Abstract
The watershed land uses in Mediterranean wetlands are essential to understand the functioning of aquatic communities. This study was designed to assess the relationship between watershed land uses, wetland characteristics and zooplankton assemblages (branchiopods and copepods) in 24 Mediterranean wetlands of the southern Iberian Peninsula, which greatly differ in both wetland land uses (olive groves, pasture, scrublands, and forest) and in their morphometric and limnological features. Firstly, results from a Principal Component Analysis allowed us to classify wetlands in two categories: impacted and non-impacted. Then, one-way Analysis of Variance was performed to test differences in zooplankton species richness and a Permutational Analysis of Variance was performed to test differences in zooplankton assemblages between categories. Lastly, a Non-metric Multidimensional Scaling analysis was chosen for the lake-by-species ordination. The results support the hypothesis that zooplankton richness and composition were negatively affected by watershed land uses, mainly agriculture practices. Moreover, species zooplankton assemblages were clearly linked to the two different wetlands categories. The present study puts forward the important role of zooplankton community for testing land use effects in Mediterranean wetlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wetlands are ecosystems with high environmental values, constituting one of the major features of the landscape in almost all parts of the world (Mitsch & Gosselink, 2000). Perhaps for this ubiquity, from ancient times men and wetlands have always been faced; so anthropogenic pressures have had strong impacts on these ecosystems (Naveh & Liberman, 1994). Among them, a reduction of their biodiversity, an increase in the eutrophication, and a growing demand of water supply have been worldwide described (Brinson & Málvarez, 2002; Dudgeon et al., 2006). Land uses in wetland catchments are also important factors to consider when evaluating the effects on diversity and composition of their aquatic communities (Rhazi et al., 2001; Dodson et al., 2005; Parra et al., 2005; Angeler et al., 2008; García-Muñoz et al., 2010) since they might play an important role in the viability of the populations, leading in some cases to the extinction of organisms on a local and regional scale.
In Mediterranean region, agricultural practices are the most important human activity inducing a drastic transformation of the landscape, with a great loss rate and degradation of wetlands (Brinson & Malvárez, 2002; Gallego-Fernández et al., 1999; Zacharias et al., 2007; Casas et al., 2011). In this sense, Casado & Montes (1995) denote that more than 60% of the Spanish wetlands have disappeared in the last fifty years. In spite of the recent acceptance of the ecological importance of wetlands in delivering ecosystem services (EPCN, 2008), this trend has not yet been reversed. Therefore, wetlands are appropriate ecosystems in which to study the land use practices effects because they interact across spatiotemporal hierarchies with the structure of the surrounding terrestrial landscapes (Angeler et al., 2008; Van Egeren et al., 2011). In this context, the source ecosystem (watershed) affects the sink ecosystem (wetland), so much as the increment of the edge/volume ratio, making it more exposed to substances exchange with the surrounding terrestrial landscapes (Gergel, 2005; Moreno-Mateos et al., 2008; Moreno-Mateos & Comín, 2010).
Lately, there exists an increasing interest to select optimal indicators for straightforward monitoring and assessment of wetlands (Angeler & Moreno, 2007). Zooplankton is an important component of wetland biodiversity, especially in Mediterranean region where it frequently acts as a keystone community, whose removal may engender dramatic changes in the structure and functioning of the aquatic ecosystem (De Leo & Levin, 1997; Waterkeyn et al., 2010). Studies of changes in zooplankton communities (species richness and species composition) are, therefore, useful tools for evaluating natural and anthropogenic wetland changes (Lougheed & Chow-Fraser, 2002; Allen & Dodson, 2011; Dodson et al., 2005, 2009). Among the zooplankton components, branchiopods and copepods are excellent models to evaluate these changes (Angeler et al., 2008). These invertebrates have passive dispersal capacities and are more adversely affected by anthropogenic impacts because they are not able to keep away from this stress situation by migrating to other wetlands (Dodson et al., 2005). Therefore, these resident species remain in dormant stages and their emergence from wetland resting egg bank decreases as a result of wetland degradation, with the subsequent impact on wetland ecological integrity (Angeler & García, 2005). However, up to date, these consequences are just beginning to be explored in Mediterranean wetlands, although they have crucial outcomes for wetland conservation and restoration (Angeler et al., 2007).
