Abstract
This study was conducted to determine the phytotoxicity of 6 nm γ-Fe2O3 nanoparticles (IONPs) in terms of root elongation and the physiological performance of rice plants. Rice seeds (Oryza sativa L. var. Koshihikari) exposed to IONPs at 500, 1,000 and 2,000 mg/L, had a significantly higher root elongation than the control and its bulk counterparts (IOBKs), indicating that the effect can be nanospecific. In a 14-week greenhouse pot experiment, the CO2 assimilation rate in IOBK and IONP-treated pots (500 and 1,000 mg/pot) decreased over time, with the decline (maximum 42.5 %) being less pronounced for IONPs, indicating that the effect cannot be inferred from the toxicity of nanoscale size iron oxide. Excessive adsorption of IONPs onto soil colloids with subsequent low water extractable iron was responsible for the unremarkable phytotoxic nature of IONPs in the rice plants. Amendment of IONPs coated with 20 mmol citric acid (IONPs-Cit) significantly diminished the CO2 assimilation rate and the decrease was similar to its bulk counterpart (IOBKs-Cit). However, maximum shoot growth inhibition (37 %) was associated with the application of IOBKs-Cit. It was concluded that massive accumulations of Fe plaque on the root surfaces of IOBKs-Cit treatments due to a decline in the pH of rhizoplane soils compared to the IONPs-Cit treatments were responsible for the remarkable shoot growth reduction. This study provided evidence of the phytotoxicity of γ-Fe2O3 nanoparticles, demonstrating the lower toxicity of nanosized iron oxide compared to a microsized preparation under reductive conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The essence of nanotechnology is based on the demonstrated fact that materials at the nanoscale have chemical, electrical, magnetic, mechanical and optical properties that are quite different from bulk materials. The rapid increase in the production and use of engineered nanoparticles has outpaced the technical capacity for monitoring their presence in the environment (Sellers et al. 2009). Without scientific investigations, it is not possible to fully evaluate whether the production of nanoparticles is accompanied by significant ecological or human health risks. Based on the evidence from the most recent published studies, it is believed that some level of toxicity of nanoparticles to living systems is inevitable (Barrena et al. 2009; Ghodak et al. 2011).
In this regard, the release of nanoparticles into the environment can be much more significant if the particles enter water bodies, especially river water, which is directly utilized for irrigation purposes in agricultural areas. Therefore, an evaluation of the phytotoxicity of nanoparticles in flooded soil mediums where the plant roots are submerged in irrigated water needs to be considered. Toxicity assays of nanoparticles indicate that they may have both positive and negative effects on seed germination and root elongation of plants. It has been reported that titanium oxide, silver, gold, cobalt and zinc oxide nanoparticles produce various inhibitory effects on the transpiration rate, seed germination and root elongation of willow cuttings, onion, cucumber, and lettuce (Seeger et al. 2009; Barrena et al. 2009; Ghodak et al. 2011). Conversely, multiwall carbon nanotubes and titanium oxide at certain concentrations have been found to accelerate both the germination rate of tomato and aged spinach seeds and their germination vigor (Khodakovskaya et al. 2009; Lei et al. 2005). The amendment of maghemite or magnetite nanoparticles to soil can potentially stimulate bacterial growth and change the bacterial community structure (He et al. 2011). In particular, iron oxide nanoparticles (magnetite or maghemite) have been used extensively in many fields including microelectronics, biomedical imaging and the detection and visualization of various phases and interfaces in oil reservoirs. To achieve water solubility and stability of dispersed particles, various surface coatings including polymers, surfactants, or low molecular weight organic acids (e.g., citric acid) have been used. It has been shown previously that citrate-coated iron oxide nanoparticles can form stable superparamagnetic nanoclusters at high salinity (680 mM NaCl) and thus achieve a strong magnetic force for the purpose of magnetomotive acoustic imaging of oil reservoirs (Kotsmar et al. 2010). The citrate-coating of particles is frequently used in clinical exposure settings, where the low solubility of iron oxide nanoparticles gives rise to precipitation and agglomeration under physiological conditions that can block blood vessels. Therefore, there is a strong possibility that this particular nanomaterial will ultimately enter aquatic ecosystems through wash off and waste water discharges.
