Abstract
Zooplankton assemblages of 51 lacustrine environments located in the middle of the Mediterranean Region were analysed to evaluate the existence of an ‘age effect’ in determining their structure. The analysed datasets refer to two different geographic areas, one comprising 30 natural and artificial lakes in Sicily and the other an arrangement of 21 analogous aquatic ecosystems located at the bottom of the Italian Peninsula, a more pristine area called Southern Apennine region. Most of the natural lakes are of post-glacial origin. The artificial lakes in both datasets were built in the last century and offer the opportunity to evaluate the possible short-term effects of ageing on the structure of their zooplankton. A comparison of assemblages in the two regions by PERMANOVA and nMDS revealed that they are quite different; therefore they were analysed separately. An explorative analysis on the possible relationship between biological data and environmental data (including lake age) was performed on both datasets using DISTLM. The presence of an ‘age effect’ emerged only in the subset of artificial lakes of the Southern Apennine region; accordingly it was tested more in detail with an a posteriori PERMANOVA analysis in the subset of reservoirs that resulted positive in the first test. SIMPER allowed us to single out the main species responsible of changes in the zooplankton along the selected age groups. No age effect was evident in the Sicilian water bodies, where other variables, such as conductivity, trophic state, urbanisation and water level fluctuations proved to have a major role in shaping zooplankton assemblages. The results showed that the age effect is: (i) detectable only at a time scale of decades; (ii) masked by the human impact in the watershed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Why are there so many kinds of animals? It is more than 50 years that the paper published by Hutchinson in 1959 keeps awake a large number of ecologists, who have been putting lifelong efforts trying to answer this question (see Thomaz et al., 2010). Since the antiquity, all those involved in studying the Nature have been fascinated by the amazing diversity of organisms. The ‘Homage to Santa Rosalia’ have undoubtedly opened new perspectives on the general theory of biodiversity and set the scene for a new concept of biological diversity. In the same year, the first mathematical model to explain species diversity was published (Hutchinson & MacArthur, 1959). These papers along with the ‘Paradox of the plankton’ (Hutchinson, 1961) have represented a milestone for aquatic ecologists investigating the structure and dynamics of organisms inhabiting (freshwater) ecosystems as well as their distribution and dispersal mechanisms. Moreover, they paved the way to a series of studies aimed at clarifying the processes and the rules at the base of community assembly.
Species accumulation in a given ecosystem starts with repeated colonisation processes and lasts until reaching a balance between competition and coexistence (Sommer & Worm, 2002). Beside the long debate on equilibrium and non equilibrium hypotheses (see Naselli-Flores et al., 2003), some studies on assembly rules revealed that the structure of a community may depend on a priority effect (Ward & Thornton, 2000) modulated by complex hierarchical factors (Rodrigo et al., 2009). Hierarchy theory is a formal approach to the complex influences of scale, and suggests that different phenomena (e.g. long term processes and disturbances) influence systems on different scales (Allen & Starr, 1982). In addition, the different scales (phenomena) are nested and interact. Thus, the community formation history (Padisák, 1992; Drake et al., 1999) is nested and interacts with the frequency and intensity of disturbances (Reynolds et al., 1993), including anthropogenic ones (Dodson et al., 2005) and with top–down and bottom–up processes as predation and competition for resources (e.g. Brooks & Dodson, 1965; Carpenter & Kitchell 1993; Cottingham, 1999; Ortega-Mayagoitia et al., 2002).
Among the factors influencing the zooplankton species richness in a given lake, the ‘age effect’ has been indicated in the last years as one of the possible causes. The dispersal mechanisms allowing colonisation of a new environment are rather unpredictable and thus, as underlined by Dodson et al. (2007), it is a plausible assumption that they may be linked to the age of a given water body: shortly after they are filled, newly constructed lakes have few or no zooplankton species; as time since first filling increases, species accumulate over time via standard dispersal routes, such as wind and waterfowl (Jenkins & Buikema, 1998) until the available space is saturated and other phenomena as those listed above starts shaping the structure of the assemblage.
Reservoirs are recently built lacustrine ecosystems and may offer a unique opportunity to investigate the early stages of community formation. To test the hypothesis that the age effect may play an important role in shaping the structure of zooplankton in aquatic ecosystems, data collected in 51 water bodies, both natural and artificial, located in the Southern part of Italy and in Sicily were analysed. In this respect, we offer our thankful homage to G.E. Hutchinson for his stimulating ideas and to Stanley I. Dodson, who inspired this work and enthusiastically contributed to disseminate Hutchinson’s heritage.
Materials and methods
Biological and environmental data
The two studied areas, the Southern Apennine and Sicily, are both located in the middle of the Mediterranean Region. The first area represents the southernmost part of the Italian Peninsula and is separated from Sicily, the largest island of the Mediterranean Sea, by the 3 km wide Strait of Messina. These areas are considered to form two distinct zoogeographical provinces: the Southern sector of the Apennine province and the Sicilian province, respectively (Ruffo & Stoch, 2005).
A detailed description of Sicilian climatic and geographic features can be found in Marrone et al. (2006) and (2009). Limnological characteristics of its water bodies were summarised by Naselli-Flores (1999) and (2003).
The Southern Apennine area couples faunal elements coming from both the Balkan and Tyrrhenian paleoareas, which were overlapped during the Quaternary by North European and West Palaearctic faunal elements. Nowadays, this area is characterised by xero-thermophilic Mediterranean species, dominating over the Northern ones; the latter contributes to the mountain-Mediterranean features of the area (Minelli et al., 2004).
The Southern Apennine area is insufficiently studied and no information on entire taxa (as Calanoida) is available (see Belmonte et al., 2006).
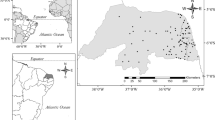
Two different datasets consisting of environmental variables, species composition and abundance of zooplankton assemblages were compared in this study. One dataset refers to 30 Sicilian lakes (25 artificial and 5 natural ones) sampled in 1987–1988, the second to 21 lakes (17 artificial and 4 natural) of the Southern Apennine area, sampled in 2005–2007 (Fig. 1).