In this context, our main goal was to test the hypothesis that zooplankton assemblages (branchiopods and copepods) are strongly affected by the catchment land uses in Mediterranean wetlands. For that, we assess the relationship between wetland limnological characteristics and their different catchments land uses (olive groves, pasture, scrublands, and forest) with zooplankton assemblages in a set of 24 wetlands.
Materials and methods
Study site
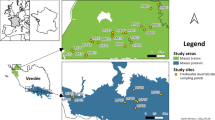
The Alto Guadalquivir region (14,020 km2) is located in the southern Iberian Peninsula (northeastern Andalusia), with a Mediterranean continental climate (AEMET, 2011). Agricultural practices are the main anthropogenic pressure in this region. The major proportion of soil is occupied by olive tree groves, with a less percentage of pastures, scrubland, and forest vegetation (Ortega et al., 2006). In this study, 24 wetlands were selected (Fig. 1) with different land uses in their drainage basin and consequently distinct degree of anthropogenic pressures. Many of them are singular for housing important zooplankton fauna, highlighting their value for regional biodiversity (Gilbert et al., 2014). The above-mentioned wetland selection represents an adequate example of the land uses heterogeneity in the study area.
Geographical location of the Alto Guadalquivir region and of the main rivers, cities, and study wetlands. Numbers represent the study wetlands (see Table 1 for wetland identification). Gray zones correspond to forested areas, while that white ones indicate agriculture areas. Please note that forested areas are coincident mainly to the highest altitude areas
Data analysis
Data employed in this study were obtained from the previous published data (Ortega et al., 2006; García-Muñoz et al., 2010; Gilbert et al., 2014, 2015a) and topographic maps (scale 1:10,000). The information comprises 11 variables related to wetland morphology, catchment characteristics, and watershed land uses (see Table 1 for more detailed information) and a zooplankton species richness list (Gilbert et al., 2015a) from which a presence–absence data matrix was constructed for further analysis (Table 2).
Firstly, the environmental data (Table 1) were analyzed by applying a Principal Component Analysis (PCA) using a correlation matrix, because the variables are measured in different scales (Borgognone et al., 2001). PCA is an adequate linear technique that allows us to discriminate wetlands in different categories (Quinn & Kenough, 2002). PCA was performed with PC-ORD version 4.0 software (McCune & Mefford, 1999).
Secondly, one-way Analysis of Variance (ANOVA) was carried out to test statistically significant differences in zooplankton species richness between the wetland categories previously obtained. Zooplankton species richness was used as the dependent variable and wetland category as the independent variable in the analysis. Statistical analysis was performed with Statistica version 7.0 (StatSoft, 2004). These univariate comparisons (ANOVA) were complemented with a multivariate analysis on zooplankton communities using a Permutational Analysis of Variance (PERMANOVA). This analysis was performed with 9999 permutations on Jaccard index for presence–absence matrix to test differences in the zooplankton assemblages between the different categories. Significant differences were inferred at a α-level of 0.05. PERMANOVA was run with PAST 3.06 software.
Finally, Non-metric Multidimensional Scaling (NMS) was used as an appropriate indirect method to examine patterns of wetland-by-species ordination (Dodson et al., 2009). NMS is recommended for presence–absence matrices with large number of zeros (>50%—McCune et al., 2002). Because NMS is relatively insensitive to distortion from common or rare species, all species were included in the analysis (McCune et al., 2002). NMS was run with Sørensen distance measure (PC-ORD version 4.0; McCune & Mefford, 1999). Significance of the axes was determined using a Monte Carlo test. Lastly, scores of the 24 wetlands along the first two PCA axes were correlated (Pearson correlation test) with the NMS axes of zooplankton ordination.
Data were transformed using log10 (x + 1) for PCA and ANOVA analysis to minimize the effects of the measurement scale, and to satisfy the assumptions of homoscedasticity and normality of the data.