Most of the research undertaken regarding the phytotoxicology of nanoparticles has been performed using seed germination tests and hydroponic cultures. To the best of Authors’ knowledge, there is no published information concerning the effects of iron oxide nanoparticle (IONPs) and its citrate-coated form (IONPs-Cit) on root elongation and the physiological performance of rice plants. In this study, the effect of IONPs and IONPs-Cit on root elongation in the germination stage and the photosynthetic characteristics of pot-grown rice plants were investigated. Treatments were applied at the panicle initiation stage because at this stage rice has the strongest aerenchyma formation, and hence has the greatest capacity for iron oxidation. For toxicity assessment, photosynthetic parameters were evaluated because a decrease in leaf chlorophyll content and photosynthesis performance is a typical symptom of elevated levels of iron in anaerobic conditions. The effects of IONPs were also compared to those of their micrometer size bulk counterparts (IOBKs). At harvest, the fractionated rhizosphere soils, as well as non-rhizosphere soils, were further sampled to determine the root-induced changes of pH in response to the treatments.
Materials and methods
Nano and microsized iron oxide particles
Iron oxide nanoparticles (99.9 % purity and average particle size of 6 nm) were purchased from Nano-Oxides, INC, USA. According to data provided by the manufacturer, the nanoparticles had a crystal phase of maghemite (cubic), specific surface area of 180 m2/g, and true density of 5.25 g/cm3. Microsized iron oxide particles were purchased from Wako Pure Chemical Industries, Inc. (Tokyo, Japan). An optical microscope (Olympus Vanox AH, Japan) at 40-fold magnification was calibrated against a stage micrometer to measure particle sizes. The bulk iron oxide had an average particle size of approximately 1–2 μm.
Stabilization of IONPs by citric acid
The method of stabilization of IONPs used in this study was based on a method in Kotsmar et al. (2010). Briefly, the IONPs (500 and 1,000 mg) were mixed with 20 mmol citric acid (3.84 g citric acid in 1,000 ml distilled water) and stirred for 90 min at 90 °C. After bath sonication, the pH value of the solution was carefully adjusted to pH 5 using 0.01 M NaOH or HCl. At this pH value, at least two carboxyl groups for citric acid molecules were dissociated and formed a carboxylate complex with iron atoms on the particle surface. Large iron oxide aggregates were removed and discarded from the dispersion before use. The bulk iron oxides were exposed to the same treatment.
Seed germination
Rice seeds (Oryza sativa L. var. Koshihikari) were immersed in a 10 % sodium hypochlorite solution for 10 min to ensure surface sterility (USEPA 1996) and were then rinsed three times with DI-water. Seeds were then transferred to filter papers placed in 140 × 25 mm Petri dishes. Fifteen milliliters of both IONPs and their bulk counterpart solution were added to each Petri dish (10 seeds per dish) at concentrations of 50, 100, 250, 500, 1,000 or 2,000 mg/L. Except addition of nano and bulk iron oxide, no macro or micronutrients (as nutrient solutions) were applied during rice seed germination. A volume of 15 mL of suspension was chosen because this was sufficient to submerge the seeds. For the homogeneous exposure of treatments to seeds and roots and to maintain sufficient contact, Petri dishes were swirled gently by hand for 1 min every four hours and were periodically weighed and watered with DI-water to bring them back to the original weight. Control groups received only DI-water. Petri dishes were covered and sealed with plastic tape. After 5 days storage in dark conditions at room temperature, seed germination was halted and seedling radicals were measured.
