Abstract
Few studies have examined zooplankton assemblages associated with grey willow (Salix cinerea) invasions in wetlands. Our aim was to quantitatively examine zooplankton composition among S. cinerea stands within the South Taupō Wetland, New Zealand, to determine whether these assemblages are affected by willow growth and willow control treatment using the herbicide metsulfuron (C14H15N5O6S). Alternatively, we examined whether wetland hydrology had an over-riding influence. Sampling was performed on three occasions (late-summer, mid-winter, and early-summer). Using Multidimensional Scaling and ANOSIM, we found no significant differences in zooplankton composition or environmental variables among native vegetation, live and dead S. cinerea sites, except for a difference in willow canopy density between late summer and winter. However, zooplankton composition differed on either side of a sand bar, suggesting areas separated by this barrier functioned independently. Overall, we found zooplankton communities to be regulated more by wetland hydrology than by willow presence. A limited willow effect was possibly due to the wetland being at an early stage of invasion, representing stand-alone individuals, with a continuous canopy not yet having formed. Alternatively, willows have lesser effects on invertebrates in wetlands than in streams. Ground control treatment of S. cinerea using metsulfuron had no apparent impact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the Northern Hemisphere, plant species of the genus Salix provide various ecological benefits; they are seen as ideal for river training and erosion control, and their wide spreading fibrous root systems help bind soil on stream- and hill-sides (Russell 1994). In the Southern Hemisphere, however, Salix species are considered ‘invasive’, having become widespread, with substantial ecological and economic impacts on wetland ecosystems (Adair et al. 2006). The introduction of Salix species to New Zealand was deliberate, with different species planted along waterways to provide erosion protection for riverbanks and for soil conservation purposes (Williams and West 2000). Salix cinerea L. (a.k.a., grey willow) was introduced by the 1870s (Thompson and Reeves 1994), and grows across a wide soil fertility range, from nutrient rich swamps to peat bogs, with only saline or high-altitude sites beyond its limits (Partridge 1994). The dispersal of small seeds adapted to long-distance wind dispersal, vegetative propagation, an ability to tolerate a variety of environmental conditions, and rapid growth rates, has resulted in their widespread distribution (Webb et al. 1988). Further, grey willow exhibits high germination rates during flooding, siltation and fire events (Champion 1994). There are thus few wetlands in New Zealand that have not been colonised by S. cinerea, and many of those invaded have high density growths (Webb et al. 1988; de Winton and Champion 1993). New Zealand wetlands have a high risk of weed invasion, in part due to their low stature native vegetation communities (Owen 1998). Salix cinerea is typically found in areas of open water, which are naturally dominated by lower growing reeds such as raupō (Typha orientalis C.Presl), sedges such as Carex secta Boott, small wetland shrubs, and herbs. Invasion of tall S. cinerea can thus lead to displacement of native plant communities, as the willows’ dense canopy shades the low growing species (Eser 1998; Partridge 1994; Thompson and Reeves 1994).
Intensive willow control programmes are perceived as an important option for restoring wetland vegetation in New Zealand. Tools for wetland willow control include combinations of mechanical and chemical ground-based treatments, which often have limited success and various disadvantages (Williams and West 2000; Husted-Andersen 2002). Emphasis on Salix control throughout New Zealand wetlands has been based on willow kill rates, and restoring and maintaining native wetland vegetation types. Few studies, however, have examined the impacts of willow, or its control, on other aquatic life (Collier 1994; Serra et al. 2013; McInerney et al. 2016). Aquatic invertebrates inhabit the bottom substrate, swim in the water column, or live on the surface of the water, providing an important link between primary producers and high trophic levels (e.g., fish and birds) in aquatic foodwebs (Hornung and Foote 2006; Suren and Sorrell 2010). Research undertaken on the impacts of willows on invertebrates in New Zealand is to date primarily limited to their effects on benthic macroinvertebrates in stream and river ecosystems, rather than wetlands (Collier 1994; Glova and Sagar 1994; Lester et al. 1994). These studies have demonstrated that willow density can determine their ecological impacts; densely willow lined sections can be detrimental to aquatic invertebrates, whereas moderate plantings of riparian willow can improve aquatic invertebrate habitat conditions.
