Abstract
Chromium (Cr), being a persistent toxic heavy metal, triggered the retardation of plant’s metabolic processes by initiating changes in rhizospheric zone. Current study focused the Cr accumulation potential of two tomato (Solanum lycopersicum Mill.) cultivars through alterations of rhizospheric pH and exudation of organic acids together with plant’s ionomics and morpho-physiological responses. Four-week-old seedlings of tomato cultivars (cv. Nakeb and cv. Nadir) were maintained in hydroponic solutions supplemented with 0, 100, 200, and 300 mg/L K2Cr2O7 and a start pH of 6.0. The pH of the growth medium was monitored twice a day up to 6 days as well as mineral contents and morpho-physiological attributes were recorded by harvesting half of plants after 1 week. The remaining half plants were shifted to rhizoboxes for the collection of root exudates. After 6 days, cv. Nakeb exhibited medium acidification by 0.7 units while cv. Nadir showed basification by 0.6 units under 300 mg/L treatment. Increase in applied Cr levels enhanced the root and shoot Cr accumulation in both cultivars with concomitant reduction in growth and accumulation of nutrients (Fe, Zn, K, Mg, and Ca). However, this reduction in biomass and nutrient acquisition was predominant in cv. Nakeb as compared to cv. Nadir. The release of organic acid exudates (citric, acetic, maleic, tartaric, and oxalic acids) was also recorded higher in cv. Nadir at 300 mg/L applied Cr level. This enhanced production of organic acids caused greater retention of mineral nutrients and Cr in cv. Nadir, probably due to growth medium basification. Enhanced exudations of di- and tri- carboxylic organic acids together with accumulation of mineral nutrients are the physiological and biochemical indicators which confer this genotype a better adaptation to Cr polluted biotic systems. Furthermore, it was perceived that organic acid and rhizospheric pH variation response by studied tomato cultivars under Cr stress is an important factor to be considered in food safety and metal remediation programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is a silvery white heavy metal which is highly abundant and mobile in earth crust, has no known biological functions, exists in different oxidation states, and readily available for uptake in soil-plant systems (Bilal et al. 2018; Singh and Prasad 2019). In Pakistan, soil Cr contents ranges from 100 to 150 mg/kg which is higher than the global average soil Cr contents of 60 mg/kg (Waseem et al. 2014). Naturally, the Cr enters the environment via rocky soil erosion and volcanic dust while it has many anthropogenic sources such as tanneries, metallurgical processes, dyeing, paints, electroplating, sewage water, steel production, and textile industries (Banerjee et al. 2019; Sharma et al. 2020). Chromium have several oxidative forms but trivalent and hexavalent forms are two most stable ones existing in soil (Khatun et al. 2019). Plants are able to take up Cr easily via reduction of trivalent form into hexavalent form either through symplastic pathway or by complex formation with essential plant nutrients (Bilal et al. 2018; UdDin et al. 2015). Hexavalent form of Cr is the most toxic form for plants. Chromium in plants affect various metabolic processes by generating oxidative stress due to overproduction of reactive oxygen species (ROS) which ultimately impaired the rate of photosynthesis, damaged the biological membranes, and reduced the production of antioxidant enzymes which in response caused cell death or reduction in cell division (Achmad and Budiawan 2017; Gill et al. 2016; Singh et al. 2020). Plants are able to deposits excess Cr into their fruits which leads to its accumulation in the food chain (Achmad and Budiawan 2017). Ingestion of Cr through food may induce various health risks including cancers, bronchial asthma, skin allergies, ulcers, and reproductive and developmental disorders in humans (Shekhawat et al. 2015).
Plants, in response to Cr toxicity, develop several mechanisms to ameliorate its toxic effects which include the increased biosynthesis of antioxidative enzymes (Gupta et al. 2020a, b; Sharma et al. 2020). The enhanced exudation of organic acids in rhizosphere of plants, in combination with other metabolites, alters the rhizospheric pH and nutrient uptake which is also helpful in coping metal stresses (Lapie et al. 2019; Tanwir et al. 2015). Organic acids are important byproducts of glyoxylate pathway, Krebs cycle, CAM, and C3 photosynthesis in plants (Leegood et al. 2006). Chromium accumulation in plants significantly alters the rhizospheric pH, triggers root exudation activity in rice plants, and is associated positively with Cr bioavailability (Zeng et al. 2008). The released citric, maleic, tartaric, and oxalic acids establish binding with metals to form chelates which mediate the bioavailability of heavy metals in plants (Shahid et al. 2017; Osmolovskaya et al. 2018).
Tomato (Solanum lycopersicum) is globally utilized as an important commercial vegetable crop and Pakistan ranks at 34th position regarding annual tomato production (Qasim et al. 2018). In Pakistan, the tomato production was recorded 10.2 tons/ha during the years 2017 and 2018, which is much lower than other countries (Agricultural Statistics of Pakistan 2019). Heavy metal pollution including Cr toxicity is one of the basic reasons behind lower tomato production.
The current study was aimed to assess the Cr retention potential of two tomato cultivars by organic acid-mediated pH changes in plant rhizosphere, together with alterations in morpho-physiological attributes and nutrients acquisition while growing under Cr toxicity regimes. The major objectives behind this study were (a) to investigate the food safety aspects of tomato production at Cr contaminated sites by finding the possible Cr tolerant tomato cultivar, (b) to assess the modulation of rhizospheric pH in tomato depending upon the exudation of organic acids for reducing Cr uptake, and (c) also to determine the linkages between morpho-physiological attributes and minerals accumulation with pH changes and their contribution for Cr remediation as well as its reduction in edible plant parts under Cr stress.
