production for both bays is 3,000 tons of mussels and less than 1,000 tons of oysters [15].
The relative importance of both activities is well reflected on the population labor distribution per activity: 70% is dedicated to agriculture and 15% to shellfish production [14]; however, these two activities are not very compatible. Aquatic organisms are currently being exposed to multiple chemical and environmental stressors with different mechanisms of toxicity, each contributing to a final overall adverse effect [16]. Recently, the shellfish farmers in the Ebro River delta have complained about a loss of production in the periods of rice cultivation that they attribute to the heavy pesticide loads discharged after rice field treatment, and this has raised also public concern about the quality of the water in this area.
1.3 Pesticide Contamination
This high agriculture activity compromises the chemical and ecological status of the Ebro delta. Pesticides used in the area migrate into the delta building up, together with the pollution produced by some industries, a contamination that can be dangerous to its both fauna and flora, and this contamination increases when water from the rice fields is drained into the delta [17].
Many studies have been conducted along the Ebro River to assess the impact of pesticide use on its fauna. For instance, between 1989 and 1991 Solé et al. [3] conducted a study to investigate the bioaccumulation and ecotoxicity of various organophosphorous (OP) pesticides (and other pollutants) in bivalves cultured in the delta. A seasonal fluctuation of pollutants that was related to the biological cycle of the organisms and to the management of the waters in the rice crop fields of the delta was observed. However, levels were in general low and did not reach toxicity thresholds for bivalves, thus concluding that OP pesticides did not pose a threat to bivalves. The authors stressed though that pesticides might be harmful to other more sensitive species such as crustaceans, which in fact are not successfully farmed in the area, and that precautions should be taken during and shortly after field treatment with pesticides [3]. More recently, Damásio et al. [16] have identified endosulfan, propanil, and phenylureas as major pesticides affecting bivalve species exposed to agricultural pollution in the Ebro delta.
Other studies have focused on fish and reptiles. In 2004, Lavado et al. [18] provided the first evidence of endocrine disruption in fish from the Ebro river. Important alterations were detected in carps from Flix, a heavily industrialized area, and in Aragón, an agricultural area. In 2009, Santos and Llorente [19] reported a decline of the viperine snake Natrix maura in the delta. This reptile that in 1995 was common in the rice fields (0.93 animals/ha) was no longer found in 2008. In the authors’ opinion, this decline would have been caused by the combined effect of the following factors (1) the transformation and degradation of the habitat; (2) the increase in population densities of natural predators, such as herons; (3) the decrease in prey availability (frogs and fish were also observed to decline); (4) the massive use of pollutants in the rice fields; and (5) snake death on local roads and directly by human persecution; and also in the authors’ opinion, recovery of the N. maura population would require an integral change in agricultural management, including the reduced use of pollutants, among other measures [19].
Because of this massive use of agrochemicals and the ecological effects observed, the study of the occurrence of pesticides (herbicides, insecticides, plant growth regulators, fungicides, etc.) in this area has increased sharply in the last years [13, 20–26], thanks to the support of the water agencies in charge. The two most recently published works [27, 28] investigated temporal and geographical variations for a set of 30 pesticides and industrial compounds in surface waters and sediments along the Ebro river from source to mouth during the period 2004–2006, and found that, first, pesticides have a point source origin in the Ebro delta area (where concentrations reached up to 2,575 ng/L), and, second, the concentration of pesticides follows a seasonal trend, which is characterized by higher levels over the spring–summer period following agriculture application.
1.4 Legislation on Pesticides
The presence of pesticides in water has been subjected to regulation in the European Union (EU) for many decades. In 2008, the Directive 2008/105/EC [29], offspring of the Water Framework Directive (WFD) [30], established environmental quality standards (EQSs), both annual average (AA) and maximum allowable concentrations (MACs), for a series of pesticides and other priority substances (33 in total) in inland and other surface waters, as a first measure to protect surface water against pollution and deterioration. However, it is clear that in the aquatic environment there are many other pollutants and pressures, and that these pollutants and pressures vary geographically. For this reason, the same directive leaves it to the Member States to lay down, where necessary, EQSs for other site-specific pollutants, as well as EQSs for sediment and/or biota, at national level. To this end and for an integrated assessment of the status (chemical and ecological) of the water bodies, the combination of data on chemical concentrations and toxicological evaluation is essential.