Zooplankton are particularly sensitive to environmental conditions (Attayde and Bozelli 1998; Duggan et al. 2002; Lougheed and Chow-Fraser 2002). Nevertheless, they have rarely been studied in wetlands globally (Schoenberg 1988; Lougheed and Chow-Fraser 2002; Medley and Havel 2007). Composition and dynamics of zooplankton in wetlands are regulated by a diverse and complex range of biotic and abiotic factors such as wetland vegetation type, hydrologic fluctuations, depth of water column, local climate and food web traits (Ortega-Mayagoitia et al. 2000; Duggan 2001; Medley and Havel 2007; Lucena-Moya and Duggan 2011). Zooplankton are an important component of wetland foodwebs, as they provide a vital link for energy flow connecting primary producers of plants and algae to secondary consumers, such as fish and birds (Lougheed and Chow-Fraser 1998; Lougheed and Chow-Fraser 2002). The aim of this research was to quantitatively examine zooplankton community composition among S. cinerea stands within the South Taupō Wetland, New Zealand, to determine whether zooplankton assemblages are affected by willow growth and willow control treatment. As variability in zooplankton community composition is commonly found to be influenced by hydrological conditions, we determined the relative importance of this compared with vegetation type.
Methods
Study Area
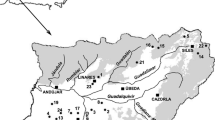
The South Taupō Wetland is one of the largest wetlands in the North Island, New Zealand (area 1500 ha), and is situated on the southern shores of Lake Taupō (Fig. 1). The wetland was formed following the last Taupō eruption, c. 1800 years ago, by the deposition of tephra onto the surrounding landscape, which was eroded and transported by the Tongariro, Waiotaka and Waimarino Rivers. This process formed the Tongariro Delta, many oxbows, and a series of beach ridges and hollows that run parallel to the lake edge, resulting in low lying waterlogged area (Singers 2009). The hydrology of the wetland is influenced by regular flooding by the three main rivers, annual rainfall of approximately 1.2 m to 3.0 m (Cromarty and Scott 1995), groundwater from surrounding areas discharging into the wetland, and water level fluctuations from the adjacent lake (Eser 1998).
The major current threat to the South Taupō Wetland is the rapid invasion of Salix cinerea and its displacement of indigenous wetland vegetation (Cromarty and Scott 1995; Eser 1998; DOC 2002; Singers 2009). Salix cinerea was first observed in the late 1970s, and by 1984 a dense forest had covered 67.2 ha and a further 220.2 ha was colonised by young, scattered S. cinerea shrubs. By 1996, various densities of S. cinerea had covered a total area of 432 ha throughout the wetland (Eser 1998). The Waiotaka Scenic Reserve, part of the South Taupō Wetland, is 29.18 ha in size, a low gradient wetland bordering the shore of Lake Taupō, created with beach ridges, pumice and greywacke alluvium. The hydrosystem is riverine, influenced by the Waiotaka River floodplain. The reserve consists of tī kōuka (Cordyline australis (G.Forst.) Endl.) and kānuka (Kunzea ericoides (A.Rich) Joy Thomps.) forest on the dune ridges, sedge rushland (Machaerina rubiginosa (Spreng.) Koyama) peat bog, raupō (Typha orientalis) reedland, mānuka (Leptospermum scoparium J.R.Forst & G.Forst.) shubland, flax (Phormium tenax J.R.Forst & G.Forst.) land, toetoe (Austroderia toetoe N.P.Baker & H.P.Linder), tussockland and open water (Department of Conservation 2002). The reserve has been invaded by a variety of non-native plants, including Salix cinerea. The site chosen for the current study consists of two blocks divided by a sandbar, which provides a hydrological disconnection between each side. Block 1 has an area of 8.4 ha, located parallel to State Highway 1. Ground control of S. cinerea took place in Block 1 in summer 2007/2008 using a variety of methods, including vehicle mounted spraying, cut and gel, and drill and inject. Block 2 has an area of 6.3 ha, located closest to the lake shore, separated from the lake by a car park and boat access. Block 2 had received no willow control prior to this study.
Seven native sites (N1-N7) were chosen in both Block 1 and 2 that represented indigenous wetland plant species that were not encroached by willow. These sites consisted of raupō (Typha orientalis), Austroderia toetoe and sedges, including Machaerina rubiginosa and Carex secta, and open water. This mix of low statured native vegetation reflects the most favourable communities for S. cinerea to potentially invade. Seven native sites were selected based on permanently wet areas and located close to live or dead S. cinerea trees. Seven live S. cinerea sites (L1-L7) were chosen in Block 2 that had never been treatedcinereal; individual trees selected were taller than 2 m, scattered throughout the block and located in permanently wet areas. Dead S. cinerea sites (D1-D7) were chosen in Block 1, which contained S. cinerea that were treated in summer 2007/2008. Seven dead S. cinerea individuals taller than 2 m, scattered throughout the block and located in permanently wet areas, were selected for this study.