The zooplankton checklists used in this study are based on a careful critical revision of the data collected by Calvo et al. (1993) and Alfonso (2007). Some samples have been re-investigated in order to check for the identity of the species whose previous identification was controversial. The species nomenclature adopted by Calvo et al. (1993) has been in some cases corrected and updated according to the more recent taxonomical works.
Sicilian lakes were sampled in spring, summer and autumn 1987 and in winter 1988, in fixed stations at the deepest point of each water body. Zooplankton samples were collected with two 21 cm diameter plankton nets of 75 μm and 125 μm mesh size. Three vertical tows were made from the bottom to the surface. Filtered water volumes were estimated by calculating the volume of a cylinder having the basal area equal to the net opening and with a height corresponding to the length of the vertical tow. The collected specimens were immediately concentrated on a 60 μm mesh gauze and fixed in 95% ethanol (Calvo et al., 1993).
The Southern Apennine lakes were sampled in June and October 2005, January 2006 and April 2007. Samples were collected by vertical tows with a 25 cm diameter net of 200 μm mesh size, from the bottom to the surface; 11 lakes were also sampled using a 50 μm net. This subset was used to analyse more in detail the effect of the altitudinal range among the examined lakes (Alfonso, 2007). Filtered water volumes were measured with a flux-meter fixed on the net mouth and samples were fixed in situ with 4% buffered formalin. Samples were collected in each lake with at least three replicates for each sampling date.
As regards crustacean plankton, we assumed that the information coming from the 200 μm net used to sample the 21 Southern Apennine lakes is comparable to that obtained by using a 125 μm net in the 30 Sicilian ones. Similarly, the net of 50 μm mesh size used for 11 Southern Apennine lakes and the 75 μm mesh size in the 30 Sicilian lakes, were considered reliable for collecting data on rotifers. The representativeness of the seasonal sampling frequency is normalised by the equal sampling effort devoted to both the studied areas.
Ergasilidae copepods were identified to species level in the Southern Apennine environments (Alfonso & Belmonte, 2010). Conversely, they were recorded but not identified in Sicily and thus they were excluded from the analyses.
Coordinates were obtained in situ using a portable GPS. Other 12 environmental factors, common to both datasets, were considered for analysis: age of the water body, altitude, average surface, average volume, maximum depth, average depth, total average conductivity, presence of urban centres in the catchment area, presence of aquatic macrophytes, water catchment area, agricultural soil (as percentage of the water catchment area), trophic state index. The age of reservoirs is calculated in reference to 2005 for the Southern Apennine lakes, to 1987 for the Sicilian ones. Depth measures were obtained directly in situ by a portable depth finder in each sampling dates, average values are referred to the four sampling dates. Area and volume measures were obtained from the management boards of the respective lakes. Conductivity was measured at each sampling date by a multiparametric probe (Idronaut Ocean Seven mod. 401 for Sicilian lakes; Idromar Multiprobe for Southern Apennine ones). Presence of macrophytes was pointed out in situ for each sampling date. Trophic state index (TSI) was calculated according to Carlson (1977) considering Chl a values of the autumnal data. For Sicilian water bodies, the presence of urban centres and the percentage of agricultural soil in the catchments are those reported in the ‘Annual of Statistics 1986’ (ISTAT, 1986). Urbanisation and the percentage of agricultural soil in the catchments of the Apennine lakes were estimated through the analysis of aerial photographs referred to the sampling period. Water catchment areas were provided by the management boards.
Each site was identified with a code in which three letters indicate the geographic area: SIC for Sicily, BAS for Basilicata and CAL for Calabria. Basilicata and Calabria belong to Southern Apennine faunal province, Sicily to the Sicilian one (Stoch, 2006). A progressive number from 001 to 030 identifies the Sicilian sites in alphabetical order. A progressive number from 001 to 021 indicates the Southern Apennine sites from North to South (Supplementary material—Appendix 1).
Statistical analyses
For statistical analysis PRIMER v6 (Clarke & Gorley, 2006) was used. The tests used were PERMANOVA (permutational multivariate analysis of variance) and DISTLM (distance-based multivariate analysis for a linear model). PERMANOVA with 4999 permutations was performed on Bray–Curtis triangular matrix of yearly presence–absence data (cumulative of the four sampling dates) to investigate the taxonomic differences between the two datasets (Sicilian lakes versus Southern Apennine lakes). The experimental design was set up to the factor ‘dataset’ at two levels: Sicilian lacustrine environments 1987–1988 and Southern Apennine lacustrine environments 2005–2007 in order to estimate their degree of similarity. The first set was represented by 30 replicates (corresponding to the Sicilian lakes), the second by 21 replicates (the Southern Apennine lakes). In this design, we considered the lakes as replicates of the respective dataset ‘Sicilian lacustrine environments 1987–1988’ or ‘Southern Apennine lacustrine environments 2005–2007’. This could be done because PERMANOVA results are not affected by unbalanced (e.g. differing in the number of replicates) datasets.
The non-metric multi dimensional scaling (nMDS) plots were used to visualise similarity/differences of zooplankton assemblages from both geographic areas. The plots were performed on Bray–Curtis triangular matrix of the presence–absence data of all taxa from all replicates of datasets (lakes). The PERMANOVA test and the nMDS plots were performed on all taxa, and also separately on Crustacea and Rotifera.
Because of the remarkable taxonomical differences between the two datasets, as depicted by PERMANOVA, further statistical analyses were performed on Sicilian and Southern Apennine data separately.
