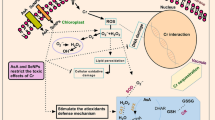
Abstract
Chromium (Cr) is a venomous heavy metal and environmental concern which threatened the global food safety due to its carcinogenic and mutagenic effects on biological systems. Some researchers have investigated the ameliorative role of ZnO-NPs to manage heavy metal toxicity in agricultural systems; however, their protective role has never been explored. Therefore, the present research was performed to explore the protective role of ZnO-NPs application under severe Cr toxicity in soybean seedlings. Soybean seedlings were supplemented with 100 µM Cr stress which caused deleterious effects on seed germination, plant growth, biomass, photosynthetic rate, disturbed PSII system, total soluble sugar, total soluble protein, nutrient acquisition by increasing the lipid peroxidation, and electrolyte leakage. Moreover, Cr stress also negatively affected the enzymatic antioxidative activities (SOD, POD, and CAT), and non-enzymatic activities (GR, GSH, GSSH) activities in soybean seedlings due to increased metal accumulation. However, foliar spray of ZnO-NPs mitigated Cr stress by reducing root and shoot Cr uptake as well as plant concentration of oxidative stress markers (MDA and H2O2). Moreover, a higher biomass, altered enzymatic and non-enzymatic antioxidant activities and increased nutrient uptake was observed in ZnO-NPs treated plants. This protective role might be due to the competitive superiority of ZnO-NPs over Cr to use similar in-planta transport channels. Our results revealed that the application of ZnO-NPs can alleviate Cr toxicity in soybean and offers a sustainable solution of crop growth in Cr-contaminated agricultural soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soybean (Glycine max L. Merril) is an essential legume that is a crucial source for edible oil production. It is also consumed as a protein concentrate for animals feeding worldwide (Sadak et al. 2020). Soybean seeds comprise approximately 20% edible oil, 35% protein, 35% carbohydrates (17% dietary fiber), and 5% ash as well as vitamins besides minerals (Rehal et al. 2019; Thomas and Erostus 2008). Soybean is cultivated across China and major cultivated zones are located in Southern Province (Bakhoum et al. 2019). The rapid industrialization and mining activities in adjacent areas resulted in the accumulation of toxicants in agricultural soil causing decreased crop yield and net production (Bakhoum et al. 2019). Besides, heavy metals toxicity modifies the soil physicochemical properties and is noxious for plant growth and development which may subsequently enter the edible plant parts and imposes serious health disorders in humans and animals (Bansal et al. 2002).
Chromium (Cr) being non-essential to plants is deliberated as 2nd utmost copious element on the earth crust (Mao et al. 2018). Cr occurs in trivalent Cr (III) or hexavalent Cr (VI) form inside the soil and is considered detrimental to both humans, and animals (Amin et al. 2019; Dubey et al. 2010). Cr contamination in agricultural soils is a foremost environmental restraint that imperils the development as well as the safety of agricultural products (Basit et al. 2021). Plants have evolved several defense mechanisms to cope Cr mediated toxicity such as enhanced antioxidant activities and metal ion conjugation but at higher levels, Cr stress can affect morpho-physio biochemical processes and may hinder the overall growth and development of plants (Gong et al. 2017). Morpho-physiological changes include inhibition of seed germination, impaired seedling development, maturation, and biochemical changes involving inhibitions of enzymes, depletion of photosynthesis pigments as well as cellular structures due to Cr-induced ROS production in plants (Basit et al. 2022).
Continued exposure to Cr stress interferes with nutrient uptake and affects the metabolic functions in plants which subsequently causes cell death in plants (Javed et al. 2021). Several physical and chemical remediation techniques are being used to overcome heavy metal contamination and to immobilize/remove HMs from the soils. Although, these approaches are quick to perform, and are not more cost-effective (Venkatachalam et al. 2017). However external use of nanoparticles is considered more efficient, environmentally friendly, as well as cost-effective to mitigate Cr stress (Gong et al. 2017). Nanotechnology infers the use of different devices and techniques to formulate nanoparticles, which have the potential to counter various abiotic stresses and can be used to enhance crop growth. Several researchers have reported the use of (NPs) as HMs chelators which limit metal uptake/translocation and stimulate plant development in metal-polluted environments (Venkatachalam et al. 2017).
The use of ZnO NPs seems to be a valuable approach for plant science, with promising aspects for plant growth and yield enhancements, which is one of the most significant solutions for the world's rapidly growing population (Chanu and Upadhyaya, 2019). However, it prompted a variety of negative effects on plant growth and crop yield at large doses and extended periods, and also become a threat to human, and animal health when it becomes a part of the food chain. Additionally, various plants show distinctive responses to ZnO NPs, some plants show beneficial effects while others demonstrate toxic, but the behind mechanism is still uncertain. (Thounaojam et al. 2021). While, various studies have revealed that the NPs size and dose are the foremost factors that determine their upshots. Substantially, investigations are conduction to produce environmentally safe ZnO NPs, with functionalization being one of the most successful strategies for bringing the NPs into a stable, less toxic, and more efficacious state. Extensive research and more effective solutions are still required to overcome the increasing exploitation and risk of ZnO NPs (Chanu and Upadhyaya 2019; Thounaojam et al. 2021).
Zinc oxide nanoparticles (ZnO-NPs) showed a positive effect on different crop plants when used in optimum concentrations as nano fertilizers. For example, ZnO-NPs application alleviated the metal stress in Leucaena leucocephala plants, and enhanced plants growth, biomass and antioxidant levels (Venkatachalam et al. 2017). In addition, it was documented that ZnO-NPs reduce arsenic-induced toxic effects in wheat cultivars, and regulates its biochemical activities by decreasing the uptake of arsenic (Ahmad et al. 2020). Lately, studies have shown that ZnO-NPs reduce the venomous effects of cadmium toxicity in Triticum aestivum and Zea mays L. by lowering the oxidative impairment via improving the antioxidant defense system inside plants (Khan et al. 2019; Rizwan et al. 2019b). Moreover, ZnO-NPs application through seed priming efficiently improved photosynthetic pigments, as well as ultrastructural damage persuaded by cobalt disclosure (Salam et al. 2022). Besides, as per our knowledge, the use of nanoparticles (NPs) particularly ZnO-NPs for the amelioration of Cr stress in Soybean crops is poorly discussed. So in this study, we selected the soybean genotype to investigate its response to different ZnO-NPs and to investigate their relationship to the changes in morpho-physio biochemical attributes and Cr accumulation under Cr stress. Henceforth, current research work hypothesized that different concentrations of ZnO-NPs may alleviate Cr-induced phytotoxicity in soybean seedlings by improving growth and thereby decreasing Cr uptake and accumulation in Soybean. The objectives were to find insights into the protective role of ZnO-NPs to alleviate Cr induce oxidative damage by evaluating the germination traits, nutrient acquisition, antioxidant system, and Cr accumulation under the Cr stress regime in soybean plants.