Materials and methods
Experimental plan
Four-week-old seedlings of two commonly grown tomato cultivars (cv. Nakeb and cv. Nadir) from Pakistan were maintained in a hydroponic experiment consisting of 32 black plastic containers (300 ml), each containing 250 ml (1/4 strength) Hoagland solution (Eliasson 1978). Six tomato seedlings, mounted in Styrofoam, were grown in each container and different Cr levels (0, 100, 200, 300 mg/L K2Cr2O7) containing 0, 35.4, 70.8, and 106.2 mg/L Cr (VI) respectively were applied (Kurshid et al. 2016). The experiment was arranged in completely randomized design with four replicates for each Cr treatment. These containers were placed in a growth chamber with day/night temperature of 25/18 ± 4 °C, relative humidity 75 ± 10%, and 16-/8-h light/dark length. Initial pH of growth medium was set to 6.0 and allowed to change freely, whereas nutrient solutions were not replenished for a period of 6 days. The pH of the experimental solution was noted after every 12 h up to 6 days with a pH meter (ISTEK Model 4005-08007 Seoul, South Korea). Three tomato plants were harvested after 7 days of cultivation, rinsed with distilled water and preserved in a freezer for physio-biochemical analysis after recording the fresh weights. For dry biomass and mineral analysis, tomato seedlings were washed twice with deionized water, dipped into 20 mM EDTA solution, and again washed twice with distilled water in order to remove the metallic ions from plant surfaces (Mohanty et al. 2015). These plants were then oven dried at 105 °C for 24 h and stored for the analysis of mineral elements.
Collection of root exudates
Remaining three tomato seedlings from hydroponics were shifted to nylon nets in the rhizoboxes for the collection of root exudates (Javed et al. 2013; UdDin et al. 2015) from rhizospheric (a zone surrounding the root) solution. After 48 h, the tomato plants of both the cultivars were taken from nylon net and washed with deionized water to collect the organic acid exudates attached with root surfaces. These exudates were filtered by using a 0.45-μm filter (Millex- HA, Millipore), mixed with NaOH solution (0.01 M), poured into Eppendorf tubes (Greger and Landberg 2008), and preserved at − 80 °C in a freezer for further analysis. The samples used for analysis of oxalic acid were not treated with NaOH.
Growth and physio-biochemical analysis
Leaf area measurement
The leaf area meter (L12000, L1-COR, USA) was utilized for the measurement of leaf area of individual seedlings growing in aqueous medium.
Seedling chlorophyll and carotenoid contents
Total chlorophyll and carotenoid contents were estimated by using methodology of Arnon (1949) for that frozen leaves were grinded in 80% acetone and kept overnight. Supernatants were separated through centrifugation at 10,000 ×g for 15 min at 4 °C. The absorbances were quantified spectrophotometrically (Hitachi U-2910, Tokyo, Japan) at wavelengths of 645 and 663 nm for chlorophyll and 480 nm for carotenoid contents.
Anthocyanin contents
Frozen plant material (0.1 g) was homogenized in 500 μl mixture containing 79:20:1 of methanol, distilled water, and HCl respectively and kept for 1 h at 4 °C after gentle shaking (Mancinelli 1984). The mixture was clarified by centrifugation at 14,000 ×g for 15 min at 4 °C and the absorbances of supernatants were measured at 530 and 657 nm by spectrophotometer (Hitachi U-2910, Tokyo, Japan). Quantification of anthocyanin was accomplished by following equation;
Determination of ascorbic acid contents
Methodology of Mukherjee and Choudhuri (1983) was used for the estimation of ascorbic acid contents. Frozen plant material (0.25 g) was extracted in 6% TCA (5 ml). Plant extract (4 ml) was poured in 2 ml dinitrophenyl hydrazine (2%) in acid followed by the addition of few drops of ethanol (70%) and thiourea in reaction mixture. After boiling in water bath for 20 min, sulphuric acid (80%) was poured into cooled mixture. The absorbance of the reaction mixtures was measured at 530 nm by using spectrophotometer (Hitachi U-2910, Tokyo, Japan). Ascorbic acid standards were used for establishment of standard curves to determine AsA concentrations.
Total free amino acids
Free amino acids of seedlings were measured by following the method of Hamilton and Van Slyke (1943). About 0.1 g of frozen leaf material was homogenized in 5 ml phosphate buffer and centrifuged at 12000 ×g for 15 min to separate the supernatant. The supernatant (1 ml) was taken in test tubes and 1 ml of both pyridine (1%) and ninhydrin (2%) were added, heated in water bath (30 min), and raised volume up to 50 ml and absorbance was recorded at 570 nm.
Determination of total proteins
One milliliter aliquote of frozen plant material extracted in phosphate buffer was mixed with Bradford reagent (2 ml), heated for 30 min in water bath, and followed by the measurement of optical density at 595 nm with a spectrophotometer (Hitachi U-2910, Tokyo, Japan) (Bradford 1976). The BSA (bovine serum albumin) standards were used for the quantification of total proteins.
Analysis of root exudates
High-performance liquid chromatography (HPLC) was used for the quantification of organic acids with a model Flexer FX-10 UHPLC isocratic pump (PerkinElmer, MA, USA). Analysis of organic acids was executed by adding 80% ethanol to frozen samples and by loading 20 μl of sample solution to C-18 column (Brownlee Analytical C-183 μm; length 150 mm × 4.6 mm2, USA). Mobile phase of HPLC was comprised of aceto-nitrile in acid: acetic acid: H2SO4 in 15:1:4 ratio respectively with fixed pH of 4.9. The flow rate of 1 ml per minute was maintained for the analysis of organic acids for total 10-min time period. The column temperature of 45 °C and 214-nm absorption wavelength were used with a detector (UV–VIS Series 200, USA) (UdDin et al. 2015).
Analysis of plant mineral elements
The dried root and shoot materials (0.1 g) were powdered separately and taken into the digestion flasks which were poured with 2 ml solution of perchloric acid (HClO4) and nitric acid (HNO3) in the ratio of 1:10 and left overnight (Bonet et al. 1991). These acidic mixtures were poured into 50-ml flasks and were further digested for the extraction of metallic ions by using MDS-8 (Microwave Digestion Unit). The digested solution was filtered and the volume was raised up to 50 ml by addition of distilled water. Chromium and other minerals (Fe, Zn, K, Mg, and Ca) were analyzed from digested solutions by using atomic absorption spectrophotometer (Hitachi. Model 7JO-8024, Tokyo, Japan).