DISTLM calculates a multivariate multiple regression analysis of symmetric distance matrices (Anderson, 2004). It was used separately in the two datasets as an explorative method to test possible correlations between biological and environmental data. The distance matrix was created by computing Bray–Curtis similarity on presence/absence of zooplankton species in the studied lakes. The matrix containing the explanatory variables was prepared using annual average values of the log(x + 1) transformed and normalised environmental variables. The conditional test of DISTLM FORWARD was used to evaluate the percentage of variability in the zooplankton community which is explained by the whole set of environmental variables considering the correlations among them (i.e. each variable is conditioned by the others). Since only 11 Southern Apenninic lakes were sampled with two nets (and therefore information on Rotifera were exhaustive only for 11 lakes), it was necessary to analyse some subsets of lakes separately. The subsets analysed took in account also the origin of the environment (natural or artificial).
Particular attention was paid to investigate the effect of lakes’ age on the crustacean zooplankton assemblages for the lakes that already showed a correlation with the variable ‘age’ in the DISTLM analysis. The selected subset for the PERMANOVA analysis was composed by the 12 Southern Apennine reservoirs, which were never interested by a complete drainage. This was necessary in order to perform the PERMANOVA analysis on a homogenous matrix of data, as required by the test itself. The PERMANOVA design nested this dataset into the factor ‘age’ at four levels (in years): 0–20, 20–35, 35–50, 50–80. Each level of age is represented at least by two lakes and each lake has four different sampling times and three different replicates (independently collected) for each sampling time. The test was performed on the Bray–Curtis triangular matrix of 4th root transformed data using 4999 permutations. The transformation allows to account also for rare and less abundant species.
The PAIRWISE test of PERMANOVA was used to quantify the similarity/dissimilarity among groups of lakes nested into the factor ‘age’.
Non-metric multi-dimensional scaling (nMDS) plots of each Bray–Curtis similarity matrix were used to visualise patterns of detected differences. The dissimilarity percentage program (SIMPER, Warwick et al., 1990) was used to identify the main species, with their respective average abundances, responsible of the changes in the zooplankton community and the trends in the different age groups.
Results
General faunal results
123 zooplankton taxa from both datasets (74 Rotifera and 49 Crustacea) were considered in this study. Crustacea and Rotifera of both datasets are listed in the supplementary material—Appendices 2A and 2B. Table 1 summarises the main characteristics of the studied assemblages. A lower number of species was detected in the Sicilian dataset, both for Crustacea and Rotifera, and the two datasets are sharing only the 23% of the species. Among Crustacea, Cladocera is the richer taxonomical group in both the geographical areas. However, only 31% of species is shared by both datasets. Cyclopoids show a higher similarity (43.75% of species) in the two areas, whereas calanoids are different. The number of rotifers species is higher than crustacean one in Southern Apennine as well as in Sicily, and just 18% of species occur in both datasets.
The number of species occurring in each lake is reported in supplementary material—Appendix 3. No more than one calanoid species per lake was identified both in Sicilian and in Southern Apennine dataset. Cladocera ranged from 1 to 8 species per lake in the Sicilian dataset and from 2 to 9 in the Southern Apennine; Cyclopoida species were 0–2 per lake in Sicily and 1–6 in the Southern Apennine. Taking in account the total number of planktonic Crustacea, a minimum of two species and a maximum of nine species per lake were detected in the Sicilian lakes, and a minimum of five species and a maximum of 14 species per lake were detected in the Southern Apennine ones.
The PERMANOVA test, carried out to evaluate the factor ‘dataset’, revealed that the zooplankton assemblages of the Sicilian dataset 1987–1988 are significantly different from those belonging to the Southern Apennine dataset 2005–2007 (Table 2). Similar significant values were obtained also considering Crustacea and Rotifera separately. This is clearly visible in the nMDS plots, which always show two separated clouds (Fig. 2) both considering all the zooplankton taxa, and Crustacea and Rotifera separately.
Environmental variables
Supplementary material—Appendix 1 shows geographical coordinates of the lakes and the average values of the 12 environmental variables used for statistical analyses. Four natural lakes in Sicily (SIC003, SIC004, SIC013, SIC018) and two natural lakes in Southern Apennine (BAS014, BAS016) are estimated to be no older than 10,000 years old since they are of post-glacial origin. The Southern Apennine lake BAS003 is estimated to be at most 100,000 years old, a period that corresponds to the end of the activity of the volcano on which it lies. BAS008, even though artificially enlarged in the 1980s of the last century, is an old site whose history is known from Middle Age. We assigned it an approximate age of about 1,000 years. The Sicilian SIC028 (Lake Soprano) is a natural lake formed after a karstic collapse of the surface rocks after the erosive activity of rain waters; in 1987–1988 it was estimated to be about 100 years old. The age of the 25 artificial lakes of the Sicilian dataset in 1987–1988 ranged from 1 to 64 years, the age of the 17 artificial lakes in the Southern Apennine dataset (2005–2007) ranged from 3 to 78 years.
The results of the DISTLM tests, performed separately on the two datasets, showed conductivity as the variable best correlated with zooplankton assemblages in the Sicilian dataset (Table 3). Among all the environmental variables, conductivity was found to have a significant effect on the zooplankton communities, when considering both the entire Sicilian dataset and the Sicilian subset containing only artificial lakes. Conductivity was also the best correlated variable considering all of the zooplankton assemblage or the Crustacea and Rotifera separately. As regards the latter group, the variable ‘urbanisation’, which reflects the presence of untreated urban waste reaching the lake, is also significantly correlated with rotifer species richness when considering the entire set of Sicilian lakes. Conductivity explained about 12% of biological variance for crustacean zooplankton both in all the 30 Sicilian water bodies and in the subset formed by the 25 artificial lakes only. The variables ‘altitude’ and ‘average depth’ were also significantly correlated considering the set containing all the 30 lakes (cumulative percentage of variance ~28%). TSI was correlated, along with conductivity, when only crustacean zooplankton in the 25 artificial lakes was considered.
More heterogeneous results were obtained when the DISTLM test was performed for the Southern Apennine lakes (Table 4). In this case, the results including the Rotifera were computed only for those 11 lakes sampled with two different mesh sized nets. In this subgroup of lakes, the best correlated variable with the zooplankton is the ‘volume’ of the water body (percentage of variance ~27.8%). Moreover, when only the seven artificial lakes of this subgroup (i.e. those sampled with two nets of different mesh size) were considered, the ‘age’ of the lakes appeared to be a significant variable explaining above the 30% of the variance of the zooplankton species richness.