Results
The results of PCA showed three axes that accounted for 65.27% of the total variance among wetlands (Table 3). The first PCA axis was negatively correlated to altitude, scrubland and forest vegetation area, and cathment:wetland ratio, and positively related to olive tree cultivation area and nutrient enrichment. The second PCA axis was negatively correlated to water mineralization, wetland vegetation heterogeneity, and water turbidity. The third PCA axis was negatively correlated to maximum wetland depth and urban surface area. The ordination of sampling sites with the two first PCA axes is shown in Fig. 2. This scatterplot allows us to make the following classification (see quadrants of PCA in Fig. 2): (I) wetlands located in high altitudes, without or with low agriculture pressure (or high presence of scrubland and forest vegetation), low external nutrient inputs, high catchment:wetland ratio, low mineralized, transparent waters, and high habitat heterogeneity; (II) wetlands with low altitudes, with high agricultural pressures (more than 70% of olive tree cultivation or low presence of scrubland and forest vegetation), high external nutrient inputs, low catchment:wetland ratio, low mineralized, transparent waters, and high habitat heterogeneity; (III) wetlands with the same characteristic that those located in the first group, but with high mineralized and turbid waters and low habitat heterogeneity (there are no wetlands in this quadrant); and (IV) wetlands with the same characteristic that those located in the second group, but with high mineralized and turbid waters and low habitat heterogeneity. According to this classification, we established two wetland categories: non-impacted wetlands, those included in the first quadrant of the PCA (Ardal, Castillo, Orcera, Pedernoso, Perales, Santisteban and Siles), and impacted wetlands, included in the second and fourth quadrants of the PCA (rest of wetlands).
PCA ordination diagram showing the environmental variables (arrows) described in Table 1 and the position of all samples in the plane of the first two axes. Symbols indicate wetlands categories: non-impacted wetlands (gray squares) and impacted wetlands (black squares)
With those categories, we have performed an ANOVA that showed the existence of significant differences in the number of zooplankton species between them (ANOVA: F (1, 23) = 4,91, P < 0.05), with a highest zooplankton richness in non-impacted wetlands. The results of PERMANOVA revealed that zooplankton species assemblages that appeared in non-impacted wetlands are significantly different from impacted wetlands (F 1, 23 = 1.689, P < 0.05).
The results obtained from NMS analysis revealed that a solution of three axes was the optimal dimensionality with a final stress value of 16.32, significantly lower than stress value for Monte Carlo simulation (P < 0.05). Figure 3 shows the ordination diagram of the two first axes of the NMS analysis, with zooplankton species and wetlands. Table 4 shows the most highly correlated species with each NMS axis. The first axis represents a transition from species of impacted sites (Metacyclops minutus) to less impacted sites (Simocephalus exspinosus, Ceriodaphnia quadrangula, and Ceriodaphnia dubia). The second axis represents a change from late successional species (Daphnia magna and Bosmina longirostris) to pioneer species (Chirocephalus diaphanus, Simocephalus vetulus, and Metacyclops minutus). Finally, the third axis represents a shift from species of less mineralized waters (Ceriodaphnia laticaudata, Simocephalus vetulus, and Neolovenula alluaudi) to saline species (Cletocamptus retrogressus and Arctodiaptomus salinus). In addition, the first two PCA were significantly correlated (P < 0.05) with NMS axes: the first PCA axis was correlated with the two first NMS axes, while the second PCA axis was correlated with the third NMS axis.
Discussion
Previous studies have assessed the effects of watershed land uses on the water quality, wetlands conservation, and biotic communities in the Mediterranean region (Angeler & Álvarez-Cobelas, 2005; Ortega et al., 2006; García-Muñoz et al., 2010; Gilbert et al., 2015b). However, up to date, scarce studies focussed on the effects on zooplankton community (Angeler et al., 2008). Our data reveal that zooplankton community is a good tool for testing land uses effects on wetlands located in Mediterranean areas, where agricultural activities have the greatest impact at the landscape level. The variables used in this study are not necessarily the only variables that explain the zooplankton community composition, but are essential to understand the functioning and complexity of biotic structure and the effect of human impacts on them.