Materials and methods
ZnO-NPs characterization and concentration screening
The zinc oxide nanoparticles were obtained from Alfa Aesar (Massachusetts, USA), has an average diameter 15–30 nm and a precise surface area (50 m2/g). These NPs were highly pure with ~ 99% purity as well as density of 5.605 g/cm3.Transmission electron microscopy (TEM) was used for imaging ZnO NPs by using TEM microscope (Model H-7650, Hitachi, Japan). Various concentrations of ZnO NPs used for screening were (0, 25, 50, 75, 100, 150, and 200 mg/L) sprayed after 2 weeks of germination with the given plant nutrient solutions subsequently sonication for nearly 30–35 min (Table S1). Based on preliminary experiment, the three levels of ZnO NPs concentrations such as 0, 50, 75, and 100 mg/L were selected.
Seed germination assay
The soybean cultivar Xudou-18 (XD-18) was used in this experiment and the seeds were taken from the Zhejiang Nongke Seeds CO., LTD. Hangzhou, Zhejiang Province, China. Initially, soybean seeds were sterilized by submerging them in a 5% sodium hypochlorite (NaClO) solution for 5 min and soaked hastily with ddH2O to remove impurities. Afterward, for seed germination assay seeds with the living embryo (50 seeds per box (12 cm × 18 cm) with three different replications for each treatment) were sown. For 14 days, all germination boxes were placed in a growth room at 25 °C with an 8-h illumination and 16-h dark cycle. (Cadavid et al. 2020), and incubated seeds were treated with foliar application of ZnO-NPs (0, 50, 75, and 100 mg/L) as well as 0 and 100 µM chromium stress from the day 1st to estimate the germination analysis. On the fifth day of germination, germinated seeds were evaluated for germination energy (GE) and the percentage of germination (GP) was calculated on day 14th. Germination Index (GI), Mean Germination Time (MGT), as well as Vigour Index (VI), was measured by following formulas (Zheng et al. 2006).
Gt is considered as the total germinated seeds on day t, Gt is the total calculated number of germinated seeds on day t, and Tt is the corresponding time to Gt in days (Zheng et al. 2006).
Experimental lay out and harvesting
Simultaneously, another lot of seeds was germinated in plastic trays on wet paper towels under the above-mentioned conditions. The seeds on trays were thinned to get equal-sized germinated seedlings, which were sprayed with 0, 50, 75, and 100 mg/L ZnO-NPs as a protective measure. The seedlings were transferred in 2L planting plastic boxes having 100 µM or 0 µM Cr in nutrient media solution. The various concentrations of ZnO-NPs were treated such as 0, 50, 75, and 100 mg/L. The solution was changed after every two days. Plants with no exogenously applied Cr stress as well as application of ZnO-NPs were deliberated as control (CK). After 21 days, samples were harvested, and to conduct more analysis, samples were frozen with liquid nitrogen and frozen at -80 °C.
Determination of morphological parameters
The treated and untreated soybean seedlings were harvested on the 21st day. Then, the measurements of various parameters such as plant length (PL), fresh weight (FW), and dry weight (DW) were carried out (Plucknett and Simmonds 1976).
Determination of photosynthetic attributes
The determination of photosynthetic pigments i.e. chlorophyll a, b, total chlorophyll (Chl-a + b), and carotenoids were carried out by following the protocol of (Lichtenthaler and Wellburn 1983). Fresh samples of leaf tissues (0.2 g) were standardized in 3 mL ethanol (95%, v/v). Later, the centrifugation of the sample at 5000×g was conducted for 10 min and the supernatant was collected. Afterward, 9 mL ethanol was poured in 1 mL aliquots and the absorbance was calculated at the particular wavelengths i.e. 665, 649, and 470 nm via atomic absorption spectrophotometer (iCAT-6000-6300, Thermo Scientific, USA). The following equations were utilized for the cunning of pigment amounts:
The quantity of photosynthetic pigments was approximated as milligrams per liter of plant extract. The observation of gas exchange parameters was carried out according to the methodology used by (Sheteiwy et al. 2018). The gas exchange traits such as net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (Tr), the intercellular concentration of CO2 (Ci), and the photochemical efficiency of PS II (Fv/Fm) in the leaves were calculated on the complete uppermost wholly extended leaf subsequently 2 h of acclimatization in a growth chamber at 18 °C, 1000 μmol/(m2 s) light intensity and 60% relative humidity.
Estimation of electrolyte leakage, total soluble sugar and total protein content
To determine the electrolyte leakage as EL (dS/m), a 5 g sample was used for each treatment with a minimum of three replications. Consequently, the seedlings were dipped in 25 mL ddH2O and nurtured (25 °C) for 24 h. The sample was shifted to an alternative blank beaker and ddH2O was poured to make a volume of 25 mL, EL was expressed in dS/m according to the method of (Ista et al. 2004).
To determine the total soluble sugar (TSS), the shoot fresh sample (0.5 g) was homogenized in extraction buffer followed by centrifugation at 12,000×g for 15 min. Afterward, the supernatant was used for quantification of TSS followed by the methodology of the phenol–sulfuric acid assay (Dubois et al. 1951) as well as a total soluble protein (TSP) was determined by following the methodology of Bradford (1976).