Calculations of bioaccumulation and translocation factor
Bioaccumulation factor (BAF) and translocation factor (TF) were calculated by following the method of Parveen et al. (2020). Bioaccumulation factor was calculated by taking the ratio of Cr contents in plant tissues and Cr contents in the nutrient medium by the following formula:
Whereas TF was measured by assessing the Cr content in one plant part with respect to other parts as follows:
Statistical analysis of experimental data
The analysis of variance (ANOVA) was applied to collected experimental data by using statistical software, CoStat V 6.2, Cohorts Software, 2003, Monterey, CA, USA. The treatment means were compared by least significant difference method (Fisher’s LSD) at p ≤ 0.05 level. Pearson correlations and principal component analysis biplots of studied variables were established by using the software, XL-STAT 2010.
Results
pH modulation response of tomato cultivars under Cr stress
Time scale studies revealed that after 48 h of all Cr treatment levels, both the cultivars initiated differential acidosis and basification response for growth medium pH which was initially adjusted to 6.0 (Table 1). At 200 mg/L treatment, acidification response diminishes in cv. Nakeb while cv. Nadir maintained medium basification after 4 days. On day 6, cv. Nakeb exhibited a pH decrease of 0.92 units, whereas cv. Nadir caused an acidosis of growth medium by 0.125 units with concomitant rise in H+ ion concentrations at 300 mg/L Cr stress. However, differential time scale response for growth medium basification was recorded in both cultivars where the cv. Nadir exhibited a rise in pH by 0.7 units up to day 4 when stressed by maximum Cr level while cv. Nakeb by 0.65 units at day 2 under 100 mg/L Cr stress.
Growth and physio-biochemical attributes of tomato cultivars under Cr stress
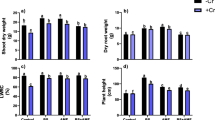
The results were indicating that increased Cr toxicity levels significantly decreased the plant biomasses, leaf area, total chlorophyll, and carotenoid contents of both tomato cultivars in a dose-dependent manner (Table 2). Maximum fresh and dry biomasses were recorded in tomato plants maintained without Cr stress. Reduction in shoot fresh and dry weights was dependent on applied Cr levels and 51.76 and 72.58% higher decrease was recorded in cv. Nakeb than cv. Nadir (35 and 43.47%) at 300 mg/L treatment in comparison to untreated controls.
Likewise root fresh and dry weights of tomato cultivars were significantly decreased at all Cr stress levels as compared to controls. Root fresh and dry weight reduction was 54.56 and 56.33% respectively for cv. Nakeb while 41.28 and 38.33% in cv. Nadir under maximum 300 mg/L Cr stress as compared to untreated controls.
Leaf areas of both tomato cultivars were significantly reduced after exposure to increasing Cr treatment levels. Decrease in leaf area was cultivar specific where prominent reduction of 40.86% was recorded in cv. Nakeb and 19.55% in cv. Nadir at highest Cr treatment level in comparison to control plants.
The degradation of photosynthetic pigments (chlorophylls and carotenoids contents) was increased significantly in both the cultivars due to enhanced Cr toxicity levels in a cultivar-specific and dose-dependent manner. Under highest applied Cr stress level of 300 mg/L, significant reduction of chlorophyll and carotenoid contents by 31.41 and 26.38% respectively was recorded in cv. Nakeb and by 29.44 and 20.23% in cv. Nadir as compared to untreated controls. Carotenoid contents of cv. Nakeb were significantly reduced at 200 and 300 mg/L Cr treatments while for cv. Nadir reduction was recorded at 300 mg/L treatment only.
A consistent pattern of increased anthocyanin levels of both cultivars was observed under various Cr stress levels predominantly in cv. Nadir as compared to cv. Nakeb. The increased pigment level was dose dependent where highest anthocyanin production (55% and 45%) was recorded in cv. Nadir at 200 and 300 mg/L Cr treatments respectively while 49.7% in cv. Nakeb at maximum applied Cr stress as compared to controls.
Chromium stress negatively affected the leaf ascorbic acid contents of both the tomato cultivars when compared to untreated controls. A significant decline of 5.63% was recorded in cv. Nakeb while 3.17% reduction in ascorbic acid content was observed in cv. Nadir in comparison to untreated plants when subjected to 300 mg/L Cr treatment.
Plant amino acid and protein biosynthesis was highest when grown without the application of Cr stress. A prominent reduction in amino acid and protein contents was observed at highest applied Cr concentration. At 300 mg/L applied Cr stress, maximum reduction in amino acid (31.57, 22.82%) and protein (48.53, 20.23%) was observed in cv. Nakeb and cv. Nadir respectively as compared to control plants.
Chromium accumulation and translocation in tomato cultivars under Cr stress
A consistent increase in plant Cr accumulation and translocation was recoded in both the cultivars with increasing levels of applied Cr stress levels (Fig. 1a). Root Cr uptake and translocation revealed an increase of 93.33 and 89.86% respectively in cv. Nakeb while 94.29 and 92.48% in cv. Nadir at 300 mg/L Cr treatment in comparison to untreated controls. However, only a small proportion of Cr was translocated to shoots rendering its higher retention in roots of both cultivars. The cultivar-specific significant variations were recorded in BAF and TF values and the cv. Nadir exhibited higher values for both BAF and TF (0.023, 0.463) respectively than cv. Nakeb (0.017, 0.419) at 300 mg/L Cr treatment as compared to untreated controls.