Environmental Measurements
Sampling was undertaken in February (late summer), July (winter) and December (early summer) 2011, to encompass seasonal variation. During these times S. cinerea was in late summer leaf, had lost their leaves (winter), or was in early summer bloom, respectively. Sampling was undertaken by wading, with the wetland accessible during high water depths using chest waders. Temperature (°C), dissolved oxygen (mg/L), specific conductance (μS/cm @25 °C) and pH were measured at each sampling site, using a multiparameter probe (model YSI 85) and a pH probe (model Oakton Waterproof pHTestr10). Water depth (cm) was measured with a wooden ruler from the substrate to the surface of the water. Canopy cover (%) of dead and living S. cinerea was measured using a Spherical Densiometer Model A instrument by noting whether overhead shade occurred on each of the 25 squares, as described in Harding et al. (2009). Percentage ground cover of vegetation at each site was visually estimated for plant species at each site. While 21 samples were collected in February and June 2011, only 17 sites could be resampled in December due to lower water levels; three live willow sites (L2, L3 and L4) and one dead willow site (D1) had inadequate volumes of water to sample.
Chlorophyll a was analysed by collecting filtrate of 60 ml of undisturbed water from the water column at each site, through a 0.45 μm glass fibre microfilter. The filter was then folded in half, wrapped in aluminium foil and stored immediately on ice until returned the laboratory, where it was stored frozen in the dark until analysis. To extract and measure chlorophyll a the method of Arar and Collins (1997) was followed. Each filter paper was steeped in 90% acetone solution (buffered with magnesium carbonate) for 12 h, and ground. Samples were then centrifuged and measured for fluorescence using a 10-AU fluorometer for chlorophyll a.
Zooplankton Sampling
For zooplankton collection, 10 L of undisturbed water was collected using a 2 L plastic jug from each site and filtered through a 40 μm mesh. Each sample was placed in a 250 ml container and immediately filled with 95% ethanol to attain a final concentration of at least 50% ethanol for preservation. In the laboratory, zooplankton samples were diluted to a known volume dependant on the amount of sediment and detrital matter in the sample, to facilitate ease of counting. Subsamples of 5 ml were taken using an autopipette, placed in an open-topped Perspex counting tray (50 mm × 80 mm) on a moveable microscope stage and enumerated using a stereo microscope (model Nikon SM2800). Successive subsamples were counted until at least 300 individuals total were obtained, or until the entire sample was counted if less were encountered. Species were identified using a compound microscope (model Olympus BX50 microscope) and appropriate literature (Chapman and Lewis 1976; Shiel 1995).
Data Analysis
In each season, environmental variables were compared between 1) natural sites (N1-N7), live willow sites (L1-L7), and dead willow sites (D1-D7), using ANOVA, and between 2) Block 1 and Block 2 sites using t-tests. Datasets were tested for normality using Shapiro-Wilk’s tests and for homogeneity of variances using Levene’s tests; in the case of non-normality or non-homogenous variance, the data were log-transformed (x + 1) to meet this assumption. On two occasions, normality and homogeneity of variances were not achieved following transformation. In these cases (canopy density in July and water depth in December), a non-parametric Kruskal-Wallis test was performed. Due to multiple comparisons being undertaken, we considered only values of p < 0.01 to be significantly different for environmental data.
Multidimensional scaling (MDS) and Analysis of Similarities (ANOSIM) were used to identify patterns in community composition and to determine which environmental variables were associated with underlying trends in species distribution. MDS ordinations were constructed based on a ranked Bray-Curtis similarity matrix (Clarke et al. 2014). Species data were log-transformed (x + 1) to reduce the influence of highly abundant taxa. Rare taxa were removed from the analysis in order to remove the influence of species potentially sampled by chance. Common taxa were defined as those present in two or more samples found in the sampling season being analysed. Copepod nauplii (likely all from cyclopoid copepods) were included in multivariate analyses, separate from adults and copepodites (grouped as ‘cyclopoid copepods’), as nauplii are smaller, and use different resources than the later stages, and are thus likely to be influenced by different environmental variables. Sites were removed from analyses where very low numbers of individuals were recorded (total counts less than 5 per L found in a sample). As sub-adult copepodite stages of cyclopoid copepods were difficult to assign to species, these were treated as a single group (‘cyclopoid copepods’) in analyses. To determine the effects of willow growth and willow control on zooplankton community composition, sample periods were divided into three seasonal groups (February, July and December 2011), and the two blocks (Block 1 and Block 2). Associations between zooplankton community composition and vegetation type (live willow, dead willow, native vegetation) or block in each sampling period were investigated firstly by superimposing these variables onto the MDS ordinations. ANOSIM was then undertaken on the Bray-Curtis similarity matrix to test whether the community differences observed between the vegetation types and blocks were statistically significant. ANOSIM is a non-parametric permutation test used with multivariate data to test a priori hypotheses (Clarke et al. 2014). The analysis provides a measure of the dissimilarity of groups of samples shown by an R-statistic that usually lies between 0 and 1. Values close to one indicate that the groups are dissimilar and those closest to zero demonstrate that groups are similar. Where significant ANOSIM results were found, a SIMPER analysis was conducted to determine which taxa were primarily responsible for the dissimilarity between sites. This was done by determining the species that most greatly discriminated between groups; we considered only species that contributed greater than 10% to the dissimilarity between groups (Clarke et al. 2014). All multivariate analyses were undertaken using PRIMER 6.0 (Plymouth Marine Laboratory).