Mediterranean wetlands are typically characterized by a higher catchment:wetland ratio (watershed surface area:wetland surface area—WS/S) than the temperate lakes, which implies a stronger interaction between the aquatic system and surrounding terrestrial habitat (Álvarez-Cobelas et al., 2005; Declerck et al., 2006; Van Egeren et al., 2011), and consequently a stronger influence of land uses on wetlands and on their aquatic communities (Trigal et al., 2007; Angeler et al., 2008). In the present study, the olive orchards are the main land use affecting biodiversity by (i) recurrent tillage in their catchment increasing soil erosion (Calero et al., 2013), and therefore increasing lake water turbidity; (ii) increasing nutrients runoff (Dodson & Lille, 2001); (iii) reducing habitat heterogeneity (García-Muñoz et al., 2010); and (iv) the impairment of wetland by increasing agrochemicals runoff, that once they reach aquatic ecosystems may cause direct toxic effects on zooplankton taxa (Parra et al., 2005; Del Arco et al., 2015, 2016). Other land uses such as herbaceous crops or/and pasture, scrubland and forest have much less adverse impacts on zooplankton communities. This idea is in concordance with the results of Jones et al. (2004), Dodson et al. (2007), and Angeler et al. (2008), who found that the runoff processes associated to land uses occurring in the watershed are the main contributors to the impairment of wetland ecological integrity. In this case, impacted wetlands tend to be located at lower altitudes where agricultural practices have been traditionally developed (Alfonso et al., 2010). By contrast, non-impacted wetlands are located at higher altitudes where natural vegetation is still preserved. Therefore, these differences in landscape context imply the amplification of the aforementioned effects. In this sense, significant differences have been observed on zooplankton species richness between non-impacted and impacted wetlands. Therefore, as suggested by others authors, the taxonomic richness could be used as a good community variable to evaluate their ecological integrity (Della Bella & Mancini, 2009). This result is also in agreement with other authors’ insights who found that wetlands in agricultural landscapes had lower species richness compared to wetlands with less agricultural impacts (Dodson & Lille, 2001; Hoffmann & Dodson, 2005; Dodson et al., 2005).
Although it is difficult to assign each taxon with different wetland categories, the obtained results show the existence of different species pools in each category (33.33% for non-impacted and 43.33% for impacted wetlands), with only 23.33% of shared species, which suggests that communities from the two categories are different from each other (see Table 2). This result is in disagreement with other authors who found a homogenization of biota with human pressures; in other words, the species composition in impacted sites is a subset of species found in non-impacted wetlands (Dodson & Lille, 2001; Lougheed et al., 2008). A likely explanation could be the idiosyncrasy of Mediterranean wetlands that are mainly temporary wetlands where the egg bank plays an important role in the zooplankton biodiversity (Angeler, 2007; Angeler et al., 2008). In this sense, a previous study reveals the existence of connectivity among the studied wetlands (Gilbert et al., 2014). Therefore, the continuous dispersal of individuals inside each wetland category increases the differences in the zooplankton community composition between categories. In this sense, there are some bibliographic evidences (Dodson et al., 2005; Angeler et al., 2008; Frisch et al., 2009) that found that in an agricultural landscape the viable propagule bank is less diverse and smaller than in natural regions. Moreover, taxa in seed bank reduced their emergence from wetland soil as result of wetland degradation (Angeler et al., 2007), contributing to these differences in zooplankton assemblages among wetland categories. These communities differ, as it is shown by the NMS analysis, in a transition between species of agricultural impacted sites, to less agricultural impacted sites, with S. exspinosus and C. quadrangula as typical species in the second one. This agrees with Dodson & Lille (2001) who found that S. vetulus (a congeneric species of S. exspinosus) and C. quadrangula are species that normally appear in non-agriculture impacted wetlands. On the other hand, the transition of wetland watershed alteration observed in the first axis of the PCA analysis is also correlated in the NMS analysis with a change of late successional species to pioneer species, since nutrients is a factor that appears to affect pioneering species assemblages (Ruhí et al., 2009). A more dynamic vision of the PCA results could also consider that it is possible a transition of wetlands between the two identified groups: impacted and non-impacted. In this way, the application of restoration and management policies in the catchment area of wetlands as Tobaruela could propitiate in a future re-evaluation the change from impacted to non- or less impacted wetlands. Accordingly, the percentage of agricultural land use could be a good indicator to establish quickly the position of a wetland in this continuous gradient, and to take the decision on which wetlands are appropriate to apply restoration and/or management actions.
Finally, it is important to consider that aquatic communities from Mediterranean wetlands are affected not only by human impacts (agriculture or urban development), but also by other features such as age of ponds, wetland surface area, or hydroperiod (Bagella et al., 2010). The two last one are closely correlated with global climate change involving an amplification of the water level fluctuation pattern, with minor water permanence and a reduction in the surface area of wetlands (Álvarez-Cobelas et al., 2005). These changes affect the species composition with a possible increase of the extinction rates and an important loss of biodiversity (McCarty, 2001). The knowledge of the species composition and diversity that support these systems is crucial for implementing management policies and efficient conservation strategies that might mitigate future effects of global climate change (Angeler, 2007).