Roots and shoots Cr (a), Fe (b), Zn (c), K (d), Mg (e), and Ca (f) contents of two cultivars of Solanum lycopersicum Mill. (cv. Nakeb and cv. Nadir) cultivated under different Cr treatments (0, 100, 200, and 300 mg/L K2Cr2O7) in hydroponics. Significant differences for root and shoot mineral contents are represented by different lower and upper case letters. (n = 4, ± SE)
Nutrient acquisition of tomato cultivars under Cr stress
Mineral nutrients (Fe, Zn, K, Mg, and Ca) in roots and shoots were analyzed to investigate the effects of applied Cr levels on nutrient acquisition patterns of both tomato cultivars (Fig. 1b–f). Accumulation of these minerals was maximum in plants grown without Cr stress while Cr treatments conferred a significant reduction in uptake and translocation of studied nutrients in a dose-dependent manner giving the reduction order of 300 ˃ 200 ˃ 100 mg/L Cr stress. The root Fe contents of both cultivars were significantly decreased at Cr treatment of 300 mg/L. The reduction in Fe contents was cultivar specific where 32.04 and 47.54% decrease of root and shoot Fe was observed in cv. Nakeb while 24.40 and 35.59% reduction in cv. Nadir at 300 mg/L Cr treatment as compared to untreated controls (Fig. 1b). Under Cr stress, root and shoot Zn contents of both the cultivars were significantly reduced but prominent reduction was observed in cv. Nakeb as compared to cv. Nadir. At 200 and 300 mg/L Cr stress, cv. Nakeb exhibited 34% and 55.73% reduction in root Zn contents, whereas 46% and 54.28% reduction of root Zn was found in cv. Nadir as compared to untreated tomato plants (Fig. 1c). The K uptake in both cultivars was decreased significantly under various Cr applied stress levels. The maximum decrease in root K contents of 28 and 45.05% as well as 16.6 and 28.54% respectively was recorded in cultivars Nakeb and Nadir at 200 and 300 mg/L Cr treatments as compared to controls. A similar trend was observed in shoot K contents for both the cultivars under Cr stress (Fig. 1d). Without Cr application, non-significant difference of root Mg content was observed in both the tomato cultivars, whereas increasing Cr stress impaired Mg accumulation by 29.22 and 35.45% in cv. Nakeb and cv. Nadir respectively at highest Cr treatment as compared to controls (Fig. 1e). Applied Cr stress reduced the shoot Mg content in both cultivars giving a reduction of 41.17 and 80.20% in cv. Nakeb while 50.25 and 72.79% reduction was noted in cv. Nadir at 200 and 300 mg/L Cr stress. Both the cultivars exhibited similar levels of Ca accumulation in control plants maintained without Cr application. The Ca contents in roots of both cultivars were decreased significantly with increasing levels of applied Cr treatments. The reduction in Ca contents was more prominent in cv. Nakeb where 38.26 and 55.54% decrease in root and shoot Ca was recorded at 300 mg/L Cr as compared to cv. Nadir which exhibited 45.88 and 53.44% reduction at same Cr level as compared to untreated controls (Fig. 1f).
Exudation of organic acids by tomato cultivars under Cr stress
Root exudation of organic acids was minimum in plants grown without Cr application. At all applied Cr stress levels, both tomato cultivars secreted different organic acids which exhibited a steady increase with increasing Cr treatment levels (Table 3). At mild Cr treatments (100 and 200 mg/L), both the cultivars exhibited mild increase in organic acids secretion. The maximum exudation was recorded in cv. Nadir giving 24.13, 20.2, 24.2, 9.35, and 21.31% higher contents of citric, acetic, maleic, tartaric, and oxalic acids respectively than cv. Nakeb at 300 mg/L Cr stress in comparison to untreated tomato plants. Enhancement of Cr-mediated organic acids exudation depicted an increasing order of maleic acid ˃ citric acid ˃ oxalic acid ˃ acetic acid ˃ tartaric acid.
Correlations and principal component analysis bioplots
Pearson correlations and principal component analysis biplots of different experimental treatments and plant morpho-physiological attributes of both tomato cultivars were established under Cr stress which indicated significant positive and/or negative relationships among studied parameters (Fig. 2 and Table 4). Correlation biplots exhibited variations of 91.75% and 90.12% for cv. Nadir and cv. Nakeb respectively (Fig. 2). The variables present in the same quadrant and very close to each other were positively correlated. Red dots indicate correlation among the parameters while the blue dots represent correlations among the applied Cr treatments. Change in pH on day 2 and day 6 was positively correlated with plant Ca (0.79*** and 0.74***) and Mg (0.75*** and 0.76***) contents respectively (Table 4). Plant Cr contents were positively correlated with citric acid (0.98***), acetic acid (0.98***), maleic acid (0.96***), tartaric acid (0.92***), and oxalic acid (0.99***) as well as anthocyanin contents (0.99***). Growth attributes like plant fresh and dry weights, leaf area, chlorophylls, ascorbic acid, and protein contents were positively correlated with plant Ca and Mg contents. Plant Cr contents exhibited negative correlations with pH change on day 2 and day 6 as well as plant fresh and dry weights, leaf area, chlorophyll, ascorbic acid, and protein contents.