Results
Environmental Variables
For water depth, water temperature, pH, specific conductance and chlorophyll a, no significant differences were observed among vegetation types or between blocks throughout the study (P > 0.01; Fig. 2). Average dissolved oxygen concentrations were not found to differ among dead willow, live willow and native sites. However, in February (late-summer; t = 4.22) and July (winter; t = 4.60), dissolved oxygen concentrations were found to be higher in Block 1 than Block 2 (P < 0.001). Canopy density was greater among live willow during February (t = 6.01; P < 0.001) and July (Kruskal-Wallis H = 9.8; P < 0.005), but not in December (P > 0.05). Average vegetation ground cover for native sites consisted of 75% Machaerina rubiginosa and 20% open water, with a mix of Carex secta, Typha orientalis and Austroderia toetoe. Live willows comprised 40% M. rubiginosa and 31% open water, with a combination of C. secta, A. toetoe, Apodasmia similis (Edgar) Briggs et L.A.S.Johnson, Coprosma robusta Raoul and T. orientalis making up the remainder. Dead willows sites comprised 44% open water and 27% M. rubiginosa, with limited quantities of A. similis, C. robusta, Phormium tenax and young shoots of S. cinerea.
Environmental variables measured in Waiotaka Scenic Reserve, for February (left panels), July (middle), and December (right panels). NS indicates no significant difference was found in variables among vegetation type or between wetland blocks. Significance levels are shown where significant differences were found (P < 0.01)
Zooplankton Composition and Dynamics
Eighteen rotifer taxa were recorded during the study, and eight cladoceran taxa (Table 1). A range of cyclopoid copepod species were identified from adults, Acanthocyclops robustus, Diacyclops bicuspidatus, Eucyclops serralatus, Mesocyclops australiensis, Paracyclops fimbriatus and Tropocyclops prasinus; however, as most individuals collected were sub-adult copepodites, and therefore unidentifiable, these were treated as a single group (‘cyclopoid copepods). Additionally, tardigrades, gastrotrichs and ostracods were also recorded. All taxa identified had previously been recorded in New Zealand, except for the rotifer Tetrasiphon hydracora (Shiel and Green 1996).
In the MDS ordinations, samples that have similar species composition are placed closer together, and those that are dissimilar further apart. In the MDS ordination for February 2011, samples from dead willow sites were distributed primarily on the top left of the ordination, while live willow samples were primarily across the bottom of the ordination; samples from natural sites were distributed throughout the ordination (Fig. 3A). ANOSIM indicated no significant differences in zooplankton composition among the vegetation types (Global R = -0.006; P > 0.05). Considering sites in Block 1 and 2 of the wetland, Block 1 samples were distributed on the top left of the ordination while Block 2 sites were distributed on the bottom and right; natural sites on either side of the sandbar were most closely associated with willows from the same side (Fig. 3B). ANOSIM results for differences in zooplankton composition between Blocks 1 and 2 was statistically significant (R = 0.212; P = 0.02). SIMPER indicated the taxa most responsible for differences were, in order of importance, Simocephalus vetulus, ostracods, copepod nauplii (likely all comprising cyclopoid nauplii), Chydorus sp. and cyclopoid copepods (copepodites and adults), which were all in higher abundances in Block 2 than 1 (Table 2). The MDS ordination for July followed the same trend, with samples from dead willow distributed primarily on the top left of the ordination, while live willow samples were primarily near the bottom and right (Fig. 3C). Again, ANOSIM found no significant difference among the vegetation types (Global R = 0.109; P > 0.05). Block 1 samples were distributed more clearly on the top left, and Block 2 samples on the bottom right, with natural sites grouping among willow sites from the same sides of the sandbar (Fig. 3D). ANOSIM again indicated a significant difference in zooplankton communities between Blocks 1 and 2 (Global R = 0.563; P = 0.02). SIMPER analysis indicated Chydorus sp., copepod nauplii and S. vetulus were all in higher abundance in Block 1 than 2, and cyclopoid copepods and ostracods were higher in Block 2 than 1; for Chydorus sp., cyclopoid copepod nauplii and S. vetulus, these were the opposite of trends from that found in February (Table 2). The MDS ordination for December showed no clear patterns for zooplankton communities among vegetation types or Blocks (Fig. 3D, E). ANOSIM results for vegetation types (Global R = 0.066; P > 0.05) and Blocks (Global R = 0.016; P > 0.05) indicated no differences in community composition among groups in this season.