Conclusion
Our results support the idea that agriculture in the watershed promotes drastic changes in wetland zooplankton community. Consequently, the monitoring of zooplankton assemblages might be a very useful and less cost-effective management tool to improve our capacity for understanding the effects of watershed land uses on Mediterranean wetlands under future global change. We are agreeing with many authors (REFRESH project, 2010; Haberman & Haldna, 2014; Van Hoey et al., 2010; Jeppesen et al., 2011; Van den Broeck et al., 2015; Alfonso et al., 2016) who suggest that zooplankton could be considered as a biological quality element (BQE) in the European Water Framework Directive (WFD; 2000/60/EC) to determine the quality status of water bodies.
The results also highlight the need for replacing the traditional agriculture strategies by other options more respectful with wetlands and zooplankton species inhabiting in them. It is necessary the integration of conservation science and conservation policy to reduce the negative effects of socio-economic development (Pullin et al., 2009; Fuentes-Rodríguez et al., 2013). In this sense, the implementation of agricultural policies that promote environmental-friendly practices, such as organic agriculture, and the use of vegetated buffer zones around the wetlands (Angeler et al., 2008) should be the main future tendencies in the Mediterranean region. The social agents responsible for the conservation policies should understand that all these strategies are used for an effective wetland management that includes a restoration at landscape scale rather than an approach to an individual wetland scale (Angeler et al., 2010). In our study area, a reference state might be established attending to changes in agricultures practices, taking into account a most respectful soil management in the olive orchards that allow the presence of vegetation under the canopy of trees (reduction of erosive processes). In the same way, it is also interesting to stimulate the heterogeneity of land use in the watershed, with the presence of natural hedges and the reduction of toxic substances used in these agricultural practices. Moreover, and considering that Mediterranean region in the future climate change scenario will likely be a zone with strong limitations of freshwater, a reduction in the use of this resource should also be considered by, for instance, limiting the current increasing tendency of irrigated croplands and therefore the consequent increase exploitation of the groundwater sources.
References
AEMET, 2011. Atlas climático ibérico. Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid, España.
Alfonso, G., G. Belmonte, F. Marrone & L. Naselli-Flores, 2010. Does lake age affect zooplankton diversity in Mediterranean lakes and reservoirs? A case study from southern Italy. Hydrobiologia 653: 149–164.
Alfonso, G., L. Beccarisi, V. Pieri, A. Frassanito & G. Belmonte, 2016. Using crustaceans to identify different pond types. A case study from the Alta Murgia National Park, Apulia (South-eastern Italy). Hydrobiologia 782: 53–69.
Allen, P. E. & S. I. Dodson, 2011. Land use and ostracod community structure. Hydrobiologia 668: 202–219.
Álvarez-Cobelas, N., C. Rojo & D. G. Angeler, 2005. Mediterranean limnology: current status, gaps and the future. Journal of Limnology 64: 13–29.
Angeler, D. G., 2007. Resurrection ecology and global climate change research in freshwater ecosystems. Journal of the North American Benthological Society 26: 12–22.
Angeler, D. G. & G. García, 2005. Using emergence from soil propagule banks as indicators of ecological integrity in wetlands: advantages and limitations. Journal of the North American Benthological Society 24: 740–752.
Angeler, D. G. & J. Moreno, 2007. Zooplankton community resilence after press-type anthropogenic stress in temporary ponds. Ecological Applications 17: 1105–1115.
Angeler, D. G. & M. Álvarez-Cobelas, 2005. Island biogeography and landscape structure: integrating ecological effects in a landscape perspective of anthropogenic impacts in temporary wetlands. Environmental Pollution 138: 421–425.
Angeler, D. G., A. J. Boulton, K. M. Jenkins, B. Sánchez, M. Álvarez-Cobelas & S. Sánchez-Carrillo, 2007. Alternative states and temporary wetlands: research opportunities for understanding effects of anthropogenic stress and natural disturbances. In Clarkson, A. (ed.), Environmental Research Advances. Nova Science Publishers Inc, New York: 5–17.