Pearson correlation biplots for pH changes, mopho-physiological attributes, organic acids, and mineral ions of cv. Nadir (a) and cv. Nakeb (b) under Cr stress. Red dots are showing correlation among the studied parameters while the blue dots represent correlations among the treatments. pH 2, pH on day 2; pH 4, pH on day 4; pH 6, pH on day6; P FW, plant fresh weight; P DW, plant dry weight; LA, leaf area; T Chl, total chlorophyll; Antho, anthocyanin; ASA, ascorbic acid; ASA, ascorbic acid; Prot, protein; Car, carotenoids; CA, citric acid; AcA, acetic acid; Ma, maleic acid; TA, tartaric acid; OA, oxalic acid; Cr P, chromium contents of plant; Mg P, magnesium contents of plant; Ca P, calcium contents of plant; BAF, bioaccumulation factor
Discussion
Initially, increase in Cr toxicity levels triggered both the tomato cultivars to cause the medium basification up to day 4 which was culminated with time on day 6 where an acidification response was recorded (Table 1). Rise in pH with increasing Cr treatment levels in the rhizosphere of rice has already been reported (Zeng et al. 2008). After 6 days of Cr treatment at highest level, cv. Nadir caused a collective medium basification of 0.6 units while cv. Nakeb resulted in an acidification of 0.7 units with concomitant alterations in proton concentrations. It was anticipated that both the cultivars possess differential Cr tolerance ability which triggered medium basification and acidification responses under Cr stress accordingly. Our results corroborate the findings that eggplant cultivars initiated different pH modulations of growth medium under applied Pb stress (Javed et al. 2019). Presented results pointed out the existence of H+ consuming or OH−1 secreting mechanisms, in cv. Nadir which increase the medium pH and are lacking in cv. Nakeb grown under Cr stress. Alterations in medium pH were prominent with increasing Cr treatment levels. Plant roots secrete protons when they uptake higher amount of cations than anions whereas take up protons when the opposite occurs (Curtin and Wen 2004). Such differential rates in accumulation of cations and anions by plants may stimulate the secretion of buffering agents which ultimately results in rise of medium pH as observed for cv. Nadir. The H+-ATPase enzyme together with antiporters and symporters regulates cellular charge balance by pumping protons in or out at plasmalemma. Another reason for altered rhizospheric pH can be owing to Cr interference with proton-pumping activity of H+-ATPase (Shanker et al. 2005) and likely to occur predominantly in cv. Nakeb as evident from marked reduction of its growth attributes.
In present study, a significant reduction in plant fresh and dry biomasses in both tomato cultivars was recorded with increasing levels of Cr treatments (Table 2; Fig. 2). Higher decrease in fresh and dry biomasses of cv. Nakeb as compared to cv. Nadir pointed out that Cr stress exerted more negative impact on photosynthetic efficiency and water relations of former tomato cultivar. Plant cell cycle and cell division were negatively influenced by Cr stresses which ultimately affects the root and shoot development. In present study, decline in tomato biomasses might result from impaired root development due to enhanced Cr accumulation which resulted in reduced nutrient uptake and lower shoot development (Singh et al. 2016). Reduced plant growth may result from the fact that Cr toxicity inhibits the chlorophyll synthase activity, protein biosynthesis, water uptake, and transpiration rate of tomato plants (Ahmad et al. 2017; Singh and Prasad 2019; Singh et al. 2016). Poor root development under Cr stress represents lower root to shoot translocation of mineral nutrients which leads to retarded leaf growth (Table 2, Fig. 2) as Cr treatment of maize plants has been reported to decrease cell volume, intracellular spaces, and formation of metal-protein complexes and ultimately inhibiting plant development (Anjum et al. 2017).
Anthocyanin contents of tomato plants increased with increasing Cr treatment levels predominantly in cv. Nadir which plays an important role in metal stress detoxification (Javed et al. 2019). Enhanced biosynthesis of anthocyanin in tomato plants probably originates from Cr-mediated glutathione S transferase enzyme as reported for Brassica juncea L. (Handa et al. 2019).
Photosynthetic pigments (chlorophyll and carotenoid contents) of tomato were significantly decreased with increasing level of Cr stress predominantly in cv. Nakeb (Table 2). Chromium-mediated reduction in photosynthetic pigments might result from lower retention of iron contents and degradation of chlorophyll synthase enzyme contributing to impaired photosynthesis and growth rate (Gupta et al. 2020a) of studied tomato cultivars. Furthermore, our results coincide with the findings that Beta vulgaris L. plants exposed to Cr stress resulted in significant disruption of electron transport chain in PSI and PSII (Pandey et al. 2017) as well as the alterations in rubisco activity and substitution of Mg2+ which leads to depletion of chlorophyll activity (Gomes et al. 2017). With increasing applied Cr levels, a steady decline in plant protein and amino acid contents was recorded and the effect was prominent in cv. Nakeb (Table 2). Similar results of soluble protein and amino acid decline, triggered by Cr uptake, in Pistia stratoites L. has been reported (Ganesh et al. 2008) and likely to originate from Cr-mediated ROS production and oxidative burst which degrades DNA, protein, and amino acid biosynthesis (Sharma et al. 2016). Furthermore, it was revealed that increased Cr uptake and decreased essential nutrients accumulation through roots affected the protein biosynthesis negatively (Ali et al. 2013).
Application of Cr significantly induced higher root exudation of citric, acetic, maleic, tartaric, and oxalic acids in cv. Nadir than cv. Nakeb as compared to untreated controls (Table 3). The differential root exudation response of Cr-stressed cv. Nadir and cv. Nakeb has been perceived to depend on their Cr tolerance levels. The toxic elements disturb the metabolism of root cells associated with accumulation and release of different organic acids. Current study depicted the exudation of different organic acids from roots of Cr-stressed tomato plants which could have significant role in ameliorating the Cr toxicity (Fig. 2) by metal detoxification, nutrient stabilization, and enhanced plant growth as reported for Solanum nigrum L. and Parthenium hysterophorus L. (UdDin et al. 2015). Our results coincide with the findings of Khatun et al. (2019) who reported that exogenous application of citric acid enhanced the activity of antioxidants, thereby restricting Cr accumulation in rice plant. Exudation of organic acids took place by anion channels (Zhu et al. 2011), and their release should be balanced by efflux of cations/protons as reported for poplar roots under metal stress (Qin et al. 2007). Chromium stress impaired the proton-pumping activities of H+-ATPase in plants which inhibited H+-efflux (Shanker et al. 2005). Under such scenario, the exuded organic acid anions bind protons which probably explain the medium basification observed up to day 4 in present study particularly for cv. Nadir. Time course studies revealed that pH modulation response of tomato cultivars was diminished with Cr exposure time and is likely due to elemental toxicity. This might be explained by the fact that secretion of organic acids stimulates ammonification which release protons and ultimately causes a decrease in medium pH. Exudation of organic acids is stimulated from plant roots by metal-induced activation of genes (Ma et al. 2000; Javed et al. 2018) and/or by various mechanisms which remained constant with time (Zhao et al. 2003). The former pattern is likely to work in tomato which means that its roots secrete different organic anions and Cr stress further expedites the secretion of citric, acetic, maleic, tartaric, and oxalic acids.