The DISTLM test restricted to the crustacean zooplankton only, and computed in all the 21 lakes of the Southern Apennine dataset, showed that the ‘water catchment area’, ‘maximum depth’ and ‘volume’ together explained more than 45% of the variance. However, when considering only the 17 artificial lakes of the Southern Apennine dataset, the best correlated variable is the ‘age’ of the lakes, explaining more than 18% of the variance. In this case, ‘water catchment area’ is the second best correlated variable, explaining 15.5% of the variance. Performing the same test on the 12 strictly permanent reservoirs, the ‘age’ is always the variable that has a significant effect on the crustacean assemblages, explaining 20.06% of variance. The variable ‘age’ is the best correlated for crustaceans also in the test performed on the subgroup of seven artificial lakes. In this last case, the highest value of explained variance (~39.3%) for a single variable was obtained among all the DISTLM tests performed.
The age effect
The results of DISTLM test revealed a positive match between biological matrix and the water bodies’ age only for the Southern Apennine reservoirs; thus, these were used to test the effect of age on the zooplankton assemblage using PERMANOVA test. Only man-made, strictly permanent reservoirs (12 sites) were selected for the test because this test requires homogeneous data. Since data on Rotifera were considered not exhaustive because of the uneven use of the 50 μm mesh-sized net, they were not considered in this test.
The PERMANOVA test singled out the lake age as a main constraints affecting crustacean zooplankton assemblage structure (Table 5). The Pairwise test, based on the performed PERMANOVA, showed the group of lakes with an age of 50–80 years as the most dissimilar when compared to the other groups of age (Table 6). This was also clear in the nMDS plot (Fig. 3), where the points corresponding to samples collected in 50–80 years old lakes are grouped more closely. Conversely, points of lakes in the group 20–35 years are more spread on the plot and they partially overlap with other groups.
Table 7 shows the main zooplankton species detected by the SIMPER analysis as responsible of the differences related to the groups of age; these are 12 cladocerans, 2 cyclopoids, and 1 calanoid. Bosmina longirostris, Ceriodaphnia pulchella, Acanthocyclops trajani and Cyclops vicinus were detected in all the age groups; Daphnia parvula and Daphnia galeata were absent only in the oldest age group. The latter included Alona affinis and Daphnia longispina, which are missing in the other groups. The calanoid Eudiaptomus vulgaris and the ctenopod Diaphanosoma lacustris were found in lakes belonging to the age groups ranging from 20 to 50 years of age. Moina micrura, Ceriodaphnia reticulata and Ceriodaphnia dubia belonged to the 35–50 years age group.
The SIMPER analysis also showed the average dissimilarity among groups of age, related to species abundances (Table 8). In accordance to the results obtained from the Pairwise test of PERMANOVA, the higher dissimilarity values were found between the group including lakes with an age of 50–80 years and all the others (Table 6). In particular, the highest value of dissimilarity was recorded between the group 50–80 and 20–35. The general trends for Cladocera and Copepoda (Fig. 4a, b) showed an increase of both abundances and species richness until 50 years of age, followed by a less pronounced decrease of values between 50 and 80 years since impoundment.
Discussion
The idea of a possible age effect structuring zooplankton communities goes back to Dussart (1966), who indicated the unknown aspects of the evolution of waterbodies along time. Some authors (Reid, 1961; Bertoni, 2006) suggested a kind of water-system ageing, based on a general increase of their trophic state that could be accelerated by human activities. Moreover, it was Hutchinson himself (1957) who first focused on ‘certain peculiarities of man-made lakes’ that are of great interest to the limnologists, offering chances for testing hypotheses due to their relatively young age when compared to natural lakes. In this article, the possible effects exerted by ageing on zooplankton assemblages’ composition and richness in 51 artificial and natural lakes were analysed. The studied environments are located in the same Mediterranean climatic area, have comparable morphological features but are subjected to different degrees of disturbance. In particular, human activities as agriculture and urbanisation in the watersheds of man-made lakes were recognised as adverse factors on zooplankton species richness, likely masking the structuring effect of lake age on zooplankton assemblages (Dodson et al., 2007). In this respect, Sicily and Southern Apennine area profoundly differ: Sicily is one of the most intensively inhabited and cultivated area in the Mediterranean Region, whereas the Southern Apennine is much less impacted both in terms of number of inhabitants and agriculture activities. This is partly due to the high percentage of landscape safeguarded by protected areas and National Parks and partly to the asperity of the territory itself, crossed by several mountain ranges that make its exploitation for agriculture purposes difficult. In addition, our results could be conditioned by the 20 years of difference between the timing of the collections carried out in Sicily (1987–1988) and in the Southern Apennine (2005–2007). However, this supports the hypothesis that human disturbance may affect species richness since the collections in Sicily were carried out in a period in which environmental protection in Italy was not yet affirmed.
Faunal composition and brief biogeographical remarks
In both the study areas, anomopod cladocerans were the most represented crustaceans in the open water (16 species occur in the Southern Apennine versus 14 in Sicily) followed by cyclopoid copepods (14 vs. 9), calanoid copepods (4 vs. 3) and ctenopod cladocerans (2 vs. 1). The order Haplopoda is only present in the Southern Apennine area with the predacious species Leptodora kindtii.
Three non-indigenous species have been recorded: two anomopod cladocerans (i.e. Daphnia ambigua and D. parvula), and one calanoid copepod (Boeckella triarticulata). The first two taxa, whose native distribution range lies in the Nearctic region, are already known to occur in the Italian peninsula and Sicily (Margaritora, 1985; Riccardi et al., 2004; Marrone et al., 2005), and their invasion in Europe from the second half of the twentieth century is well documented (e.g. Flössner, 2000, and references therein). As regard D. parvula, Gherardi et al. (2008) report that it was introduced in Italy in 2002. However, it was already present in the 1987 samples collected in Sicily and thus its introduction in Italy likely happened along with that of D. ambigua. B. triarticulata is an Australasian centropagid copepod first reported in fish ponds in northern Italy (Ferrari et al., 1991), whose Italian distribution range is expanding southwards at a fast pace (cf. Ferrari & Rossetti, 2006; Alfonso & Belmonte, 2008), although it has not been recorded in Sicily to date (Marrone et al., 2006).