Angeler, D. G., O. Viedma, S. Sánchez-Carrillo & M. Álvarez-Cobelas, 2008. Conservation issues of temporary wetland Branchiopoda (Anostraca, Notostraca: Crustacea) in a semiarid agricultural landscape: what spatial scales are relevant? Biological Conservation 141: 1224–1234.
Angeler, D. G., C. Trigal, S. Drakare, R. K. Johnson & W. Goedkoop, 2010. Identifying resilence mechanisms to recurrent ecosystem perturbations. Oecologia 164: 231–241.
Bagella, S., S. Gascón, M. C. Caria, J. Sala, M. A. Mariani & D. Boix, 2010. Identifying key environmental factors related to plant and crustacean assemblages in Mediterranean temporary ponds. Biodiversity and Conservation 19: 1749–1768.
Borgognone, M. G., J. Bussi & G. Hough, 2001. Principal component analysis in sensory analysis: covariance or correlation matrix? Food Quality and Preference 12: 323–326.
Brinson, M. M. & A. I. Malvárez, 2002. Temperate freshwater wetlands: types, status, and threats. Environmental Conservation 29: 115–133.
Calero, J., M. P. Cordovilla, V. Aranda, R. Borjas & C. Aparicio, 2013. Effect of organic agriculture and soil forming factors on soil quality and physiology of olive trees. Agroecology and Sustainable Food Systems 37: 193–214.
Casado, S. & C. Montes, 1995. Guía de los lagos y humedales de España. J. M, Reyero, Madrid, España.
Casas, J. J., J. Toja, S. Bonachela, F. Fuentes, I. Gallego, D. Juan, M. León, P. Peñalver, C. Pérez & P. Sánchez, 2011. Artificial ponds in a Mediterranean region (Andalusia, southern Spain): agricultural and environmental issues. Water and Environment Journal 25: 308–317.
De Leo, G. A. & S. Levin, 1997. The multifaceted aspects of ecosystem integrity. Conservation Ecology 1: 3.
Declerck, S., T. De Bie, D. Ercen, H. Hampel, S. Schrijvers, J. Van Wichelen, V. Gillard, R. Mandiki, B. Losson, D. Bauwens, S. Keijers, W. Vyverman, B. Goddeeris, L. De Meester, L. Brendonck & K. Martens, 2006. Ecological characteristics of small farmland ponds: associations with land use practices at multiple spatial scales. Biological Conservation 131: 523–532.
Del Arco, A. I., F. Guerrero, F. Jiménez-Gómez & G. Parra, 2015. Effects of nitrate concentration within legal limits on natural assemblages of plankton communities. Fundamental and Applied Limnology 187: 1–10.
Del Arco, A. I., F. Jiménez-Gómez, F. Guerrero & G. Parra, 2016. Can a copper sulphate pulse below toxic threshold change plankton communities? Aquatic Ecosystem Health and Management 19: 64–73.
Della Bella, V. & L. Mancini, 2009. Freshwater diatom and macroinvertebrate diversity of coastal permanent ponds along a gradient of human impact in a Mediterranean eco-region. Hydrobiologia 634: 25–41.
Dodson, S. I. & R. A. Lille, 2001. Zooplankton communities of restored depressional wetlands in Winconsin, USA. Wetlands 21: 292–300.
Dodson, S. I., R. A. Lillie & S. Will-Wolf, 2005. Land use, water chemistry, aquatic vegetation, and zooplankton community structure of shallow lakes. Ecological Applications 15: 1191–1198.
Dodson, S. I., W. R. Evenhart, A. K. Jandl & S. J. Krauskopf, 2007. Effect of watershed land use and lake age on zooplankton species richness. Hydrobiologia 579: 393–399.
Dodson, S. I., A. L. Newman, S. Will-Wolf, M. L. Alexander, M. Woodford & S. Van Egeren, 2009. The relationships between zooplankton community structure and lake characteristics in temperate lakes (Northern Wisconsin, USA). Journal of Plankton Research 31: 93–100.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A. H. Prieur-Richard, S. Soto, M. L. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
EPCN, 2008. The pond manifesto. European Pond Conservation Network (www.europeanponds.org).
Frisch, D., A. Arechederra & A. J. Green, 2009. Recolonisation potential of zooplankton propagule banks in natural and agriculturally modified sections of a semiarid temporary stream (Doñana, Southwest Spain). Hydrobiologia 624: 115–123.