Chromium accumulation in roots and shoots of both tomato cultivars increased with increasing treatment levels of Cr in the growth medium (Fig. 1a). Our results corroborates the findings of Gupta et al. (2020a, b) who reported that Cr uptake has increased in tomato plants due to dose-dependent bioavailability of Cr. Higher root and shoot Cr contents have been recorded in various species including Oryza sativa L. (Zeng et al. 2011) and Brassica napus L. (Gill et al. 2015) with increasing Cr stress levels. Shahid et al. 2017 reported that Cr accumulates predominantly in plant root tissues with very limited acropetal translocation. Higher root Cr retention in cv. Nadir as compared to cv. Nakeb might result from higher exudation of organic acids. Differential Cr uptake response of cv. Nadir and cv. Nakeb may also result from plant-induced pH effect on Cr speciation (Szabó et al. 2018). Gupta et al. (2020a) reported the increased phytoremediation ability of tomato associated with PGPR inoculation under Cr stress. In present study, exuded organic acids by both tomato cultivars were significantly correlated with plant Cr accumulation (Table 4) and could be a detoxification mechanism for tomato through complexation of organic anions with Cr cations. For example, citrate and malate have a strong affinity to establish stable complexes with metallic cations due to the presence of tri-carboxyl groups resulting in formation of 5- or 6-member chelate rings (Qin et al. 2007). Various studies explained the interaction effects of metals and organic acids for increased mobility achieved either by direct competition for cation adsorption sites or by reduction of negative electrostatic potential (Collins et al. 2003). For example, study by Srivastava et al. (1999) depicted enhanced Cr uptake in tomato owing to interaction effects between Cr and organic acids. Despite Cr accumulation, reduction of shoot growth was partially ameliorated by enhanced secretion of organic acids in studied tomato cultivars. The ameliorative effect could be strong if the organic acids are produced in higher concentrations as recorded for cv. Nadir.
Root and shoot Fe, Zn, K, Ca, and Mg contents in both tomato cultivars were affected antagonistically with increasing Cr stress levels but the response was cultivar specific. Results revealed that Cr stress conferred lower retention of mineral nutrients where more reduction was recorded in cv. Nakeb than cv. Nadir in comparison to untreated controls (Fig. 1, Table 4). Such outcomes could arise from higher Cr accumulation which displace the mineral nutrients from binding sites and thereby limit their uptake and translocation (Gupta et al. 2020b). For example, increase in Cr uptake might compete with Fe binding sites and causes significant reduction in plant Fe uptake (Tripathi et al. 2018). Likewise, reduction in K contents may be associated with xylem blockage and inhibition of plant root growth under Cr stress (Sundaramoorthy et al. 2010). Similar results for Zn and Ca were reported by Zeng et al. (2010) that Cr stress decreased plant Zn and Ca levels resulting in impaired growth of rice. Chromium stress resulted in impaired Mg uptake in tomato roots and shoots which caused a decline in chlorophyll biosynthesis (Hayat et al. 2012) predominantly in cv. Nakeb and might be associated with negative root development. Higher nutrient accumulation in cv. Nadir than cv. Nakeb might result from higher medium basification effect caused by Cr stress. A pH increase by roots of cv. Nadir reduces the interaction effect between protons and nutrient ions at uptake sites in solution (Marschner 2011), and thereby increases accumulation of cationic nutrients (Fe, Zn, K, Mg, and Ca). However, medium acidosis induced by cv. Nakeb under Cr stress diminishes nutrient accumulation in this cultivar due to uptake competition among protons and nutrient ions. The uptake of nutrients may also be facilitated by organic acids, however, not in present study as organic acids were negatively correlated with plant Ca and Mg contents in both cultivars.
Conclusions
It was concluded that tomato cultivars (cv. Nakeb and cv. Nadir) responded to Cr stress by triggering differential pH changes in growth medium and organic acids exudation which is likely to depend on their Cr tolerance potential. After 6 days of Cr application, cv. Nadir exhibited cumulative basification of 0.6 units, augmented growth, and exudation of various organic acids. Plant Cr contents were positively correlated with the exudation of citric, acetic, maleic, tartaric, and oxalic acids. It was envisioned that Cr stress triggered higher exudation of organic acids in cv. Nadir which initiate growth medium basification followed by greater retention of Cr and nutrients (Fe, Zn, K, Mg, and Ca). The reported biochemical changes in tomato plants and rhizosphere enabled this genotype for better adaptation towards Cr stress as deciphered by growth augmentation. The organic acid-based pH response of both cultivars under Cr toxicity is a probable feature which would be used for food bio-safety programs as well as for phytoremediation after fruit harvesting. Current study focused the short-term Cr effects on organic acid release, pH modulation, and growth response of tomato cultivars in nutrient medium. Hence, long-term field studied should be executed to draw parallels amongst root exudates, pH modulation response, metal stress, nutrients transport, plant growth, and food bio-safety.
Data availability
Not applicable.