Multidimensional scaling (MDS) ordination plots illustrating similarities in zooplankton community composition among sites in Waiotaka Scenic Reserve, for February (A, B), July (C, D), and December (E, F). In the left-hand panels, sites are superimposed with symbols indicating whether they were dominated by native vegetation, or associated with live or dead willow. In the right-side panels, sites are superimposed with symbols indicating position in the wetland (Block 1 or Block 2)
Discussion
No significant differences in zooplankton community composition were found between the native, living, and dead willow sites in any season, indicating that willow (growth and control) had no effect on zooplankton community composition in South Taupō Wetland. Further, these results also indicate that ground control application of metsulfuron resulted in no significant longer-term (3–4 years) changes in zooplankton composition. We did, however, find differences in species composition on either side of the sand bar, suggesting that the hydrology of Blocks 1 and 2 functioned independently, and that hydrology is a more important driver of zooplankton composition in the wetland than the invasion of willows.
Zooplankton community composition differed between Block 1 and 2, on either side of a sand bar in the wetland. In the months where differences in zooplankton communities were found, February (late-summer) and July (mid-winter), the same species were found to be important in determining these differences according to SIMPER. However, for these species, each month exhibited differing trends, with the cladocerans Simocephalus vetulus and Chydorus sp., and cyclopoid copepod nauplii, all higher in abundance in Block 2 than Block 1 in late-summer, but lower in Block 2 than Block 1 in July. Unmeasured hydrological differences and variations in water depth are likely to have played a role in these patterns, as the cladocerans and copepod nauplii are more free-swimming taxa, and will prefer deeper water. Although water depth differences were not found to be significant, Block 2 had higher measured water levels than Block 1 in February, when the adjacent Lake Taupō levels were high, while Block 2 had lower water levels than Block 1 in July, when rainfall was high and Lake Taupō water levels were lower; this inconsistency, along with having a sandbar separating them, indicates that these blocks act hydrologically independently. Among constructed treatment wetlands, drainage ditches and adjacent lakes in rural areas in New Zealand, Eivers et al. (2018) found that depth of habitat was an important predictor of zooplankton composition, with cladocerans more important in deeper habitats. Other studies have also found hydrology (including flooding frequency and hydroperiod) to have significant effects on zooplankton community composition within or among wetlands; for example, in floodplain ponds in Missouri, USA (Medley and Havel 2007), and in a semi-arid wetland in Spain (Ortega-Mayagoitia et al. 2000). Oxygen concentrations were found to differ between blocks, but not in a manner consistent with changes in zooplankton communities; dissolved oxygen concentrations were higher in Block 1 than Block 2 in both late-summer and winter. Nevertheless, the separation of Block 1 than Block 2 by a sandbar is likely responsible for such differences.
The presence of grey willow, dead or alive, had little effect on zooplankton community composition. This finding is in common with Wech et al. (2018), who found no significant changes in macroinvertebrate community composition (including of cladocerans and copepods) in the year following aerial application of glyphosate over the swamp willow canopy, in Whangamarino wetland, New Zealand. Kratzer and Batzer (2007) found little variation in aquatic macroinvertebrate communities in Okefenokee Swamp, Florida, USA, despite sampling in different plant habitats, including areas with and without trees; this result was attributed to water quality not varying greatly throughout the wetland. We similarly found no significant differences in environmental variables among native, live and dead S. cinerea, with the exception of canopy density in February and July. Aquatic macroinvertebrate communities have been found to differ in New Zealand, Australian and South American streams and rivers in the presence of dense willow (e.g., Read and Barmuta 1999; Serra et al. 2013; McInerney et al. 2016). In New Zealand, Lester et al. (1994) observed significantly lower invertebrate densities in willow-lined sections of streams in Otago than in nearby open sections in summer, autumn, and winter. This was attributed to a decrease in average substrate size and/or a lowering of food production through shading effects. Conversely, Glova and Sagar (1994) found that diversity and abundance of benthic macroinvertebrates to be greater in the willowed than the non-willowed sections of streams in the North and South Islands, which they related to the degree of periphyton growth; these authors argued that in open sections of stream, dense periphytic growth may have reduced the presence of grazing invertebrates relative to willowed sections. In our study, the lack of variability in zooplankton among native, live and dead willow sites (as seen by Wech et al. (2018) for macroinvertebrates), may indicate that willows have lesser effects on aquatic invertebrates in wetlands than streams. Nevertheless, this does not appear to be the case for terrestrial invertebrates, with beetle composition found to differ between willow and native sites in New Zealand wetlands, and for composition to change following treatment (Watts et al. 2012, 2015). Conversely, in our study, we may have seen limited effects due to the S. cinerea trees representing stand-alone individuals, which is typical of early stages of invasion, with a continuous canopy not yet formed; when such a canopy has formed, environmental variability across the wetland is likely to be enhanced.