Fuentes-Rodríguez, F., M. Juan, I. Gallego, M. Lusi, E. Fenoy, D. León, P. Peñalver, J. Toja & J. J. Casas, 2013. Diversity in Mediterranean farm ponds: trade-offs and synergies between irrigation modernisation and biodiversity conservation. Freshwater Biology 58: 63–78.
Gallego-Fernández, J. B., M. R. García-Mora & F. García-Novo, 1999. Small wetlands lost: a biological conservation hazard in Mediterranean landscapes. Environmental Conservation 26: 190–199.
García-Muñoz, E., J. D. Gilbert, G. Parra & F. Guerrero, 2010. Wetlands classification for amphibian conservation in Mediterranean landscapes. Biodiversity and Conservation 19: 901–911.
Gergel, S. E., 2005. Spatial and non-spatial factors: when do they affect landscape indicators of watershed loading? Landscape Ecology 20: 177–189.
Gilbert, J. D., I. de Vicente, R. Jiménez-Melero, G. Parra & F. Guerrero, 2014. Selecting priority conservation areas based on zooplankton diversity: the case of Mediterranean wetlands. Marine and Freshwater Research 65: 857–871.
Gilbert, J. D., I. de Vicente, F. Ortega, R. Jiménez-Melero, G. Parra & F. Guerrero, 2015a. A comprehensive evaluation of the crustacean assemblages in southern Iberian Mediterranean wetlands. Journal of Limnology 74: 169–181.
Gilbert, J. D., F. Guerrero, R. Jiménez-Melero & I. de Vicente, 2015b. Is the bioproduction number a good index of the trophic state in Mediterranean wetlands? Knowledge and Management of Aquatic Ecosystems 416: 05.
Haberman, J. & M. Haldna, 2014. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: long term study of Lake Võrtsjärv. Journal of Limnology 73: 263–273.
Hammer, U. T., 1978. The saline lakes of Saskatchewan. III. Chemical characterization. Internationale Revue der gesamten Hydrobiologieund Hydrographie 63: 311–335.
Hoffmann, M. D. & S. I. Dodson, 2005. Land use, primary productivity, and lake area as descriptors of zooplankton diversity. Ecology 86: 255–261.
Jeppesen, E., P. Nõges, T. A. Davidson, J. Haberman, T. Nõges, K. Blank, T. L. Lauridsen, M. Søndergaard, C. Sayer, R. Laugaste, L. S. Johansson, R. Bjerring & S. L. Amisnck, 2011. Zooplankton as indicators in lakes – a scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 676: 270–297.
Johnes, P., B. Moss & G. Phillips, 1996. The determination of total nitrogen and total phosphorus concentrations in freshwaters from land use, stock head age and population data: testing of model for use in conservation and water quality management. Freshwater Biology 36: 451–473.
Jones, J. R., M. F. Knowlton, D. V. Obrecht & E. A. Cook, 2004. Importance of landscape variables and morphology on nutrients in Missouri reservoirs. Canadian Journal of Fisheries and Aquatic Sciences 61: 1503–1512.
Lougheed, V. L. & P. Chow-Fraser, 2002. Development and use of a zooplankton index of wetland quality in the Laurentian Great Lakes basin. Ecological Applications 12: 474–486.
Lougheed, V. L., M. D. McIntosh, C. A. Parker & R. J. Stevenson, 2008. Wetland degradation leads to homogenization of the biota at local and landscape scales. Freshwater Biology 53: 2402–2413.
McCarty, J. P., 2001. Ecological consequences of recent climate change. Conservation Biology 15: 320–331.
McCune, B. & M. J. Mefford, 1999. PC-ORD. Multivariate Analysis of Ecological Data, Version 4. Gleneden Beach, MJM Software Design.
McCune, B., J. B. Grace & D. L. Urban, 2002. Analysis of Ecological Communities. MJM Software Design, Gleneden Beach.
Mitsch, W. J. & J. G. Gosselink, 2000. Wetlands, 3rd ed. Wiley & Sons, New York.
Moreno-Mateos, D. & F. A. Comín, 2010. Integrating objectives and scales for planning and implementing wetland restoration and creation in agricultural landscapes. Journal of Environmental Management 91: 2087–2095.