References
Achmad RT, Budiawan AEI (2017) Effects of chromium on human body. Annu Res Rev Biol 13(2):1–8
Agricultural Statistics of Pakistan report (2019) Ministry of national food security and research
Ahmad H, Hayat S, Ali M, Ghani M I, Zhihui C (2017) Regulation of growth and physiological traits of cucumber (Cucumis sativus L.) through various levels of 28-homobrassinolide under salt stress conditions. Can J Plant Sci 98(1):132–140
Ali S, Farooq MA, Jahangir MM, Abbas F, Bharwana SA, Zhang GP (2013) Effect of chromium and nitrogen form on photosynthesis and anti-oxidative system in barley. Biol Plant 57(4):758–763
Anjum SA, Ashraf U, Khan I, Tanveer M, Shahid M, Shakoor A, Longchang W (2017) Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 27(2):262–273
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Banerjee S, Misra A, Chaudhury S, Dam B (2019) A Bacillus strain TCL isolated from Jharia coalmine with remarkable stress responses, chromium reduction capability and bioremediation potential. J Hazard Mater 367:215–223
Bilal S, Khan AL, Shahzad R, Kim YH, Imran M, Khan MJ, Lee IJ (2018) Mechanisms of Cr(VI) resistance by endophytic Sphingomonas sp. LK11 and its Cr (VI) phytotoxic mitigating effects in soybean (Glycine max L.). Ecotox Environ Safe 164:648–658
Bonet A, Poschenrieder C, Barcelo J (1991) Chromium III-iron interaction in Fe-deficient and Fe-sufficient bean plants. I. Growth and nutrient content. J Plant Nutr 14:403–414
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254
Collins RN, Merrington G, Mclauglin MJ, Morel JL (2003) Organic ligand and pH effects on isotopically exchangeable cadmium in polluted soils. Soil Sci Soc Am J 67:112–121
Curtin D, Wen G (2004) Plant cation-anion balance as affected by the ionic composition of the growing medium. Plant Soil 267:109–115
Eliasson L (1978) Effects of nutrients and light on growth and root formation in Pisum sativum cuttings. Physiol Plant 43(1):13–18
Ganesh KS, Baskaran L, Rajasekaran S, Sumathi K, Chidambaram ALA, Sundaramoorthy P (2008) Chromium stress induced alterations in biochemical and enzyme metabolism in aquatic and terrestrial plants. Colloids Surf B 63(2):159–163
Gill RA, Ali B, Cui P, Shen E, Farooq MA, Islam F, Ali S, Mao B, Zhou W (2016) Comparative transcriptome profiling of two Brassica napus cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genomics 17:885
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Gomes MA, Hauser-Davis RA, Suzuki MS, Vitoria AP (2017) Plant chromium uptake and transport physiological effects and recent advances in molecular investigations. Ecotoxicol Environ Saf 140:55–64
Greger M, Landberg T (2008) Role of rhizosphere mechanisms in Cd uptake by various wheat cultivars. Plant Soil 312(1-2):195–205
Gupta P, Kumar V, Usmani Z, Rani R, Chandra A, Gupta VK (2020a) Implications of plant growth promoting Klebsiella sp. CPSB4 and Enterobacter sp. CPSB49 in luxuriant growth of tomato plants under chromium stress. Chemosphere 240:124944
Gupta P, Kumar V, Usmani Z, Rani R, Chandra A, Gupta VK (2020b) A comparative evaluation towards the potential of Klebsiella sp. and Enterobacter sp. in plant growth promotion, oxidative stress tolerance and chromium uptake in Helianthus annuus (L.). J Hazard Mater 377F:391–398
Hamilton PB, Van Slyke DD (1943) Amino acid determination with ninhydrin. J Biol Chem 150(1):231–250
Handa N, Kohli SK, Sharma A, Thukral AK, Bhardwaj R, Abd Allah EF, Ahmad P (2019) Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ Exp Bot 161:180–192
Hayat S, Khalique G, Irfan M, Wani AS, Tripathi BN, Ahmad A (2012) Physiological changes induced by chromium stress in plants: an overview. Protoplasma 249(3):599–611
Javed MT, Akram MS, Habib N, Tanwir K, Ali Q, Niazi NK, Gul H, Iqbal N (2018) Deciphering the growth, organic acid exudations, and ionic homeostasis of Amaranthus viridis L. and Portulaca oleracea L. under lead chloride stress. Environ Sci Pollut Res 25:2958–2971
Javed MT, Habib N, Akram MS, Ali Q, Haider MZ, Tanwir K, Chaudhary HJ (2019) The effect of lead pollution on nutrient solution pH and concomitant changes in plant physiology of two contrasting Solanum melongena L. cultivars. Environ Sci Pollut Res 26(33):34633–34644
Javed MT, Stoltz E, Lindberg S, Greger M (2013) Changes in pH and organic acids in mucilage of Eriophorum angustifolium roots after exposure to elevated concentrations of toxic elements. Environ Sci Pollut Res 20:1876–1880
Khatun MR, Mukta RH, Islam MA, Huda AN (2019) Insight into citric acid-induced chromium detoxification in rice (Oryza sativa L.). Int J Phytoremediat 21(12):1234–1240
Kurshid S, Shoaib A, Javaid A (2016) Chromium toxicity to tomato (Lycopersicum esculentum Mill.) susceptible to Fusarium wilt pathogen. Curr Sci:399–404
Lapie C, Leglize P, Paris C, Buisson T, Sterckeman T (2019) Profiling of main metabolites in root exudates and mucilage collected from maize submitted to cadmium stress. Environ Sci Pollut Res 26:17520–17534
Leegood RC, Sharkey TD, von Caemmerer S (2006) (Eds.) Photosynthesis: physiology and metabolism (Vol. 9). Springer Science & Business Media.