The study described in the following section, which has been recently performed by our group in the Ebro delta, is a good example of the integration of chemical and biological tools to obtain a more comprehensive picture of the existing toxic pressures, as supported by the WFD, and of the surveillance of compounds beyond those specifically targeted in the legislation that can, nonetheless, be a problem in a specific area.
2 Case Study: Investigation of the Occurrence and Toxicity of Pesticides in the Ebro River Delta
2.1 Introduction
In response to the concern expressed by the shellfish farmers operating in the Ebro River delta about the potential positive role of pesticides on the oyster and mussel mortalities observed in the area, our group, commissioned by and with the collaboration of the Catalan Water Agency (ACA), carried out a comprehensive study in which chemical and toxicity data were combined to assess potential toxic presures present in the delta. To this end, a combined approach scheme integrating the measurement of various general physicochemical parameters in water, quantitative chemical analysis of pesticides in water and biota, and ecotoxicity assays in water was applied to a series of samples collected at springtime (between mid-April and mid-June 2008) from six selected sites of the delta: the two (northern and southern) bays (Fangar and Alfacs, respectively) and four main draining channels discharging the output water from the rice fields into the bays (Fig. 1). In total, 104 water samples, and 7 oyster and 3 mussel samples (each corresponding to a pool of 8–12 individuals collected alive from the two bays), were investigated.
Analysis of pesticides in water was performed by fully automated online solid-phase extraction-liquid chromatography–tandem mass spectrometry (SPE-LC–MS/MS) [25, 31]. These pesticides (a total of 22 belonging to the classes of triazines, OP, chloroacetanilides, phenylureas, thiocarbamates, acid herbicides, and anilides) were selected on the basis of previously published studies [20, 25], information gathered from the water authorities, and known use in rice crops.
Analysis of pesticides (eight in total, namely, molinate, propanil, fenitrothion, malathion, bentazone, cypermetrine, maloxon, and fenitrothion oxon) in biota was accomplished with a method based on pressurized liquid extraction (ASE), followed by SPE clean-up, and analysis by gas chromatography-mass spectrometry with electron impact ionization (GC/MS-EI).
Ecotoxicity assessment of water samples was carried out, in parallel to chemical analysis, using three standardized bioassays based on the micro-crustacean Daphnia magna, the algae Pseudokirchneriella subcapitata, and the bioluminescent bacteria Vibrio fischeri.
Finally, the pesticide concentrations determined in water and biota, together with the toxicity values of each individual compound, the toxicity data measured in the water samples, and the general physicochemical values were combined and analyzed together to establish potential cause–effect relationships and identify major toxicants or environmental pressures in the area of study. More details can be found in Köck et al. [12].
2.2 Results and Discussion
2.2.1 Levels of Pesticides in Water
Of the 22 pesticides analyzed in water, 21 were found to be present in some or all of the samples analyzed; cyanazine was the only undetected compound. Figure 2 shows the concentration of individual and total pesticides and their frequency of detection in the water samples collected from each of the six sampling sites monitored.
Boxplots of the concentrations of individual and total pesticides and frequency of detection (expressed in % in parentheses) in the water samples collected from each of the six sampling sites. HDAD, HDCD, HEOD, and HEIMD: draining channels; HDM and HEM: bays; DIA Deisopropylatrazine; DEA desethylatrazine
In what compliance with the stipulated legislation is concerned, only alachlor was found to exceed the EQS (MAC of 700 ng/L) in two of the samples investigated (HDAD, May 2; HEIMD, April 21) [29].
Bentazone and MCPA were the most ubiquitous compounds (detected in 100% of the samples), whereas malathion followed by MCPA and molinate were the compounds found at highest concentrations (5,825, 4,197 and 3,590 ng/L, respectively). Pesticide profiles similar to this have been observed previously in the studied area, as well as in other Mediterranean estuaries [32]. In 1990–1991, Readman et al. [32] conducted an extensive pilot survey in various selected Mediterranean locations to generate data about herbicides levels in estuarine zones, and found molinate and bentazone to be the most, or among the most abundant herbicides in the Ebro River delta (maximum concentrations 1,400 and 5,500 ng/L, respectively) and in the Po/Italy region (1,750 and 311 ng/L, respectively), which is in line with the rice cultivation activities performed in both areas. Nevertheless, in the study as a whole the most commonly encountered herbicides were atrazine, simazine, alachlor, metolachlor, and molinate. This study provided the first extensive evidence that significant concentrations of some herbicides persist through freshwater and estuarine environments and contaminate marine systems [32]. Later, other works investigating also the presence of pesticides in Mediterranean estuaries (see Table 1) have served to confirm this finding [17, 33–39].