ANOVA analysis for canopy density indicated significant differences between live and dead willow in late-summer and winter, where live willow canopy density was higher than dead willow canopy density. This was expected for February, in particular, as Salix cinerea is in full leaf form from early to late summer (Webb et al. 1988). However, this does not explain the difference in canopy density between live and dead willow in July, as the live willow had lost their leaves in winter. As the dead willow trees had been poisoned three years prior to our study, it is likely they had started to break down, with branches being lost from the main trunk. With high canopy density cover among willow during the summer period we might also have expected to see lower chlorophyll a concentrations and water temperature due to shading. For example, Glova and Sagar (1994) and Lester et al.’s (1994) studies on small rivers and streams demonstrated that willows shade out algal production. Overall, apart from shading, live and dead willows in this study seemingly made no significant difference to environmental variables in the water below them, or relative to natives. This could be due to the willow representing stand-alone individuals, with a continuous canopy not yet formed. The density of willows seems to play a major factor affecting ecological impact on streams and rivers (Collier 1994; Glova and Sagar 1994; Lester et al. 1994).
As differences were not found between areas with dead willows and other sites, ground control treatment of grey willow using metsulfuron appears to have no long-term impacts on zooplankton communities. Many herbicides persist for up to three months in freshwater, suggesting that they are capable of producing adverse effects on freshwater zooplankton, at least in the short-term (Rico-Martínez et al. 2012). For metsulfuron, the length of time required for half of the material to dissipate in water has been estimated as being >84 days when high concentrations are applied (Thompson et al. 1992). Toxicity tests with the zooplankton species Daphnia magna found the concentration that will kill 50% of the sample population over 48-h (i.e., the ‘lethal concentration’, or LC50) of greater than 150 mg/l (USEPA 1986). Nevertheless, the dead willow trees had been poisoned three years prior to our study, while application method was likely also important, with the herbicide having been applied using the ‘drill and inject’ method, ensuring the herbicide does not enter the water directly. Overall, no longer term-effects, even indirectly from the death of the willows, could be detected due to herbicide use.
Conclusions
Variation in zooplankton community composition within the South Taupō Wetland was explained more by variability in wetland hydrology than by the presence of non-native willows (Salix cinerea). Nevertheless, the limited effects by willows observed here may be a result of the trees representing stand-alone individuals, due to being at an early stage of invasion. When a continuous canopy has formed, leading to greater shading of primary producers, the effects of willow may be more pronounced. The ground treatment of S. cinerea using metsulfuron appears to have had no apparent longer-term impacts.