Quantification of malondialdehyde (MDA), hydrogen peroxide (H2O2), and superoxide radical (O2 ·‒) contents
The estimation of MDA content was conducted by taking 1.5 mL of leaf tissue homogenized in 2.5 mL of 5% TBA as well as diluted in 5% trichloroacetic acid. Later, homogenized samples were heated at 90 °C for 20 min, and earlier, it was immediately chilled on ice and absorption was measured at 532 and 600 nm wavelengths through a UV–vis spectrophotometer. (Hitachi U-2910) following the method of (Heath and Packer 1968). The MDA content was expressed as nmol/mg protein.
The hydrogen peroxide (H2O2) contents were estimated by homogenizing the plant tissues in phosphate buffer and then centrifugation at 6000×g. The 0.1% titanium sulfate comprising 20% (v/v) H2SO4 was poured into the supernatant and stirred thoroughly. The strength of the yellow color was calculated calorimetrically at 410 nm following (Kwasniewski et al. 2013). The H2O2 values were calculated by creating the standard curve with the known concentration of H2O2 and the reaction mixture of tissue sample was deliberated as control alongside its interpretations deducted from treatments. The H2O2 values were intended in terms of (µmol/g FW).
The quantification of superoxide radical (O2·‒) was done by following the protocol of Jiang and Zhang (2001) with little adjustments. Almost 0.5 g leaf tissues were amalgamated in 4 mL of 70 mM potassium phosphate buffer, followed by centrifugation at 5000×g for 12 min and 1 mL supernatant was mixed with 0.8 mL of 70 mM potassium phosphate buffer and 0.2 mL of 10 mM hydroxylamine hydrochloride. Afterward, the incubation of mixture was done for 24 h. Later 1 mL of sulphanilamide (17 mM) was followed by adding anaphthylamine (7 mM) thoroughly the mixed for 20 min at 25 °C and 1 mL of n-butanol was added and centrifuged at 15,000×g for 10 min. The absorbance was calculated at 530 nm and the production rate of superoxide radical was premeditated with a standard curve.
Additionally, the H2O2 and O2· ‒ accumulation was observed inside both shoot and root samples of soybean plants by using staining with 3,3-diaminobenzidine (DAB), and nitroblue tetrazolium (NBT), individually. Immersed shoots and roots were observed under a high digital resolution microscope camera (Leica MZ-g5, Germany).
Determination of antioxidant enzymatic, and non-enzymatic activities
The activity of superoxide dismutase (SOD) was analyzed by using the protocol of El-Shabrawi et al. (2010). The SOD activity was assayed in U (one U was the amount of enzyme required to inhibit photoreduction (50%) of NBT) (min/mg protein). For CAT determination, the extinction coefficient of 39.4 M−1 cm−1 was utilized to measure the catalase activity following the protocol of Hossain et al. (2010). The peroxidase (POD) activity was estimated according to the Change and Maehly (1955) method, and the activities of enzymes were measured in terms of l M of guaiacol oxidized g−1 FW min−1 at 25 ± 2 °C.
To determine the non-enzymatic activities, the plants tissues were collected and homogenized using a solution of 5% metaphosphoric acid and 1 mM ethylenediaminetetraacetic acid (EDTA). Following that, the homogenate was extracted by centrifugation (12,000×g for 10 min) and subjected to quantify the GR contents according to the protocol of Schaedle and Bassham (1977) with minor modifications whereas, GSH, and GSSG were estimated by following the methodology of Law et al. (1983).
Determination of Cr contents, and micronutrients in plant tissues
To measure the Cr-metal analysis, dried samples of both shoots and roots (0.2 g) for each treatment were used and assimilated by 5 mL concentrated HNO3 and HCLO4 (5:1, v/v) on a hot plate at 70 °C for approximately 5 h. The digested samples were diluted using 2% HNO3 to a final volume of 10 mL and examined. The filtrate was used for the quantification of Cr and other microelements Cu, Mn, and Zn using an atomic absorption spectrophotometer (iCAT-6000-6300, Thermo Scientific, USA) (Khan et al. 2013).
Statistical analysis
The analysis of all experimental data was performed by applying analysis of variance (One-way ANOVA) with the least significant differences (LSD) at p < 0.05 and p < 0.01 levels amongst mean values using Statistix (8.1) software. All data demonstrated in this study are the mean values of three biological replicates. And the experiment was conducted in a completely randomize design.
Results
Plant phenotypic traits
Phenotypic changes are an indicator of Cr stress on soybean seedlings and foliar spray with various levels of ZnO-NPs (0, 50 75, and 100 mg/L) under Cr toxicity showed considerable changes in comparison to Cr-stressed plants (Fig. 1). Under alone treatment of Cr, the plant height declined significantly as compared to the control. However, the plant length was significantly enhanced when subjected to 50, and 75 mg/L ZnO-NPs concentration under Cr stress as compared to the alone treatment of Cr exposure (Fig. 1). Whereas the foliar use of 100 mg/L ZnO-NPs remarkably enhanced the plant length under Cr stress in comparison to other ZnO-NPs. These phenotypical visualizations suggest that 100 mg/L ZnO-NPs can significantly promote plant growth under Cr-induced toxicity.
Seed germination, and plant growth attributes
The germination attributes of seeds such as germination energy (GE), germination percentage (GP), mean germination time (MGT), and vigor index (V.I) as well as growth traits viz plant fresh weight (F/W), plant dry weight (D/W) and plant length (P/L), were investigated to examine the effect of Cr stress on soybean seedlings. The Cr treatment for soybean seedlings caused a significant reduction in germination traits (G.E, G.P, and V.I by 89.34, 86.83, and 69.12%, respectively) as well as in plant growth such as (F/W, D/W, and P/L by 69.23, 52.38, and 43.39%, correspondingly) than control (Table 1). Although, MGT was considerably enhanced (33.67%) under Cr alone exposure (Table 1). However, the foliar treatment of ZnO-NPs (50, and 75 mg/L) significantly enhanced the germination indices such as G.E, G.P, and V.I by 28.32/30.57%, 29.63/32.42%, and 44.92/47.18%, individually under Cr exposure whereas, the increment inside the above-mentioned seed germination indices were more obvious through 100 mg/L ZnO-NPs concentration by 40.01%, 39.67%, and 62.52%, correspondingly under Cr treatment. At the same time, the MGT was decreased in 100 mg/L application of ZnO-NPs seedlings (38.02%) under Cr toxicity (Table 1). Further, the plant growth traits i.e. F/W, D/W, and P/L were augmented with the foliar treatment of ZnO-NPs (50, and 75 mg/L) under Cr toxicity by 50.18/51.85%, 42.31/49.26%, and 37.63/40.76%, individually, but the increment was more prominent with 100 mg/L ZnO-NPs as compared to the other ZnO-NPs treatment levels by 60.13%, 62,50%, and 48.65%, respectively under Cr toxicity stress (Fig. 1b, Table 1).