Overall, in the 51 sampled lakes, 49 crustacean species were recorded. 23 of these were exclusive of the Southern Apennine lakes (47%), 12 of Sicily (24%), while 15 are present in both the study areas (30.6%). It is noteworthy that the Southern Apennine area presents a higher overall species richness (19 branchiopod taxa versus 15; 18 copepod taxa versus 13) in spite of the minor sampling effort devoted (21 lakes vs. 30). The scenario is even more pronounced if we do not consider three brackish and saline lakes (i.e. Biviere di Gela, Lake Ogliastro and Lake Pergusa), an habitat typology which is present only in the Sicilian dataset: this way a single calanoid species would be present in Sicily versus four species in the Southern Apennine area. Moreover, the average crustacean species richness per lake is higher in the Southern Apennine area than in Sicily.
Focusing on the specific composition of crustacean assemblages (Supplementary material—Appendix 2), it seems that most of the species exclusive of the Sicilian sites are in fact littoral or pond species, which occupy an atypical ecological niche (i.e. the pelagic environment), which in the Southern Apennine is occupied by their ‘pure lacustrine’ counterparts (Margaritora, 1985; Alonso, 1996); the only exception is D. cucullata, a pure lacustrine species recorded in Sicily and apparently absent in the studied lakes of the Southern Apennine. However, putative hybrids galeata × cucullata were present in this area (listed as Daphnia gr. galeata in supplementary material—Appendix 2).
Based on these data, it seems that crustacean assemblages in lakes and reservoirs of Sicily are composed by an impoverished subset of the species present in the Southern Apennine, accompanied by the ingression of some pond species which found an ‘empty niche’ in the Sicilian man-made reservoirs. This arrangement could be explained by the different geographical location of the two studied areas: the lakes and reservoirs of the Southern Apennine lie in continuous matrix of natural and artificial permanent water bodies reaching northern Italy and the southern slope of the Alps. This continuous frame of permanent water bodies possibly behave as a ‘stepping stone matrix’ and\or as a network of ‘sink & source habitats’ for pure lacustrine species. Conversely, Sicilian reservoirs were built in a context where the only natural permanent water bodies were the brackish coastal lakes and the high-altitude ponds, where no pure lacustrine species occur.
Species composition differs considerably among the different types of water systems and many species show pronounced affinities with one or a few specific water body types (e.g. De Bie et al., 2008). Consequently, the pools of crustacean species that are candidate for the colonisation of lakes and reservoirs in Sicily and the Southern Apennine differ both in species richness and composition due to a peninsula effect (Wiggins, 1999). While the crustacean species inhabiting the permanent water bodies of Peninsular Italy could have easily colonised the man-made reservoirs of the Southern Apennine, only a subset of these could have crossed the Catanzaro graben and the Strait of Messina to colonise Sicilian reservoirs. In this process, some ecological niches were left empty and thus colonised in Sicily by those pond species which could take advantage of the absence of their more specialised pure lacustrine counterparts. Moreover, pond species can be further advantaged in Sicily by the wide water-level fluctuations to which these environments are subjected (Naselli-Flores, 2003). Conversely, in the Southern Apennine these species, although present (unpublished data), are confined in small ponds and in temporary water bodies being competitively disadvantaged in the permanent lakes when compared to their lacustrine congeneric species (this is the case of, e.g. Macrothrix hirsuticornis, Ceriodaphnia quadrangula and Daphnia magna).
The hypothesis that, lacking specialised taxa, some species may opportunistically colonise sub-optimal environments is confirmed by the re-colonisation in Lake Orta after its liming (Bonacina & Pasteris, 2001). The first species which appeared in the lake were Daphnia obtusa and Arctodiaptomus wierzejskii: two taxa mostly typical of ponds and temporary pools. Pond species have likely more effective dispersal strategies when compared with lacustrine taxa and are thus the first taxa which may colonise a recently built (or detoxified) water body. After a few years, the more lacustrine Daphnia longispina colonised the lake, prevailed on D. obtusa, and excluded it from the lake. The presence of the typical ‘pond species’ Daphnia magna and Eucyclops macruroides in the Sicilian Lake Soprano, can be interpreted in a similar way. Lake Soprano was formed by a landslide about one century ago and from then always interested by a noticeable anthropogenic disturbance, continuously keeping its zooplankton assemblages into an early developmental stage and thus allowing the persistence of these ‘pond species’ in the open waters of the lake (see Lampert & Sommer, 2007 and references therein).
Age effect and zooplankton structure in the studied environments
The results from the DISTLM analysis suggest that the specific features of a given territory are important factors in structuring the biological communities. Some of the Sicilian lakes and reservoirs lie on evaporite deposits of Messinian Age pertaining to the ‘Gessoso Solfifera’ formation (Madonia et al., 2006), others lie on siliceous bedrocks. Thus, a large span of conductivity values characterises these environments: from 0.083 mS cm−1 in Lake Biviere di Cesarò to 34 mS cm−1 (with summer peaks above 120 mS cm−1) in Lake Pergusa (Calvo et al., 1993). As a consequence, conductivity resulted the best correlated environmental variable in structuring the zooplankton in the Sicilian dataset. The influence of conductivity was so strong to be revealed not only on the whole zooplankton assemblage but also considering Crustacea and Rotifera separately. In accordance with Boronat et al. (2001) and Schell et al. (2001), higher conductivity values may explain the lower diversity observed in Sicilian water bodies compared to peninsular ones. Conversely, trophic state and urbanisation were found to affect zooplankton species richness in these environments. In particular, as shown in supplementary material—Appendix 1, urban centres are present in 38% of Southern Apennine watersheds against 57% in Sicily and the average percentage of agricultural soil in the catchment area is 39% in Southern Apennine against 71% in Sicily. The higher percentage of agriculture soil is directly linked to the higher use of fertilizers and ultimately to the trophic state of the recipient water bodies. Moreover, average depth also resulted to affect zooplankton diversity. In fact, this variable is indirectly linked to the wideness of water-level fluctuations. In general, lower average depths are related to stronger water level fluctuations, whose effects deeply condition the biota of Sicilian reservoirs (Naselli-Flores, 2010). This last variable was already found to be critical for zooplankton structure and dynamics (Naselli-Flores & Barone, 1994; 1997), and is linked to the wide water-level fluctuations affecting Sicilian reservoirs and lakes (Naselli Flores, 2003; Barone et al., 2010). These results are in agreement with the findings of Dodson et al. (2007), who highlighted that zooplankton communities in lakes with no riparian buffer zone, in agriculture-dominated watersheds, contained about half as many species as lakes in least-impact watersheds.