Comparison of the pesticide concentrations (ng/L) found in this study in sites HDCD and HDAD with those measured in a previous study performed in 2005 in the same sampling sites [16, 20] showed a general good agreement for all pesticides except for bentazone, MCPA, propanil, and atrazine, which presented now comparatively lower concentrations, and alachlor, malathion, diuron, and molinate, whose concentrations have increased considerably (Fig. 3).
Comparing the six sampling points monitored in this study (Fig. 4), HEIMD followed by HDAD were the most polluted ones, showing concentration levels of total pesticides above 5 μg/L in 39% and 22% of the samples analyzed, respectively. As expected, the sampling points located in the two bays (HEM and HDM) were the less polluted, due to the dilution effect of the marine water. This dilution effect may explain also the higher contamination level observed in the northern bay as compared to the southern bay, which is comparatively larger in size and depth.
2.2.2 Water Toxicity
Figure 5 illustrates the results of the water toxicity evaluation performed with each of the three assays (24–48 h immobilization of D. magna, growth inhibition of Pseudokirchneriella subcapitata, and bioluminescence inhibition of V. fischeri), and the period of observed shellfish mortality. In 8 of the 14 monitored days, high toxicity values (above 50% inhibition) were recorded for at least one organism in at least one of the six sampling sites. As it can be seen in the figure, peak toxicity values occurred mainly in mid-May coinciding with, or a few days before, the shellfish mortality episodes.
These experimental toxicity values were compared with the theoretical toxicity units calculated for each sample and organism from the pesticide concentrations measured in the samples and their toxicity (50% effective concentration, EC50) towards the corresponding test organisms [12]. This comparison, which is illustrated in Fig. 6, gives an estimation of the extent to which the pesticides measured contribute to the observed experimental toxicity as well as about their relative contribution to it.
According to the results obtained, and assuming that the simple additive approach applied is valid, malathion appears to be the main contributor to Daphnia toxicity (98% toxic contribution on average for all samples); molinate, fenitrothion, and malathion would be the most relevant pesticides in the case of V. fischeri (69, 21, and 8% toxic contribution, respectively); and the alga P. subcapitata would be more affected by the presence of some herbicides, such as diuron, simazine, and terbuthylazine (36, 26, and 17% toxic contribution, respectively).
On the other hand, as it can be seen in Fig. 6, experimental and theoretical toxicity values followed fairly similar trends. The differences between them might be due to synergism or antagonism effects, matrix effects, and/or the presence of other not investigated chemicals (such as different pesticides, heavy metals, etc).
2.2.3 Levels of Pesticides in Shellfish
In shellfish only three of the eight compounds monitored were detected: fenitrothion, which was found in a mussel sample at 3.46 mg/kg, malathion, found in an oyster sample at 53.12 mg/kg, and the malathion degradation product malaoxon, which was found in one oyster and two mussel samples at concentrations between 2.53 and 4.59 mg/kg. As it can be seen in Fig. 7, positive samples (five of ten analyzed) were found in both bays and in scattered days along the period of study, with the sample showing the highest pesticide concentration (53.12 mg/kg of malathion in oysters collected from the northern bay on May 27) coinciding with the period of shellfish mortality.
In all cases, the concentrations of malathion and fenitrothion measured in water (up to 5.8 and 1.2 μg/L, respectively) were below the LC50 (lethal concentration 50%) values reported for these compounds in oysters and mussels, which range between 2.7 and 278 mg/L in the case of malathion, and between 10.3 μg/L and 123 mg/L in the case of fenitrothion (http://www.pesticideinfo.org). However, it has to be stressed that these LC50 values express acute toxicity, that both malathion and fenitrothion might be bioaccumulated by molluscs (as their detection in biota suggests), and that aquatic organisms are exposed to a variety of contaminants, some of which could show synergetic or additive effects [40]. Further matters of concern are (1) the detection itself of malathion in water and biota, provided that since June 2007 its use in plant protection products is forbidden in the European Union (Decision 2007/389/EC) [41] and the period of grace granted for the use of existing stocks expired in December 2008, and (2) the fact that malathion is transformed in the aquatic environment to malaoxon, which is more toxic than the parent compound [42].