References
Adair R, Sagliocco J, Bruzzes E (2006) Strategies for the biological control of invasive willows (Salix spp.) in Australia. Australian Journal of Entomology 45:259–267
Arar E, Collins G (1997) In Vitro determination of chlorophyll a Pheophytin a in marine and freshwater algae by Flurescence. U.S. Environmental Protection Agency (USEPA) method 445.0, revision 1.2, September 1997
Attayde JL, Bozelli RL (1998) Assessing the indicator properties of zooplankton assemblages to disturbance gradients by canonical correspondence analysis. Canadian Journal of Fisheries and Aquatic Sciences 55:1789–1797
Champion PD (1994) Extent of willow invasion - the threats. Proceedings of the wild willows in New Zealand. Proceedings of a willow control workshop hosted by Waikato conservancy, Hamilton, New Zealand. Department of Conservation. Pp 43-49
Chapman MA, Lewis MH (1976) An introduction to the freshwater Crustacea of New Zealand. William Collins, Auckland
Clarke KR, Gorley RN, Somerfield PJ, Warwick RM (2014) Change in marine communities: an approach to statistical analysis and interpretation, 3nd edn. PRIMER-E, Plymouth
Collier K (1994) Effects of willows on waterways and wetlands - an aquatic habitat perspective. Proceedings of the Wild Willows in New Zealand. Proceedings of a Willow Control Workshop hosted by Waikato Conservancy, 24–26 November 1993, Hamilton. Department of Conservation. pp 33–42
Cromarty P, Scott DA (eds) (1995) A directory of wetlands in New Zealand. Wellington, NZ, Department of Conservation
de Winton MD, Champion PD (1993) The vegetation of the lower Waikato Lakes, volume 1: factors affecting the vegetation of the lower Waikato lakes. NIWA Ecosystems Publication No. 7
Department of Conservation (2002) Tongariro/Taupo conservation management strategy 2002–2012. Wellington, Department of Conservation
Duggan IC (2001) The ecology of periphytic rotifers. Hydrobiologia 446:139–148
Duggan IC, Green JD, Shiel RJ (2002) Distribution of rotifer assemblages in North Island, New Zealand, lakes: relationships to environmental and historical factors. Freshwater Biology 47:195–206
Eivers RS, Duggan IC, Hamilton DP, Quinn JM (2018) Constructed treatment wetlands provide habitat for zooplankton communities in agricultural peat lake catchments. Wetlands 38:95–108
Eser PC (1998) Ecological patterns and processes of the South Taupo wetland, North Island, New Zealand, with special reference to nature conservation management. Unpublished thesis, Victoria University Wellington
Glova GJ, Sagar PM (1994) Comparison of fish and macroinvertebrate standing stocks in relation to riparian willows (Salix spp.) in three New Zealand streams. New Zealand Journal of Marine and Freshwater Research 28:255–266
Harding J, Clapcott J, Quinn J, Hayes J, Joy M, Storey R, Greig H, Hay J, James T, Beech M, Ozane R, Meredith A, Boothroyd I (2009) Stream habitat assessment protocols for wadeable rivers and streams of New Zealand. Christchurch, New Zealand, University of Canterbury. 136 p
Hornung JP, Foote AL (2006) Aquatic invertebrate responses to fish presence and vegetation complexity in Western boreal wetlands, with implications to waterbird productivity. Wetlands 26:1–12
Husted-Andersen D (2002) Control of the environmental weed Salix cinerea (grey willow) in the South Taupo wetland, central North Island, New Zealand. Unpublished thesis, The Royal Veterinary and Agriculutural University, Copenhagen, Denmark
Kratzer EB, Batzer DP (2007) Spatial and temporal variation in aquatic macroinvertebrates in the Okefenokee swamp, Georgia, USA. Wetlands 27:127–140
Lester PJ, Mitchell SF, Scott D (1994) Effects of riparian willow trees (Salix fragilis) on macroinvertebrates densities in two small Central Otago streams, New Zealand. New Zealand Journal of Marine and Freshwater Research 28:267–276
Lougheed VL, Chow-Fraser P (1998) Factors that regulate the zooplankton community structure of a turbid, hypereutrophic Great Lakes wetland. Canadian Journal of Fisheries and Aquatic Sciences 55:150–161
Lougheed VL, Chow-Fraser P (2002) Development and use of a zooplankton index of wetland quality in the Laurentian Great Lakes basin. Ecological Applications 12:474–486
Lucena-Moya P, Duggan IC (2011) Macrophyte architecture affects the abundance and diversity of littoral microfauna. Aquatic Ecology 45:279–287
McInerney PJ, Rees GN, Gawne B, Suter P, Watson G, Stoffels RJ (2016) Invasive willows drive instream community structure. Freshwater Biology 61:1379–1391
Medley KA, Havel JE (2007) Hydrology and local environmental factors influencing zooplankton communities in floodplain ponds. Wetlands 27:864–872
Ortega-Mayagoitia E, Armengol X, Rojo C (2000) Structure and dynamics of zooplankton in a semi-arid wetland, the National Park Las Tablas de Daimiel (Spain). Wetlands 20:629–638