Conversely, Southern Apennine area is much less impacted by human activities than Sicily and water-level fluctuations, although typically present, are less extreme (Alfonso, 2007). In this peninsular area, conductivity does not appear to have the same importance because of the more homogeneous and lower values (<1 mS cm−1) characterising the studied lakes. Thus, other variables were better correlated, and more heterogeneous results appeared in the DISTLM test. The importance of the lake’s age clearly emerged when considering only the artificial environments in the Southern Apennine area. In particular, the age was detected as the most important factor in structuring the crustacean zooplankton assemblages of the 17 Southern Apennine artificial lakes sampled with the 200 μm net, and on the entire assemblage in the subset of the seven lakes sampled with both the 200 and 50 μm nets. The age effect seems to disappear when also more ancient natural lakes were included in the analysis. This can be regarded as a ‘Clementian effect’: Clements (1916), trying to explain the climax concept, postulated that the temporal development of a community reaches a stage that is rather persistent, self sustaining, where further ‘development’ is limited if at all possible. It can be supposed that in Southern Apennine natural lakes, long-term community dynamics accumulate such numerous episodic events that stochasticity obscures the age effect on time scales longer than decades. In addition, experimental evidence shows that inter-specific interactions determine a steady-state outcome wherein relatively few species achieve overwhelming dominance through competitive exclusion (Reynolds, 1993).
The existence of an age effect in the artificial lakes of the Southern Apennine area seems to be confirmed also in the other tests performed (PERMANOVA, SIMPER), which helped to better understand how the factor age explicates its influence on the zooplankton community. Even though the statistical result could be influenced by a relatively low number of lakes included in some age classes, a relative increase of abundances and species in the first 50 years of lake age was observed in our data. This pattern likely reflects the chance of higher rates of resource exploitation in a newly constructed environment. Obviously, a relatively short period is necessary to colonise the new lakes. In the Southern Apennine dataset, this is visible after a few years since the first filling; in 20 years an average number of six crustaceans per lake could be identified. A critical age was detected 50 years after impoundment, when both abundances and species richness begin to decrease with the ageing of the system, which is likely related to a change and re-assortment of the zooplankton community structure. This age threshold probably represents the time span after which diversity is reduced by competitive exclusion, thus opening the way to new equilibrium dynamics (see Naselli-Flores et al., 2003).
Conclusions
This study suggests that, in the absence of disturbance, planktonic communities tend to become more complex and to increase their species richness over time. On the other hand, the probability that disturbance may occur becomes higher with the increase of the age of the lakes themselves; the ‘age effect’ can thus be detectable with difficulty or, as this study indicates, only on a short time to take place.
The influence of lake age on zooplankton could be detected in the reservoirs located on the Southern Apennine range. This part of the Italian Peninsula is much less impacted than Sicily with regard to human activities in the watersheds. On the other hand, no effect of ageing was found on zooplankton assemblages in Sicilian lakes and reservoirs. This is likely due to the stronger human impacts on these environments. Agriculture, in particular, may exert a detrimental influence on freshwater biodiversity (Moss, 2008). In accordance to literature (see Lampert & Sommer, 2007 and references therein), it can be hypothesised that human disturbances continuously revert the Sicilian zooplankton assemblages to an earlier developmental stage, and do not allow to reach a more mature condition, characterised by a higher species richness, as observed in some of the Southern Apennine lakes.
Furthermore, some hypotheses to answer the question ‘Why in Sicily are there less kind of animals than in Southern Apennine?’ could be addressed. In fact, in spite of their geographical proximity to Southern Apennine lakes, Sicilian permanent water bodies show lower numbers of species per lake, and a general lower species richness in both the crustacean and rotifer zooplankton. The reasons of such lower richness can be sought in two different facts. The first is attributable to an intrinsic reason: the higher conductivity values due to the evaporite outcrops, which generally corresponds to a lower species richness. The second is related with external reasons due to human impacts in the watershed and in the lakes; these impacts have to be considered and contribute to deteriorate the natural environmental frame. Sicilian lakes and reservoirs are characterised by a higher trophic state resulting from untreated urban waste and a high percentage of agriculture land in the watersheds. Moreover, lakes, both artificial and natural, are often managed in a way that promotes and increases eutrophication processes and effects (Barone et al., 2010; Naselli-Flores, 2010). All these features may be considered adverse factors for biodiversity in Sicilian inland waters and contribute to mask any possible age effect on zooplankton richness.
References
Alfonso, G., 2007. Composizione e struttura dello zooplancton nei laghi dell’Appennino Meridionale. PhD Thesis, University of Salento (Italy).
Alfonso, G. & G. Belmonte, 2008. Expanding distribution of Boeckella triarticulata (Thomson, 1883) (Copepoda: Calanoida: Centropagidae) in Southern Italy. Aquatic Invasions 3: 238–242.
Alfonso, G. & G. Belmonte, 2010. Neoergasilus japonicus (Harada, 1930): a new non-indigenous copepod for the Italian fauna. Italian Journal of Zoology 77(2): 172–178.