2.2.4 Other Environmental Parameters
Agricultural practices are the main responsible source of surface water pollution in the Ebro River basin and this is particularly true in the lower course of the river [43, 44]. However, there are other factors that could contribute to the water conditions and possibly to the shellfish mortality episodes, such as the presence of other contaminants such as metals (copper is often used as algicide), hazardous algal blooms, and/or high temperatures. The elevated temperatures reached in these bays in mid-summer, especially in the Alfacs bay where values can exceed 28°C for several weeks, have been previously pointed out as a cause of mussel mortality [45, 46]. Thus, to investigate some of these possibilities, the water authorities in charge recorded continuously during the period of study in the sampling sites HEM and HDM (i.e., the northern and southern bays, respectively) some physicochemical parameters, including temperature, dissolved oxygen, pH, redox potential and conductivity. The first four parameters showed a normal behavior in both bays (thus excluding, for instance, anoxia episodes), but the conductivity varied notably especially in the northern bay. Such observed variations that are attributed to inputs of fresh water from the draining channels were coincident on time with peaks of pesticides, as well as with the registered mortality episodes in the northern bay, which constitutes a further support to the hypothesis of pesticides being a likely cause of shellfish impairment. Temperature was disregarded as a potential factor since the maximum values reached during the period of study were 21.8°C and 23.1°C in the northern and southern bays, respectively.
3 Conclusions
The great number and variety of environmental conditions that can be behind an adverse ecotoxicological observation makes virtually impossible to establish direct cause–effect relationships. However, the consistency of results between pesticides concentrations (in water and seafood), toxicity, and shellfish (oysters and mussels) mortality episodes in this study suggest that the pesticides used in the delta during the rice-growing season are likely responsible for the observed bivalves mortality, although the contribution of other not investigated factors, such as contamination by metals (e.g., copper) or by other agrochemicals, can obviously not be discarded. Of the various pesticides investigated, malathion and to a lesser extent diazinon and molinate appear as the most relevant compounds. Temperature in this particular case study is not believed to play an important role because it did not exceed 24°C during the monitoring period and the most remarkable mortality episodes took place in the northern bay of the delta, which was identified as the most polluted area, and where temperature was comparatively lower than in the southern bay.
Moving mussel cultivation outside the bays of the Ebro River delta has been suggested by scientists at the Institute for Agricultural Research and Technology (IRTA) and the Spanish Institute of Oceanography (IEO) as a potential solution to prevent shellfish mortality caused by pollution or high temperatures in the summer.
References
Council of European Economic Community (1992) Directive 92/43/EEC on the conservation of natural habitats and of wild fauna and flora. Off J Eur Community L 206:7
Sundseth K (2010) Natura 2000 en la región mediterránea. Unión Europea, Bruselas
Sole M, Porte C, Barcelo D et al (2000) Bivalves residue analysis for the assessment of coastal pollution in the Ebro Delta (NW Mediterranean). Mar Pollut Bull 40:746–753
Barth JAC, Grathwohl P, Fowler HJ et al (2009) Mobility, turnover and storage of pollutants in soils, sediments and waters: achievements and results of the EU project AquaTerra. A review. Agron Sustain Dev 29:161–173
Falco S, Niencheski LF, Rodilla M et al (2010) Nutrient flux and budget in the Ebro estuary. Estuar Coast Shelf Sci 87:92–102
Faria M, Huertas D, Soto DX et al (2010) Contaminant accumulation and multi-biomarker responses in field collected zebra mussels (Dreissena polymorpha) and crayfish (Procambarus clarkii), to evaluate toxicological effects of industrial hazardous dumps in the Ebro river (NE Spain). Chemosphere 78:232–240