Ma JF, Taketa S, Yang ZM (2000) Aluminium tolerance genes on the short arm of chromosome 3R are linked to organic acid release in triticale. Plant Physiol 122:687–694
Mancinelli AL (1984) Photoregulation of anthocyanin synthesis VIII. Effect of light pretreatments. Plant Physiol 75(2):447–453
Marschner P (2011) Mineral nutrition of higher plants. Academic press, London
Mohanty M, Pradhan C, Patra HK (2015) Chromium translocation, concentration and its phytotoxic impacts in in vivo grown seedlings of Sesbania sesban L. Merrill. Acta Biol Hung 66(1):80–92
Mukherjee SP, Choudhuri MA (1983) Determination of glycolate oxidase activity, H2O2 content and catalase activity. Physiol Plant 58:167–170
Osmolovskaya N, Dung VV, Kuchaeva L (2018) The role of organic acids in heavy metal tolerance in plants. Commun Biol 63(1)
Pandey S, Fartyal D, Agarwal A, Shukla T, James D, Kaul T, Reddy MK (2017) Abiotic stress tolerance in plants: myriad roles of ascorbate peroxidase. Front Plant Sci 8:581
Parveen A, Saleem MH, Kamran M, Haider MZ, Chen JT, Malik Z, Rana MS, Hassan A, Hur G, Javed MT, Azeem M (2020) Effect of citric acid on growth, ecophysiology, chloroplast ultrastructure, and phytoremediation potential of jute (Corchorus capsularis L.) seedlings exposed to copper stress. Biomolecules 10(4):592
Qasim M, Farooq W, Akhtar W (2018) Preliminary report on the survey of tomato growers in Sindh. Punjab and Balochistan:1–35
Qin R, Hirano Y, Brunner I (2007) Exudation of organic acid anions from poplar roots after exposure to Al, Cu and Zn. Tree Physiol 27:313–320
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Yan D (2020) Chromium bioaccumulation and its impacts on plants: an overview. Plants 9(1):100
Sharma P, Kumar A, Bhardwaj R (2016) Plant steroidal hormone epibrassinolide regulate–Heavy metal stress tolerance in Oryza sativa L. by modulating antioxidant defense expression. Environ Exp Bot 122:1–9
Shekhawat K, Chatterjee S, Joshi B (2015) Chromium toxicity and its health hazards. Int J Adv Res 3(7):167–172
Singh S, Prasad SM (2019) Regulation of chromium toxicity tolerance in tomato and brinjal by calcium and sulfur through nitric oxide: involvement of enzymes of sulfur assimilation and the ascorbate-glutathione cycle. Environ Exp Bot 166:103789
Singh S, Prasad SM, Singh VP (2020) Additional calcium and sulfur manages hexavalent chromium toxicity in Solanum lycopersicum L. and Solanum melongena L. seedlings by involving nitric oxide. J Hazard Mater:122607
Singh VP, Kumar J, Singh M, Singh S, Prasad SM, Dwivedi R, Singh MPVVB (2016) Role of salicylic acid-seed priming in the regulation of chromium (VI) and UV-B toxicity in maize seedlings. Plant Growth Regul 78(1):79–91
Srivastava S, Prakash S, Srivastava MM (1999) Chromium mobilization and plant availability-the impact of organic complexing ligands. Plant Soil 212:203–208
Sundaramoorthy P, Chidambaram A, Ganesh KS, Unnikannan P, Baskaran L (2010) Chromium stress in paddy: (i) nutrient status of paddy under chromium stress;(ii) phytoremediation of chromium by aquatic and terrestrial weeds. CR Biol 333(8):597–607
Szabó M, Kalmár J, Ditrói T, Bellér G, Lente G, Simic N, Fábián I (2018) Equilibria and kinetics of chromium(VI) speciation in aqueous solution—a comprehensive study from pH 2 to 11. Inorg Chim Acta 472:295–301
Tanwir K, Akram MS, Masood S, Chaudhary HJ, Lindberg S, Javed MT (2015) Cadmium-induced rhizospheric pH dynamics modulated nutrient acquisition and physiological attributes of maize (Zea mays L.). Environ Sci Pollut Res 22(12):9193–9203
Tripathi DK, Singh S, Gaur S, Singh S, Yadav V, Liu S, Dubey NK (2018) Acquisition and homeostasis of iron in higher plants and their probable role in abiotic stress tolerance. Front Environ Sci 5:86
UdDin I, Bano A, Masood S (2015) Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol Environ Saf 113:271–278
Waseem A, Arshad J, Iqbal F, Sajjad A, Mehmood Z, Murtaza G (2014) Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. Biomed Res Int 2014
Zeng F, Ali S, Qiu B, Wu F, Zhang G (2010) Effects of chromium stress on the subcellular distribution and chemical form of Ca, Mg, Fe, and Zn in two rice genotypes. J Plant Nutr Soil Sc 173(1):135–148
Zeng F, Chen S, Miao Y, Wu F, Zhang G (2008) Changes of organic acid exudation and rhizosphere pH in rice plant under chromium stress. Environ Pollut 155:284–289
Zeng FR, Zhao FS, Qiu BY, Ouyang YN, Wu FB, Zhang GP (2011) Alleviation of chromium toxicity by silicon addition in rice plants. Agric Sci China 10(8):1188–1196
Zhao Z, Ma JF, Sato K, Takeda K (2003) Different Al resistance and citrate secretion in barley (Hordeum vulgare L.). Planta 217:794–800
Zhu XF, Zheng C, Hu YT, Jiang T, Liu Y, Dong NY, Yang JL, Zheng SJ (2011) Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esculentum. Plant Cell Environ 34:1055–1064
Acknowledgments
The authors would like to thank Ayub Agricultural Research Institute (ARRI), Faisalabad, Pakistan, for providing seedlings of tomato cultivars.
Funding
Current work was funded by Higher Education Commission (HEC), Pakistan (Grant No: 20-4243/NRPU/R&D/HEC/14/885)
Author information
Authors and Affiliations
Contributions
MTJ, KT, SA, and HJC: planning of study; KT, SA, and RI: experimental work; KT, SA, and MHS: statistical analysis and critical review; MTJ. KT, MHS, HJC, and SA: data discussion. All the authors approved the final version of the manuscript. The presented data is the part of MPhil research work of Ms. Robina Iqbal.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable
Consent to publish
Not applicable.
Competing interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Javed, M.T., Tanwir, K., Abbas, S. et al. Chromium retention potential of two contrasting Solanum lycopersicum Mill. cultivars as deciphered by altered pH dynamics, growth, and organic acid exudation under Cr stress. Environ Sci Pollut Res 28, 27542–27554 (2021). https://doi.org/10.1007/s11356-020-12269-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12269-8