Allen, T. F. H. & T. B. Starr, 1982. Hierarchy: Perspectives for Ecological Complexity. University of Chicago Press, Chicago.
Alonso, M., 1996. Crustacea, Branchiopoda. In Ramos, M. A. et al. (eds), Fauna Iberica, Vol. 7. Museo Nacional de Ciencias Naturales, CSIC, Madrid.
Anderson, M. J., 2004. DISTLM v.5 Distance-based multivariate analysis for a linear model. [Available at http://www.stat.auckland.ac.nz/~mja/prog/DISTLM_UserNotes.pdf] (Accessed 12th of January 2010).
Barone, R., G. Castelli & L. Naselli-Flores, 2010. Red sky at night Cyanobacteria delight: the role of climate in structuring phytoplankton assemblage in a shallow, Mediterranean lake (Biviere di Gela, southeastern Sicily). Hydrobiologia 639: 43–53.
Belmonte, G., G. Alfonso & S. Moscatello, 2006. Copepod fauna (Calanoida and Cyclopoida) in small ponds of the Pollino National Park (South Italy), with notes on seasonality and biometry of species. Journal of Limnology 62: 107–113.
Bertoni, R., 2006. Laghi e Scienza, introduzione alla limnologia. Aracne Edizioni, Roma.
Bonacina, C. & A. Pasteris, 2001. Zooplankton of Lake Orta after liming: an eleven years study. Journal of Limnology 60: 101–109.
Boronat, L., M. R. Miracle & X. Armengol, 2001. Cladoceran assemblages in a mineralization gradient. Hydrobiologia 442: 75–88.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size, and composition of the plankton. Science 150: 28–35.
Calvo, S., R. Barone, L. Naselli-Flores, C. Fradà Orestano, G. Dongarrà, A. Lugaro & G. Genchi, 1993. Limnological studies on lakes and reservoirs of Sicily. Naturalista Siciliano 17 (Suppl.): 1–292.
Carlson, R. E., 1977. A trophic state index for lakes. Limnology and Oceanography 22: 361–369.
Carpenter, S. R. & J. F. Kitchell, 1993. The Trophic Cascade in Lakes. Cambridge University Press, Cambridge.
Clarke, K. R. & R. N. Gorley, 2006. PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth.
Clements, F. E., 1916. Plant Succession: An Analysis of the Development of Vegetation, Vol. 242. Carnegie Inst. Washington Publ, Washington: 1–517.
Cottingham, K. L., 1999. Nutrients and zooplankton as multiple stressors of phytoplankton communities: evidence from size structure. Limnology and Oceanography 44: 810–827.
De Bie, T., S. Declerck, K. Martens, L. De Meester & L. Brendonck, 2008. A comparative analysis of cladoceran communities from different water body types: patterns in community composition and diversity. Hydrobiologia 597: 19–27.
Dodson, S. I., R. A. Lillie & S. Will-Wolf, 2005. Land use, water chemistry, aquatic vegetation, and zooplankton community structure of shallow lakes. Ecological Applications 15: 1191–1198.
Dodson, S. I., W. R. Everhart, A. K. Jandl & S. J. Krauskopf, 2007. Effect of watershed land use and lake age on zooplankton species richness. Hydrobiologia 579: 393–399.
Drake, J. A., C. Zimmerman, T. Purucker & C. Rojo, 1999. On the nature of the assembly trajectory. In Weiher, E. & P. Keddy (eds), Ecological Assembly Rules. Cambridge University Press, Cambridge: 233–251.
Dussart, B., 1966. Limnologie, l’étude des eaux continentales. Gautier Villars, Paris.
Ferrari, I. & G. Rossetti, 2006. New records of the centropagid Boeckella triarticulata (Thomson, 1883) (Copepoda: Calanoida) in Northern Italy: evidence of a successful invasion? Aquatic Invasions 1: 219–222.
Ferrari, I., A. Farabegoli, A. Pugnetti & E. Stella, 1991. The occurrence of a calanoid Australasian species, Boeckella triarticulata (Thomson), in fish ponds of Northern Italy. Verhandlungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 24: 2822–2827.
Flössner, D., 2000. Die Haplopoda und Cladocera Mitteleuropas. Backhuys Publishers, Leiden.
Gherardi, F., S. Bertolino, M. Bodon, S. Casellato, S. Cianfanelli, M. Ferraguti, E. Lori, G. Mura, A. Nocita, N. Riccardi, G. Rossetti, E. Rota, R. Scalera, S. Zerunian & E. Tricarico, 2008. Animal xenodiversity in Italian inland waters: distribution, modes of arrival, and pathways. Biological Invasions 10: 435–454.
Hutchinson, G. E., 1957. A Treatise on Limnology. Vol. I Geography, Physics and Chemistry. Part 1 Geography and Physics of Lakes. Wiley, New York.
Hutchinson, G. E., 1959. Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist 93: 145–159.
Hutchinson, G. E., 1961. The paradox of the plankton. The American Naturalist 95: 137–145.
Hutchinson, G. E. & R. H. MacArthur, 1959. A theoretical ecological model of size distribution among species of animals. The American Naturalist 93: 117–125.
ISTAT, 1986. Annali di Statistica–Regione Sicilia. Istituto Nazionale di Statistica, Rome.
Jenkins, D. G. & A. L. Buikema, 1998. Do similar communities develop in similar sites? A test with zooplankton structure and function. Ecological Monographs 68: 421–443.
Lampert, M. & U. Sommer, 2007. Limnoecology, 2nd ed. Oxford University Press, Oxford.
Madonia, P., L. Naselli-Flores, P. Parello, B. Parlato & A. Viola, 2006. Geological development of a gypsum lake formed at the beginning of the 20th century in central Sicily, Italy: integration of historical data with modern survey techniques. Chemistry and Ecology 22(Suppl. 1): 333–347.
Margaritora, F. G., 1985. Fauna d’Italia: Cladocera. Ed. Calderini, Bologna.