Effect of ZnO-NPs on photosynthetic pigments and gas exchange parameters in soybean seedlings under Cr stress
Comparable to the control, the photosynthetic pigments were ominously decreased under Cr stress treatment (Fig. 2a–d). However, foliar addition of 75 mg/L ZnO-NPs caused an increase in photosynthetic traits (chlorophyll a, b, total chlorophyll, and carotenoid content) up to 25.41, 27.13, 21.53, and 43.71%, correspondingly but the increase was not a significant when compared with 50 mg/L foliar supply of ZnO-NPs in soybean seedlings. Although, the increase in photosynthetic traits was more apparent with foliar spray of 100 mg/L ZnO-NPs which showed significant increase in chlorophyll a up to (9.7%), chlorophyll b (33.27%), total chlorophyll (38.42%), and carotenoid content upto (65.95%) under Cr stress than alone treatment of Cr toxicity (Fig. 2a–d). Likewise, we analyzed that 100 mg/L concentration of ZnO-NPs significantly improved the Fv/Fm values as comparable to the control and Cr-stressed plants. It was further verified through taking the false-colored images of Fv/Fm, and Fm via using ImagingWin software (IMAGING-PAM, Walz, Effeltrich, Germany) (Fig. 3a–b).
The gas exchange parameters of soybean seedlings with foliar application of various ZnO-NPs under Cr toxicity are presented in (Table 2). Related to the control condition, there was a significant reduction in Pn, Tr, gs, and Ci values under the alone treatment of Cr but these values were noticeably enhanced with 50, and 75 mg/L application of ZnO-NPs than Cr alone treated plants. These gas exchange attributes were further augmented with a foliar spray of 100 mg/L ZnO-NPs concentrations under Cr stress in comparison to other ZnO-NPs treatment levels (Fig. 3c). These results showed that 100 mg/L concentration of ZnO-NPs can efficiently alleviate the Cr stress than 50 and 75 mg/L applications under Cr stress conditions.
Effect of ZnO NPs on MDA, O2 ·−, H2O2 production, and electrolyte leakage (EL) under Cr stress
Under Cr toxicity, a visible higher accumulation of H2O2 (82.41%), MDA (73.95%), and O2·− (79.16%) was observed in soybean plants as compared to controls (Fig. 4a–c). Whereas, under Cr-induced toxicity, the treatment of 50 and 75 mg/L ZnO-NPs diminished the accumulation of H2O2 (41.32%), MDA (39.26%), O2·− (43. 91%) per plant, correspondingly. Further, the 100 mg/L ZnO-NPs application reduced the accumulation of H2O2 by 54.68%, MDA 53.89%, and O2·− 72.19% per plant, respectively under Cr exposure as compared to the 50, and 75 mg/L ZnO-NPs application (Fig. 4a, c).
Furthermore, a significant upsurge in electrolyte leakage up to (53.31%) was observed under alone exposure to Cr toxicity (Fig. 4d) relative to the control. To some extent the 50, and 75 mg/L ZnO-NPs foliar exposure reduced the EL by (32.01, and 33.47%) respectively under Cr stress. Whereas the 100 mg/L ZnO-NPs treatment minimized the EL by 48.73% under Cr stress (Fig. 4d).
Additionally, the findings related to the accumulation of H2O2, MDA, and O2·− were further confirmed thru histochemical staining by foliar application of ZnO-NPs (0, and 100 mg/L) under Cr-induced 100 µM Cr stress (Fig. 5a, d). The Soybean shoots and roots exposed to 100 µM Cr toxicity showed dark brown and dark blue staining for H2O2 and O2·−. The 100 mg/L ZnO-NPs applications abridged the staining intensity of DAB and NBT in soybean shoots as well as roots as compared to the alone treatment of Cr exposure under Cr toxicity (Fig. 5a, d).
Effect of ZnO NPs on enzymatic, and non-enzymatic activities of soybean seedlings under Cr stress
Relative to the normal controls, the exposure to Cr stress considerably enhanced the antioxidative enzymatic (SOD, POD, and CAT) activities in soybean seedlings (Fig. 6a–c). Comparable to the Cr alone treatment, 75 mg/L ZnO NPs further stimulated the SOD, POD, and CAT (25.67, 38.31, 21.94%) in Soybean seedlings. The optimum increase in antioxidant enzymes SOD, POD, and CAT value (32.93, 46.34, and 34.61%) were noticed in plants added with 100 mg/L ZnO NPs under Cr stress as comparable to the alone disclosure of Cr stress (Fig. 6a–c).
The result indicated that the application of ZnO NPs had a positive impact on the non-enzymatic activities of Cr Stressed Soybean plants. The Cr-exposed plants showed increased activity of GR, GSH, and GSSG up to (56.72, 69.31, and 51.44%) in contrast to controls. Interestingly the treatments of ZnO NPs reduced the activities of GR, GSH, and GSSG by (34.71, 29.27, and 37.89%) and (35.02, 31.93 and 39.32%) in 50, and 75 mg/L ZnO NPs applied plants individually in comparison to the alone Cr stress condition (Fig. 7a–c). However, the maximum decrease in values of non-enzymatic antioxidant activities such as GR, GSH, and GSSG was found up to (39.92, 37.33 and 47.21%), was noticed with foliar application of 100 mg/L ZnO NPs treatment under Cr exposure (Fig. 7a–c). These results explained that 100 mg/L ZnO NPs noticeably scavenges the overproduction of reactive oxygen species under Cr-induced toxicity and improves the tolerance capability of soybean plants.