Owen SJ (1998) Department of conservation strategic plan for managing invasive weeds. Wellington, New Zealand, Department of Conservation. 86 p
Partridge TR (1994) Ecological impacts of willows in wetlands. Proceedings of the wild willows in New Zealand. Proceedings of a willow control workshop hosted by Waikato conservancy, Hamilton, New Zealand. Department of Conservation. Pp. 17-22
Read MG, Barmuta LA (1999) Comparisons of benthic communities adjacent to riparian native eucalypt and introduced willow vegetation. Freshwater Biology 42:359–374
Rico-Martínez R, Arias-Almeida JC, Pérez-Legaspi IA, Alvarado-Flores J, Retes-Pruneda JL (2012) Adverse effects of herbicides on freshwater zooplankton. Herbicides - properties, synthesis and control of weeds. In: El-Ghany Hasaneen MNA (ed) herbicides—properties, synthesis and control of weeds, pp 405–434
Russell GH (1994) Values and threats of willows in waterways - an engineer's perspective. Proceedings of the wild willows in New Zealand. Proceedings of a willow control workshop hosted by Waikato conservancy, Hamilton, New Zealand. Department of Conservation. Pp. 23-27
Schoenberg SA (1988) Microcrustacean community structure and biomass in marsh and lake habitats of the Okefenokee swamp, seasonal dynamics and responses to resource manipulations. Holarctic Ecology 11:8–18
Serra MN, Albarinõ R, Villanueva VD (2013) Invasive Salix fragilis alters benthic invertebrate communities and litter decomposition in northern Patagonian stream. Hydrobiologia 701:173–188
Shiel RJ (1995) A guide to the identification of rotifers, cladocerans and copepods from Australian inland waters. Co-operative research Centre for freshwater ecology. Murray-Darling Freshwater Research Centre, Albury
Shiel RJ, Green JD (1996) Rotifera recorded from New Zealand, 1859-1995, with comments on zoogeography. New Zealand Journal of Zoology 23:191–207
Singers N (2009) An overview of habitat types and significant sites in Tongariro Taupo conservancy. Tongariro Taupo, Department of Conservation, 139 p
Suren A, Sorrell B (2010) Aquatic invertebrate communities of lowland wetlands in New Zealand; Characterising spatial, temporal and geographic distribution patterns. Wellington, Department of Conservation, 62 p
Thompson DG, MacDonald LM, Staznik B (1992) Persistence of Hexazinone and Metsulfuron-methyl in a mixed-wood/boreal Forest Lake. Journal of Agricultural and Food Chemistry 40:1444–1449
Thompson K, Reeves P (1994) History and ecology of willows in New Zealand. Proceedings of the wild willows in New Zealand. Proceedings of a willow control workshop hosted by Waikato conservancy, Hamilton, New Zealand. Department of Conservation. Pp 3-16
USEPA (United States Environmental Protection Agency) (1986) Pesticide fact sheet number 71: Metsulfuron-methyl. Office of Pesticide Programs, Washington, DC
Watts C, Rohan M, Thornburrow D (2012) Beetle community responses to grey willow (Salix cinerea) invasion within three New Zealand wetlands. New Zealand Journal of Zoology 39:209–227
Watts C, Ranson H, Thorpe S, Cave V, Clarkson B, Thornburrow D, Bartlam S, Bodmin K (2015) Invertebrate community turnover following control of an invasive weed. Arthropod-Plant Interactions 9:585–597
Webb CJ, Sykes WR, Garnock-Jones PJ (1988) Flora of New Zealand volume IV, naturalised Pteridophytes, gymnosperms, Dicotyledons. Botany Division, Department of Scientific and Industrial Research, Christchurch, N.Z. 1365 pp
Wech J, Suren A, Brady M, Kilroy C (2018) The effect of willow control using a glyphosate formulation on aquatic invertebrates within a New Zealand wetland. New Zealand Journal of Marine and Freshwater Research 52:16–41
Williams JA, West CJ (2000) Environmental weeds in Australia and New Zealand: issues and approaches to management. Austral Ecology 25:425–444
Acknowledgments
L. Laboyrie and W. Powrie assisted with field and laboratory work, and B. O’Brien with microscopy. Department of Conservation Turangi Office, particularly L. Roberts, assisted with the planning and execution of this research. B. Clarkson (Manaaki Whenua) provided comments that improved our manuscript. YT received financial support from the Genesis Energy Committee, a Tūwharetoa Māori Trust Board Scholarship, a Motukawa Farm Trust Education Grant, the Tumate Mahuta Memorial Scholarship, Te Runanga o Ngāi Te Rangi Iwi Trust Tertiary Education Grant, a Tauwhao Te Ngare Trust Tertiary Education Grant, a Poripori Farm A Trust Tertiary Education Grant, the Rose Hellaby Postgraduate Scholarship, the Golden Plover Wetland Research Award and a Tongariro Natural History Society Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taura, Y.M., Duggan, I.C. The Relative Effects of Willow Invasion, Willow Control and Hydrology on Wetland Zooplankton Assemblages. Wetlands 40, 2585–2595 (2020). https://doi.org/10.1007/s13157-020-01359-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-020-01359-5