Marrone, F., R. Barone & L. Naselli-Flores, 2005. Cladocera (Branchiopoda: Anomopoda, Ctenopoda, and Onychopoda) from Sicilian inland waters: an updated inventory. Crustaceana 78: 1025–1039.
Marrone, F., R. Barone & L. Naselli-Flores, 2006. Ecological characterization and cladocerans, calanoid copepods and large branchiopods of temporary ponds in a Mediterranean island (Sicily, southern Italy). Chemistry and Ecology 22(Suppl. 1): 181–190.
Marrone, F., G. Castelli & L. Naselli-Flores, 2009. Sicilian temporary ponds: an overview of the composition and affinities of their crustacean biota. In Fraga, P. (ed.), Proceedings of the International Conference on Mediterranean Temporary Ponds, Consell Insular de Menorca, Maò, Menorca, Recerca 14: 189–202.
Minelli, A., C. Chemini, R. Argano & S. Ruffo, 2004. Wildlife in Italy. Touring Editore, Milan & Italian Ministry for the Environment and Territory, Rome: 448 pp.
Moss, B., 2008. Water pollution by agriculture. Philosophical Transactions of the Royal Society B 363: 659–666.
Naselli-Flores, L., 1999. Limnological aspects of Sicilian reservoirs: a comparative, ecosystemic approach. In Tundisi, J. G. & M. Straškraba (eds), Theoretical Reservoir Ecology and Its Applications. Backhuys, Leiden: 283–311.
Naselli-Flores, L., 2003. Man-made lakes in Mediterranean semi-arid climate: the strange case of Dr Deep Lake and Mr Shallow Lake. Hydrobiologia 506(509): 13–21.
Naselli-Flores, L., 2010. Mediterranean climate and eutrophication of reservoirs: limnological skills to improve management. In Ansari, A. A., S. Singh, G. R. Lanza & R. Walter (eds), Eutrophication: Causes, Consequences and Control. Springer, Dordrecht (in press).
Naselli-Flores, L. & R. Barone, 1994. Relationship between trophic state and plankton community structure in 21 Sicilian dam reservoirs. Hydrobiologia 275(276): 197–205.
Naselli-Flores, L. & R. Barone, 1997. Importance of water-level fluctuations on Cladoceran dynamics in a hypertrophic reservoir. Hydrobiologia 360: 223–232.
Naselli-Flores, L., J. Padisák, J. M. Dokulil & I. Chorus, 2003. Equilibrium/steady-state concept in phytoplankton ecology. Hydrobiologia 502: 395–403.
Ortega-Mayagoitia, E., C. Rojo & M. A. Rodrigo, 2002. Factors masking the trophic cascade in shallow eutrophic wetlands-evidence from a microcosm study. Archiv für Hydrobiologie 155: 43–63.
Padisák, J., 1992. Seasonal succession of phytoplankton in a large shallow lake (Balaton, Hungary) – a dynamic approach to biological memory, its possible role and mechanisms. Journal of Ecology 80: 217–230.
Reid, G. K., 1961. Ecology of Inland Waters and Estuaries. Reinhold Publishing Corporation, New York.
Reynolds, C. S., 1993. Scales of disturbance and their role in plankton ecology. Hydrobiologia 249: 157–171.
Reynolds, C. S., J. Padisák & U. Sommer, 1993. Intermediate disturbance in the ecology of phytoplankton and the maintenance of species diversity: a synthesis. Hydrobiologia 249: 183–188.
Riccardi, N., G. Giussani, F. Margaritora & B. Couchaud, 2004. Population dynamics of the pioneer population of Daphnia parvula, Fordyce during the invasion of Lake Candia (Northern Italy). Journal of Limnology 63: 44–52.
Rodrigo, M. A., C. Rojo, M. Segura & J. Larrosa, 2009. Mechanisms of microalgae selection during the assembly of a planktonic community. Aquatic Ecology 43: 61–72.
Ruffo, S. & F. Stoch, 2005. Checklist e distribuzione della fauna italiana. 10.000 specie terrestri e delle acque interne. Memorie del Museo Civico di Storia Naturale di Verona, serie 2. Sezione Scienze della Vita 16: 1–307.
Schell, J. M., C. J. Santos-Flores, P. E. Allen, B. M. Hunker, S. Kloehn, A. Michelson, R. A. Lillie & S. I. Dodson, 2001. Physical-chemical influences on vernal zooplankton community structure in small lakes and wetlands of Wisconsin U.S.A. Hydrobiologia 445: 37–50.
Sommer, U. & B. Worm, 2002. Competition and Coexistence. Ecological Studies 161. Springer, Berlin.
Stoch, F., 2006. L’assetto zoogeografico dell’Appennino Centro-settentrionale. Biogeographia 27: 129–150.
Thomaz, S. M., T. S. Michelan, P. Carvalho & M. L. Bini, 2010. The influence of “Homage to Santa Rosalia” on aquatic ecology: a scientometric approach. Hydrobiologia (in press). doi:10.1007/s10750-010-0342-4.
Ward, S. A. & W. B. Thornton, 2000. Chance and determinism in the development of isolated communities. Global Ecology and Biogeography 9: 7–18.
Warwick, R. M., K. R. Clarke & Suharsono, 1990. A statistical analysis of coral community responses to the 1982–83 El Niño in the Thousand Islands, Indonesia. Coral Reefs 8: 171–179.
Wiggins, D. A., 1999. The peninsula effect on species diversity: a reassessment of the avifauna of Baja California. Ecography 22: 542–547.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: L. Naselli-Flores & G. Rossetti / Fifty years after the “Homage to Santa Rosalia”: Old and new paradigms on biodiversity in aquatic ecosystems
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alfonso, G., Belmonte, G., Marrone, F. et al. Does lake age affect zooplankton diversity in Mediterranean lakes and reservoirs? A case study from southern Italy. Hydrobiologia 653, 149–164 (2010). https://doi.org/10.1007/s10750-010-0350-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0350-4