Isidoro D, Aragó R (2007) River water quality and irrigated agriculture in the ebro basin: an overview. Int J Water Resour Dev 23:91–106
Martí-Cid R, Huertas D, Nadal M et al (2010) Dietary exposure to organochlorine compounds in Tarragona Province (Catalonia, Spain): health risks. Hum Ecol Risk Assess 16:588–602
Moreno-Mateos D, Pedrocchi C, Comín FA (2010) Effects of wetland construction on water quality in a semi-arid catchment degraded by intensive agricultural use. Ecol Eng 36:631–639
Postigo C, López de Alda MJ, Barceló D (2010) Drugs of abuse and their metabolites in the Ebro River basin: occurrence in sewage and surface water, sewage treatment plants removal efficiency, and collective drug usage estimation. Environ Int 36:75–84
Benejam L, Benito J, García-Berthou E (2010) Decreases in condition and fecundity of freshwater fishes in a highly polluted reservoir. Water Air Soil Pollut 210:231–242
Kock M, Farre M, Martinez E et al (2010) Integrated ecotoxicological and chemical approach for the assessment of pesticide pollution in the Ebro River delta (Spain). J Hydrol 383:73–82
Manosa S, Mateo R, Guitart R (2001) A review of the effects of agricultural and industrial contamination on the Ebro delta biota and wildlife. Environ Monitor Assess 71:187–205
Camp J (1994) Aproximaciones a la dinámica ecológica de una bahía estuárica mediterránea. Universitat de Barcelona, Barcelona
Acuicultura I (2007) Los productores del Delta promocionan la ostra. http://www.ipacuicultura.com/ipac/noticia.php?idNoticia=2426. Accessed 2010
Damasio J, Navarro-Ortega A, Tauler R et al (2010) Identifying major pesticides affecting bivalve species exposed to agricultural pollution using multi-biomarker and multivariate methods. Ecotoxicology 19:1084–1094
Peris E, Requena S, de la Guardia M et al (2005) Organochlorinated pesticides in sediments from the Lake Albufera of Valencia (Spain). Chemosphere 60:1542–1549
Lavado R, Thibaut R, Raldua D et al (2004) First evidence of endocrine disruption in feral carp from the Ebro River. Toxicol Appl Pharmacol 196:247–257
Santos X, Llorente GA (2009) Decline of a common reptile: case study of the viperine snake Natrix maura in a Mediterranean wetland. Acta Herpetol 4:161–169
Barata C, Damasio J, Lopez MA et al (2007) Combined use of biomarkers and in situ bioassays in Daphnia magna to monitor environmental hazards of pesticides in the field. Environ Toxicol Chem 26:370–379
Claver A, Ormad P, Rodriguez L et al (2006) Study of the presence of pesticides in surface waters in the Ebro river basin (Spain). Chemosphere 64:1437–1443
Gomez-Gutierrez AI, Jover E, Bodineau L et al (2006) Organic contaminant loads into the Western Mediterranean Sea: estimate of Ebro River inputs. Chemosphere 65:224–236
Hildebrandt A, Guillamon M, Lacorte S et al (2008) Impact of pesticides used in agriculture and vineyards to surface and groundwater quality (North Spain). Water Res 42:3315–3326
Hildebrandt A, Lacorte S, Barcelo D (2009) Occurrence and fate of organochlorinated pesticides and PAH in agricultural soils from the Ebro River basin. Arch Environ Contam Toxicol 57:247–255
Kuster M, de Alda MJL, Barata C et al (2008) Analysis of 17 polar to semi-polar pesticides in the Ebro river delta during the main growing season of rice by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry. Talanta 75:390–401
Santos TCR, Rocha JC, Barcelo D (2000) Determination of rice herbicides, their transformation products and clofibric acid using on-line solid-phase extraction followed by liquid chromatography with diode array and atmospheric pressure chemical ionization mass spectrometric detection. J Chromatogr A 879:3–12
Navarro A, Tauler R, Lacorte S et al (2010) Occurrence and transport of pesticides and alkylphenols in water samples along the Ebro River Basin. J Hydrol 383:18–29
Navarro-Ortega A, Tauler R, Lacorte S et al (2010) Occurrence and transport of PAHs, pesticides and alkylphenols in sediment samples along the Ebro River Basin. J Hydrol 383:5–17
Council of the European Communities (2008) Decision 2008/105/EC on environmental quality standards in the field of water policy. Off J Eur Community L 348:84