Moreno-Mateos, D., Ü. Mander, F. A. Comín, C. Pedrocchi & E. Uuemaa, 2008. Relationships between landscape pattern, wetland characteristics, and water quality in agricultural catchments. Journal of Environmental Quality 37: 2170–2180.
Naveh, Z. & A. Lieberman, 1994. Landscape Ecology: theory and Application. Springer, New York.
Ortega, F., G. Parra & F. Guerrero, 2006. Usos del suelo en las cuencas hidrográficas de los humedales del Alto Guadalquivir: importancia de una adecuada gestión. Limnetica 25: 723–732.
Parra, G., R. Jiménez-Melero & F. Guerrero, 2005. Agricultural impacts on Mediterranean wetlands: the effects of pesticides on survival and hatching rates in copepods. Annales de Limnologie - International Journal of Limnology 41: 161–167.
Pullin, A. S., A. Baldi, O. E. Can, M. Dieterich, V. Kati, B. Livoreil, G. Lövei, B. Mihók, O. Nevin, N. Selva & I. Sousa-Pinto, 2009. Conservation focus on Europe: major conservation policy issues that need to be informed by conservation science. Conservation Biology 23: 818–824.
Quinn, G. P. & M. J. Keough, 2002. Experimental Design and Data Analysis for Biologists. Cambridge University Press, Cambridge.
REFRESH project, 2010. Zooplankton: an integrative Biological Quality Element for assessing the Ecological Status of lakes. http://www.refresh.ucl.ac.uk/webfm_send/2240
Rhazi, L., P. Grillas, A. M. Toure & L. T. Ham, 2001. Impact of land use in catchment and human activities on water, sediment and vegetation of Mediterranean temporary pools. Comptes Rendus de l’Académie des Sciences – Series III – Sciences de la Vie 324: 165–177.
Ruhí, A., D. Boix, J. Sala, S. Gascón & X. D. Quintana, 2009. Spatial and temporal patterns of pioneer macrofauna in recently created ponds: taxonomic and functional approaches. Hydrobiologia 634: 137–151.
StatSoft Inc., 2004. STATISTICA (data analysis software system), version 7. http://www.statsoft.com.
Trigal, C., F. García-Criado & C. Fernández-Aláez, 2007. Macroinvertebrate communities of Mediterranean ponds (North Iberian Plateau): importance of natural and human-induced variability. Freshwater Biology 52: 2042–2055.
Van den Broeck, M., A. Waterkeyn, L. Rhazi, P. Grillas & L. Brendonck, 2015. Assessing the ecological integrity of endorheic wetlands, with focus on Mediterranean temporary ponds. Ecological Indicators 54: 1–11.
Van Egeren, S. J., S. I. Dodson, B. Torke & J. T. Maxted, 2011. The relative significance of environmental and anthropogenic factors affecting zooplankton community structure in Southeast Wisconsin Till Plain lakes. Hydrobiologia 668: 137–146.
Van Hoey, G., A. Borja, S. Birchenough, L. Buhl-Mortensen, S. Degraer, D. Fleischer, F. Kerckhof, P. Magni, I. Muxika, H. Reiss, A. Schröder & M. L. Zettler, 2010. The use of benthic indicators in Europe: from the Water Framework Directive to the Marine Strategy Framework Directive. Marine Pollution Bulletin 60: 2187–2196.
Waterkeyn, A., B. Vanschoenwinkel, P. Grillas & L. Brendonck, 2010. Effect of salinity on seasonal community patterns of Mediterranean temporary wetland crustaceans: a mesocosm study. Limnology and Oceanography 55: 1712–1722.
Zacharias, I., E. Dimitriou, A. Dekker & E. Dorsman, 2007. Overview of temporary ponds in the Mediterranean region: threats, management and conservation issues. Journal of Environmental Biology 28: 1–9.
Acknowledgements
Our thanks go to the Consejería de Medio Ambiente (Junta de Andalucía) for permission to collect zooplankton samples in wetlands. Thanks to Francisco J. Márquez for the useful statistical comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Juan Carlos Molinero
Rights and permissions
About this article
Cite this article
Gilbert, J.D., de Vicente, I., Ortega, F. et al. Linking watershed land uses and crustacean assemblages in Mediterranean wetlands. Hydrobiologia 799, 181–191 (2017). https://doi.org/10.1007/s10750-017-3211-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3211-6