Effect of ZnO NPs on total soluble sugar and total soluble protein content under Cr toxicity
Relative to the control, the TSS, and TSP content was significantly decreased in soybean seedlings under Cr exposure (Fig. 8a, b). However, the foliar treatment of 50, and 75 mg/L ZnO-NPs enhanced the TSS, and TSP content by (38.23, 32.86%), and (38.92, 33.71%) respectively under Cr toxicity than the alone application of Cr. Moreover, the foliar treatment of 100 mg/L ZnO-NPs caused a significant increase in TTS up to (to 56.79%), and TSP (49.21%) under the Cr stress (Fig. 8a, b).
Effect of ZnO NPs on Cr content and nutrient uptake
In comparison to the control, the Cr accumulation was augmented in roots and shoots of soybean plants under exogenously applied alone treatment of Cr stress and the higher Cr accumulation was found in Soybean roots than shoots (Table 3). The foliar addition of ZnO-NPs 50, and 75 mg/L significantly decreased the Cr uptake inside roots, as well as translocation toward shoots in soybean seedlings, but the prominent reduction was seen with 100 mg/L ZnO-NPs foliar spray under Cr stress (Table 3). Further, under the alone treatment of Cr toxicity, a significant decline in micronutrient (i.e. Mn, Fe, and Zn) uptake, and translocation were observed inside soybean plants. The decline was more obvious in roots as compared to the shoots (Table 3). Interestingly, the foliar supply of 50, and 75 mg/L ZnO-NPs improved the micronutrient uptake and translocation balance inside soybean seedlings by restricting the Cr uptake, and accumulation significantly. However, a significant diminution in Cr uptake and nutrient accumulation was found in 100 mg/L ZnO-NPs treatment than alone Cr exposure under Cr-induced toxicity.
Discussion
ZnO-NPs play a vital role in plant growth by emancipating the Zn element which is intricate in cell division, and cell expansion through the synthesis of the auxin, and indole-3-acetic acid (Rai-Kalal and Jajoo 2021; Adrees et al. 2021). Chromium is a toxic heavy metal that can change its oxidation states and confers damage to living organisms when released into the environment (Sharma et al. 2022). Chromium translocation inside plant tissues can disrupt enzyme activities, damage cell wall integrity, reduce the photosynthetic process, and may cause yield loss. The exploitation of nano-biotechnology is a fast-growing approach to increase crop yield and remediate heavy metals contaminated soils for sustainable agriculture to overcome the increasing global food demand. Germination indices and plant growth are fundamental preliminary factors to detecting Cr-induced oxidative damage in plants. In the current study, Cr stress diminished the seed germination attributes (GE, GP, GI, VI), and reduced plant biomass traits such as (F/W, D/W, P/L) which might be caused by Cr-induced reticence of growth process, disturbance in water uptake balance and reduced nutrient uptake (Table 1). It was envisaged that decreased germination attribute may have resulted due to Cr accumulation inside the seed coat. Besides, reduced fresh and dry biomass might have happened due to decreased water absorption, nutrient acquisition, and Cr accumulation inside root cells (Shahid et al. 2017; Abbas et al. 2020).
The Zinc nanoparticle treatment acts as a slow Zn-fertilizer and is an important source of Zn elements that enhance the metabolic activities in plants (Li et al. 2019). Inside our research, the foliar supply of ZnO-NPs relieved soybean seedlings from Cr stress and increased the seed germination traits concurrent with plant growth as depicted in (Table 1). Our finding corroborates with (Faizan et al. 2021b) who reported that Zinc NPs substantially increased the growth and biomass in Lycopersicum esulentum under Cd stress via decreasing metal accumulation. Further, a notable increment in foxtile millet has been reported through the foliar application of Zinc NPs (Kolenčík et al. 2019). Current findings demonstrated that Cr stress significantly reduced the photosynthetic efficacy of soybean seedlings by deteriorating the chlorophyll and carotenoids content or either disrupting the chlorophyllase biosynthesis as reported in different plants (Fig. 2). The decreased chlorophyll content may have occurred due to inhibition in Fe-uptake and suppressed heme-based enzyme activities responsible for the chlorophyll synthesis which may have a deleterious influence on plant growth (Sharma et al. 2021).
Results revealed that photosynthetic traits were considerably decreased in response to Cr stress which leads toward stomatal closure and inhibition of CO2 absorption in Cr-stressed plants as presented in (Table 2). Furthermore, decreased water transport leading to reduced transpiration rate (Tr) might have occurred owing to stomatal closure (Chaves et al. 2009; Flexas et al. 2012). Heavy metal-induced oxidative stress destroyed the mesophyll cells, and caused leaf shrinkage which ultimately decreases (Gs), (Ci), and (Pn) values and leads to diminished photosynthesis (Flexas et al. 2012). Photosystem II is essential for plants to engage in photosynthesis and Fv/Fm is used to measure the activity of PS II (Maxwell and Johnson 2000). Chromium-induced stress lowered the value of Fv/Fm which represents alterations in PS II and causes photoinhibition (Pan et al. 2018). Application of Zinc nanoparticles significantly improved the (Gs), (Ci), (Pn), and Fv/Fm values of Cr-stressed seedlings which indicate growth promoting ability of ZnO NPs. Similarly, Zinc nanoparticles reportedly enhanced the development of chloroplast and repaired PS II under a stress environment (Salama et al. 2019; Spormann et al. 2021). Likewise, ZnO-NPs enhanced the photosynthetic attributes and these outcomes are concurrent to the findings in wheat against Cd exposure (Hussain et al. 2018), in soybean under arsenic (Zeeshan et al. 2021), in rice under arsenic (Wu et al. 2020), and inside wheat under lead toxicity (Raghib et al. 2020).