Council of the European Communities (2000) Directive 2000/60/EC establishing a framework for Community action in the field of water policy. Off J Eur Community L 327:1
Kampioti AA, da Cunha ACB, de Alda ML et al (2005) Fully automated multianalyte determination of different classes of pesticides, at picogram per litre levels in water, by on-line solid-phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Anal Bioanal Chem 382:1815–1825
Readman JW, Albanis TA, Barcelo D et al (1993) Herbicide contamination of mediterranean estuarine waters – results from a Med Pol pilot survey. Mar Pollut Bull 26:613–619
Feo ML, Eljarrat E, Ginebreda A et al (2010) Presence of pyrethroid pesticides in water and sediments of Ebro River Delta. J Hydrol doi: 10.1016/j.jhydrol.2010.08.012
Gamon M, Saez E, Gil J et al (2003) Direct and indirect exogenous contamination by pesticides of rice-farming soils in a Mediterranean wetland. Arch Environ Contam Toxicol 44:141–151
Comoretto L, Arfib B, Talva R et al (2008) Runoff of pesticides from rice fields in the Ile de Camargue (Rhone river delta, France): field study and modeling. Environ Pollut 151:486–493
Skoulikidis NT (2009) The environmental state of rivers in the Balkans-A review within the DPSIR framework. Sci Total Environ 407:2501–2516
Burgess RM, Terletskaya AV, Milyukin MV et al (2009) Concentration and distribution of hydrophobic organic contaminants and metals in the estuaries of Ukraine. Mar Pollut Bull 58:1103–1115
Vosniakos F, Petre J, Pascu L et al (2010) Aquatic ecosystem quality assessment of the Danube Delta in the periods April-October 2007 and 2008. Fresenius Environ Bull 19:20–29
Said TO, El Moselhy KM, Rashad AAM et al (2008) Organochlorine contaminants in water, sediment and fish of Lake Burullus, Egyptian Mediterranean Sea. Bull Environ Contam Toxicol 81:136–146
KayLynn Newhart (2006) Environmental fate of Malathion. California Environmental Protection Agency Department of Pesticide Regulation, CA, USA
Council of the European Communities (2007) Decision 2007/389/EC concerning the non-inclusion of malathion in Annex I to Council Directive 91/414/EEC. Off J Eur Community L 146:19
Aker WG, Hu XK, Wang P et al (2008) Comparing the relative toxicity of malathion and malaoxon in blue catfish Ictalurus furcatus. Environ Toxicol Chem 23:548–554
Terrado M, Barcelo D, Tauler R (2009) Quality assessment of the multivariate curve resolution alternating least squares method for the investigation of environmental pollution patterns in surface water. Environ Sci Technol 43:5321–5326
Terrado M, Barcelo D, Tauler R (2010) Multivariate curve resolution of organic pollution patterns in the Ebro River surface water-groundwater-sediment-soil system. Anal Chim Acta 657:19–27
Ramón M, Cano J, Peña BJ et al (2005) Current status and perspectives of mollusc (bivalves and gastropods) culture in the Spanish Mediterranean. Boletín Instituto Español de Oceanografía 21:361–373
Ramon M, Fernandez M, Galimany E (2007) Development of mussel (Mytilus galloprovincialis) seed from two different origins in a semi-enclosed Mediterranean Bay (NE Spain). Aquaculture 264:148–159
Acknowledgments
This work has been supported by the Catalan Water Agency and by the Spanish Ministry of Science and Innovation through the projects SCARCE (Consolider-Ingenio 2010 CSD2009-00065) and CEMAGUA (CGL2007-64551/HID). Merck is acknowledged for the gift of LC columns and Biotage for the gift of SPE cartridges. Marianne Köck gratefully acknowledges the European Social Fund and AGAUR (Generalitat de Catalunya, Spain) for their economical support through a FI predoctoral grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Köck-Schulmeyer, M. et al. (2010). Pesticides at The Ebro River Delta: Occurrence and Toxicity in Water and Biota. In: Barceló, D., Petrovic, M. (eds) The Ebro River Basin. The Handbook of Environmental Chemistry(), vol 13. Springer, Berlin, Heidelberg. https://doi.org/10.1007/698_2010_89
Download citation
DOI: https://doi.org/10.1007/698_2010_89
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-18031-6
Online ISBN: 978-3-642-18032-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)