Results demonstrated that Cr-induced stress enhanced MDA formation which is indicative of cell membrane damage, and oxidative stress is associated with (O2·− and H2O2) production as well as electrolyte leakage in soybean seedlings (Figs. 5a–c, 4a). Lipid peroxidation and injury to DNA and RNA structures concurrent with disruption of enzymatic activities might result from elevated levels of MDA and H2O2 generation (Chaves et al. 2009; Maxwell & Johnson 2000). These findings are parallel to those in rice (Basit et al. 2021; Mostofa et al. 2019), Brassica napus (Ulhassan et al. 2019), besides Brassica juncea (Kohli et al. 2018) wherein lipid peroxidation marked increased when plants were exposed to HMs stress. It was also noted that the Cr stress caused lipid peroxidation to increase than oxidative stress, indicative of membrane damage which was further confirmed through histochemical staining of both roots, and shoots of soybean seedlings (Fig. 5). Besides high dose of Zinc nanoparticles relieved the Cr toxicity in soybean seedlings through decreased MDA accumulation and scavenging H2O2 by converting them into H2O and enhanced antioxidant activities. Previous studies corroborated with our outcomes that ZnO-NPs application decrease the ROS accretion, MDA contents, and electrolyte leakage of different plant species (Bandyopadhyay et al. 2015; Hussain et al. 2018; Rizwan et al. 2019a; Zeeshan et al. 2021). Under Cr stress we observed decreased TSS and TSP content in soybean seedlings (Fig. 4b, c). Plant accumulate TSP and TSP contents to mitigate stress by facilitating metabolism thereby growth. Additionally TSS, and TSP are linked with photosynthesis activity in plants (Mostofa et al. 2019). However, Zinc nanoparticle spray facilitate the TSS and TSP accumulation in Cr stressed plants as presented in (Fig. 4b, c). The increased level of TSS and TSP assists plants to overcome Cr-induced toxicity by improving the structural stability and metabolic functions in plants (Mostofa et al. 2019).
Antioxidants (enzymatic, and non-enzymatic) are pondered as the first line of defense against abiotic stress. In the current study, soybean seedlings showed augmented levels of SOD, POD, and CAT accumulation against Cr stress. Plants tend to upregulate antioxidant levels which serve as ROS scavengers in a metal-stressed environment and diminish the oxidative hassle (Ahmad et al. 2020). Zinc nanoparticles substantially increased the levels of antioxidant levels (SOD, POD, and CAT) in Cr-stressed soybean seedlings to overcome the metal-induced damage and initiate a defense mechanism (Fig. 7a–c). The inhibitory effect on ROS biosynthesis through the Zinc NPs application has been stated in rice and Brassica napus under chromium (Ulhassan et al. 2019) toxicity. Non-enzymatic antioxidants GR, GSH, and GSSG were elevated under Cr stress in soybean plants in comparison to the control. Likewise, alterations in non-enzymatic activities were observed in soybean (Wani et al. 2018) and Vigna angularis (Ahanger et al. 2020) under metal stress conditions. The ZnO-NPs foliar spray up-regulated the non-enzymatic activities under Cr stress inferred decreased accumulation and consequent oxidative damage. A recent study (Zeeshan et al. 2021) reported that the up-regulation of the non-enzymatic system under NPs application can overcome the arsenic-induced stress. In this study, Cr contents were enhanced whereas micronutrients (Mn, Fe, and Zn) were decreased in roots and shoots of soybean seedlings without ZnO NPs application. However, it was anticipated that ZnO NPs condensed the uptake of chromium in the root and shoot tissue of soybean seedlings. The ZnO-NPs may act as a metal binding agent behind the possible mechanism of Cr sequestration and sequester it into the vacuole by forming a complex with Cr ions (Rizwan et al. 2019a). Increased Zn content concurrent with other nutrients by Zinc NPs has been reported by (Faizan et al. 2021a) which specified their association with growth augmentation, and crop protection against metal stress. The potential of ZnO NPs to decrease metal content was reported by (Faizan et al. 2021a) inside wheat (Adrees et al. 2021). Hence, this study suggests that the application of Zinc NPs can offer novel prospects to develop the formulation that can further use as a remedy for the metal stressed environment for sustainable agriculture.
Conclusion
Herein, our study demonstrated that employing ZnO-NPs can alleviate Cr stress through modulation in Cr uptake antioxidant machinery and nutrient balance. Our results confirmed that Cr-stressed toxicity significantly inhibited soybean growth, biomass, antioxidant system, and nutrient uptake. Application of ZnO-NPs (especially 100 mg/L) confers advantageous effect on growth as reflected from higher biomass, photosynthetic apparatus, enzymatic and non-enzymatic antioxidants while maintaining decreased levels of lipid peroxidation, electrolyte leakage, and Cr uptake and translocation. It was concluded that ZnO-NPs can be used as a proficient remediation tool for crop growth in Cr polluted environment for sustainable agriculture, which may be endorsed to improve the plant growth, biomass, and chlorophyll pigments in ZnO-NPs treated soybean plants against Cr stress. It is highly recommended that the ZnO-NPs fertilizers can alleviate Cr-induced phytotoxicity, and at the same time enhance soybean plants growth and development. It was further proposed that the field trials should be performed to draw parallels among cost-profit ratio, yield losses, and environmental restoration are recommended to further validate the ZnO-NPs (100 mg/L) as a remediation agent in Cr-contaminated soils.
Data availability
The data of this research can be obtained upon request to the corresponding author.
References
Abbas S, Javed MT, Shahid M, Hussain I, Haider MZ, Chaudhary HJ, Maqsood A (2020) Acinetobacter sp. SG-5 inoculation alleviates cadmium toxicity in differentially Cd tolerant maize cultivars as deciphered by improved physio-biochemical attributes, antioxidants and nutrient physiology. Plant Physiol Biochem 155:815–827
Adrees M, Khan ZS, Hafeez M, Rizwan M, Hussain K, Asrar M, Alyemeni MN, Wijaya L, Ali S (2021) Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol Environ Saf 208:111627
Ahanger MA, Aziz U, Alsahli A, Alyemeni MN, Ahmad P (2020) Combined kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in Vigna angularis by up-regulating antioxidant and nitrogen metabolism under cadmium stress. Biomolecules 10:147
Ahmad P, Alyemeni MN, Al-Huqail AA, Alqahtani MA, Wijaya L, Ashraf M, Kaya C, Bajguz A (2020) Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 9:825
Alidoust D, Isoda A (2014) Phytotoxicity assessment of γ-Fe2O3 nanoparticles on root elongation and growth of rice plant. Environ Earth Sci 71:5173–5182
Amin H, Ahmed Arain B, Abbasi MS, Amin F, Jahangir TM, N-u-A S (2019) Evaluation of chromium phyto-toxicity, phyto-tolerance, and phyto-accumulation using biofuel plants for effective phytoremediation. Int J Phytoremed 21:352–363
Bakhoum GS, Badr EAE, Sadak MS, Kabesh MO, Amin GA (2019) Improving growth, some biochemical aspects and yield of three cultivars of soybean plant by methionine treatment under sandy soil condition. Int J Environ Res 13:35–43
Bandyopadhyay S, Plascencia-Villa G, Mukherjee A, Rico CM, José-Yacamán M, Peralta-Videa JR, Gardea-Torresdey JL (2015) Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci Total Environ 515:60–69
Bansal P, Sharma P, Goyal V (2002) Impact of lead and cadmium on enzyme of citric acid cycle in germinating pea seeds. Biol Plant 45:125–127
Basit F, Chen M, Ahmed T, Shahid M, Noman M, Liu J, An J, Hashem A, Fahad Al-Arjani A-B, Alqarawi AA (2021) Seed priming with brassinosteroids alleviates chromium stress in rice cultivars via improving ROS metabolism and antioxidant defense response at biochemical and molecular levels. Antioxidants 10:1089
Basit F, Nazir MM, Shahid M, Abbas S, Javed MT, Naqqash T, Liu Y, Yajing G (2022) Application of zinc oxide nanoparticles immobilizes the chromium uptake in rice plants by regulating the physiological, biochemical and cellular attributes. Physiol Mol Biol Plants 28:1175–1190
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cadavid IC, Guzman F, de Oliveira-Busatto L, de Almeida RM, Margis R (2020) Transcriptional analyses of two soybean cultivars under salt stress. Mol Biol Rep 47:2871–2888
Change B, Maehly A (1955) Assay of catalases and peroxidase. Meth Enzymol 2:764–775
Chanu TT, Upadhyaya H (2019) Zinc oxide nanoparticle-induced responses on plants: a physiological perspective. Nanomaterials in plants, algae and microorganisms. Elsevier, Amsterdam, pp 43–64
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Dubey S, Misra P, Dwivedi S, Chatterjee S, Bag SK, Mantri S, Asif MH, Rai A, Kumar S, Shri M (2010) Transcriptomic and metabolomic shifts in rice roots in response to Cr (VI) stress. BMC Genom 11:1–19
Dubois M, Gilles K, Hamilton J, Rebers P, Smith F (1951) A colorimetric method for the determination of sugars. Nature 168:167–167
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245:85–96
Faizan M, Bhat JA, Chen C, Alyemeni MN, Wijaya L, Ahmad P, Yu F (2021a) Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol Biochem 161:122–130
Faizan M, Faraz A, Mir AR, Hayat S (2021b) Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J Plant Growth Regul 40:101–115
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193:70–84
Gong X, Huang D, Liu Y, Zeng G, Wang R, Wan J, Zhang C, Cheng M, Qin X, Xue W (2017) Stabilized nanoscale zerovalent iron mediated cadmium accumulation and oxidative damage of Boehmeria nivea (L.) Gaudich cultivated in cadmium contaminated sediments. Environ Sci Technol 51:11308–11316
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 16:259–272
Hussain A, Ali S, Rizwan M, Ur-Rehman MZ, Javed MR, Imran M, Chatha SAS, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut 242:1518–1526
Ista LK, Callow ME, Finlay JA, Coleman SE, Nolasco AC, Simons RH, Callow JA, Lopez GP (2004) Effect of substratum surface chemistry and surface energy on attachment of marine bacteria and algal spores. Appl Environ Microbiol 70:4151–4157
Javed MT, Tanwir K, Abbas S, Saleem MH, Iqbal R, Chaudhary HJ (2021) Chromium retention potential of two contrasting Solanum lycopersicum Mill. cultivars as deciphered by altered pH dynamics, growth, and organic acid exudation under Cr stress. Environ Sci Pollut Res 28:27542–27554
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Khan HAA, Shad SA, Akram W (2013) Resistance to new chemical insecticides in the house fly, Musca domestica L., from dairies in Punjab. Pakistan Parasitol Res 112:2049–2054
Khan ZS, Rizwan M, Hafeez M, Ali S, Javed MR, Adrees M (2019) The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ Sci Pollut Res 26:19859–19870
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2018) Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 255:11–24
Kolenčík M, Ernst D, Komár M, Urík M, Šebesta M, Dobročka E, Černý I, Illa R, Kanike R, Qian Y (2019) Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica l.) under field conditions. Nanomaterials 9:1559
Kwasniewski M, Chwialkowska K, Kwasniewska J, Kusak J, Siwinski K, Szarejko I (2013) Accumulation of peroxidase-related reactive oxygen species in trichoblasts correlates with root hair initiation in barley. J Plant Physiol 170:185–195
Law M, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem J 210:899–903
Li C, Wang P, van der Ent A, Cheng M, Jiang H, Lund Read T, Lombi E, Tang C, de Jonge MD, Menzies NW (2019) Absorption of foliar-applied Zn in sunflower (Helianthus annuus): importance of the cuticle, stomata and trichomes. Ann Bot 123:57–68
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd
Mao F, Nan G, Cao M, Gao Y, Guo L, Meng X, Yang G (2018) The metal distribution and the change of physiological and biochemical process in soybean and mung bean plants under heavy metal stress. Int J Phytoremediation 20:1113–1120
Mao C, Song Y, Chen L, Ji J, Li J, Yuan X, Yang Z, Ayoko GA, Frost RL, Theiss F (2019) Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. CATENA 175:339–348
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Mostofa MG, Rahman M, Ansary M, Uddin M, Fujita M, Tran L-SP (2019) Interactive effects of salicylic acid and nitric oxide in enhancing rice tolerance to cadmium stress. Int J Mol Sci 20:5798
Pan G, Liu W, Zhang H, Liu P (2018) Morphophysiological responses and tolerance mechanisms of Xanthium strumarium to manganese stress. Ecotoxicol Environ Saf 165:654–661
Plucknett D, Simmonds N (1976) Evolution of crop plants. Longman, London
Raghib F, Naikoo MI, Khan FA, Alyemeni MN, Ahmad P (2020) Interaction of ZnO nanoparticle and AM fungi mitigates Pb toxicity in wheat by upregulating antioxidants and restricted uptake of Pb. J Biotechnol 323:254–263
Rai-Kalal P, Jajoo A (2021) Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol Biochem 160:341–351
Rajput VD, Minkina T, Kumari A, Singh VK, Verma KK, Mandzhieva S, Sushkova S, Srivastava S, Keswani C (2021) Coping with the challenges of abiotic stress in plants: new dimensions in the field application of nanoparticles. Plants 10:1221
Rehal J, Beniwal V, Gill B (2019) Physico-chemical, engineering and functional properties of two soybean cultivars. Leg Res Int J 42:39–44
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, ur Rehman MZ, Waris AA (2019a) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Rizwan M, Ali S, ur Rehman MZ, Adrees M, Arshad M, Qayyum MF, Ali L, Hussain A, Chatha SAS, Imran M (2019b) Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ Pollut 248:358–367
Sadak MS, Abd El-Hameid AR, Zaki FS, Dawood MG, El-Awadi ME (2020) Physiological and biochemical responses of soybean (Glycine max L.) to cysteine application under sea salt stress. Bull Natl Res Centre 44:1–10
Salam A, Khan AR, Liu L, Yang S, Azhar W, Ulhassan Z, Zeeshan M, Wu J, Fan X, Gan Y (2022) Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J Hazard Mater 423:127021
Salama DM, Osman SA, Abd El-Aziz M, Abd Elwahed MS, Shaaban E (2019) Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal Agric Biotechnol 18:101083
Schaedle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Sharma P, Tripathi S, Sirohi R, Kim SH, Ngo HH, Pandey A (2021) Uptake and mobilization of heavy metals through phytoremediation process from native plants species growing on complex pollutants: antioxidant enzymes and photosynthetic pigments response. Environ Technol Innov 23:101629
Sharma P, Chouhan R, Bakshi P, Gandhi SG, Kaur R, Sharma A, Bhardwaj R (2022) Amelioration of chromium-induced oxidative stress by combined treatment of selected plant-growth-promoting rhizobacteria and earthworms via modulating the expression of genes related to reactive oxygen species metabolism in Brassica juncea. Front Microbiol. https://doi.org/10.3389/fmicb.2022.802512
Sheteiwy MS, Gong D, Gao Y, Pan R, Hu J, Guan Y (2018) Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ Exp Bot 153:236–248
Spormann S, Soares C, Teixeira J, Fidalgo F (2021) Polyamines as key regulatory players in plants under metal stress A way for an enhanced tolerance. Ann Appl Biol 178:209–226
Thomas D, Erostus N (2008) Soybean research in Africa for 30 years, by IITA. Research for Development, Nigeria
Thounaojam TC, Meetei TT, Devi YB, Panda SK, Upadhyaya H (2021) Zinc oxide nanoparticles (ZnO-NPs): a promising nanoparticle in renovating plant science. Acta Physiol Plant 43:1–21
Ulhassan Z, Gill RA, Huang H, Ali S, Mwamba TM, Ali B, Huang Q, Hamid Y, Khan AR, Wang J (2019) Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol Biochem 145:142–152
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma N, Sahi S (2017) Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem 110:59–69
Wani P, Sunday O, Kehinde A, Oluwaseyi L, Wasiu I, Wahid S (2018) Antioxidants and chromium reductases by Penibacillus species enhance the growth of soybean under chromium stress. Int J Environ Sci Technol 15:1531–1542
Wu F, Fang Q, Yan S, Pan L, Tang X, Ye W (2020) Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): germination, early growth, and arsenic uptake. Environ Sci Pollut Res 27:26974–26981
Zeeshan M, Hu YX, Iqbal A, Salam A, Liu YX, Muhammad I, Ahmad S, Khan AH, Hale B, Wu HY (2021) Amelioration of AsV toxicity by concurrent application of ZnO-NPs and Se-NPs is associated with differential regulation of photosynthetic indexes, antioxidant pool and osmolytes content in soybean seedling. Ecotoxicol Environ Saf 225:112738
Zheng Y, Hu J, Zhang S, Gao C, Song W (2006) Identification of chilling-tolerance in maize inbred lines at germination and seedling growth stages. J Zhejiang Univ (agric Life Sci) 32:41–45
Zschau T, Getty S, Gries C, Ameron Y, Zambrano A, Nash Iii T (2003) Historical and current atmospheric deposition to the epilithic lichen Xanthoparmelia in Maricopa County, Arizona. Environ Pollut 125:21–30
Acknowledgements
We acknowledge the contributions of Mr. Muhammad Noman for his help in data analysis and proof-reading of this manuscript.
Funding
This research was financially supported by Zhejiang Provincial Natural Science Foundation (No. LY21C130006), and National Natural Science Foundation of China (No. 32072127).
Author information
Authors and Affiliations
Contributions
Conceptualization: MS and GY. Methodology: MS, GY, FB and MT. Formal analysis and investigation: FB, MM SA, SA, MA and YC. Writing—original draft preparation: FB, YC, SJ and JH. Writing—review and editing: MS, MT, MSA, FB, SA, XL and JH. Funding acquisition: GY. Supervision: MS and GY.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Luca Sebastiani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Basit, F., Shahid, M., Abbas, S. et al. Protective role of ZnO nanoparticles in soybean seedlings growth and stress management under Cr-enriched conditions. Plant Growth Regul 100, 703–716 (2023). https://doi.org/10.1007/s10725-023-00965-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-023-00965-7