Pot trial
The pot trials were performed in a greenhouse under natural light conditions in triplicate. Field soils equivalent to 1.5 kg oven-dried weight were fertilized at a rate of N:P2O5:K2O = 100:80:100 mg/kg and placed into a 0.05 m2 plastic Wagner pot (internal dimension: 252 mm, height: 300 mm). Four rice seeds were planted in each pot and watered to 62 % of the maximum water holding capacity, which was determined using the Hilgard method prior to the experiment.
Seedlings were thinned to two vigorous plants after 2 weeks of germination. The pot experiment continued for 14 weeks. The pH (H2O) of the soil sample was measured in a suspension (soil:water = 1:2.5). The average values of pH, EC and water extractable iron concentration of the tested soil were 6.59, 0.22 dS/m and 16.2 mg/kg, respectively.
To investigate the adsorption potential of IONPs or IOBKs onto the soil colloids, 3 g soil samples were placed in a centrifuge tube and 0.35 mg of IONPs or IOBKs with 20 mmol citric acid or no citric acid were added to the soil. Fifteen milliliters of distilled water was added and the solutions were shaken for 24 h on a reciprocator. The suspension was then centrifuged (11,700 × g) for 10 min, filtered through 0.45 μm pore size filters and stored for later analysis of “water extractable iron”. The control samples received only distilled water. Iron concentrations in the soil extracts were determined using ICP-AES (ICPE-9000, Shimadzu).
In total, nine treatments received a soil amendment: a treatment using plants without the addition of nano and bulk iron oxide (control); four treatments using plants and iron oxides (500 and 1,000 mg per pot, equivalent to 349.4 and 698.7 mg iron per pot, respectively); and four treatments using plants and citric acid (20 mM) coated iron oxides. Before the treatments began, the pots were left for days until the soil was at its field capacity. To obtain better contact between the roots and the iron oxides, treatments were applied to central and marginal regions of the soil surface (soil-pot interfaces) where artificial cavities were created with a glass rod for efficient infiltration of the suspensions. The treatments were applied to the soils before panicle emergence.
Photosynthetic parameters of rice leaves were determined on the seventh day after treatment began using an Li-6400 Portable Photosynthesis Analysis System (Li-COR Inc., Lincoln, NE, USA). The CO2 assimilation rate (A n ), stomatal conductance (g s), intercellular CO2 concentration (C i ) and transpiration rate (T r) were measured under an external CO2 concentration of 380 μmol/mol supplied from a CO2 steel gas cylinder and at a light intensity of approximately 1,000 μmol/m2 s provided by an LED Red/Blue light source. Leaf chlorophyll (Chl) was monitored non-destructively with a SPAD-502 apparatus (Minolta, CO., Ltd., Japan) 1 day after soil treatments began and then on a daily basis for a period of 1 week. To account for leaf heterogeneity, SPAD measurements were taken from different locations on the leaves (n = 30). At harvest, the soil systems were differentiated into bulk, rhizosphere and rhizoplane soils based on the mechanical removal of root-adhering soil aggregates (Alidoust et al. 2012). Root fragments were carefully removed from the pots and gently shaken. The soil remaining in the pot was subsequently passed through a 1-mm mesh to trap the majority of the soil aggregates containing root segments. The sieved soil was mixed homogenously and sampled as a bulk soil fraction. Soil aggregates adhering to the root system were then spread on a glass plate. Forceps and a scalpel were then used to remove soil aggregates from the root surface, which were adjusted and fixed to a thickness of 5 mm.
This procedure was undertaken to avoid any dilution of rhizosphere soil by the bulk soil components. A similar treatment was applied to the soil aggregates (containing root segments) trapped on the 1-mm mesh during bulk soil separation. The collected aggregates were then carefully sampled using a dissecting needle to produce 1 mm soil particles, which were considered to represent the rhizosphere soil fraction (Fox et al. 1990; Gobran and Clegg 1996). The soil layer of <1 mm thickness adhering to the root surface was referred as the rhizoplane soil fraction and was sampled using a soft brush. Root debris was then removed from all soil samples with tweezers and a further treatment involving static electricity was used to collect rhizoplane soils.
Statistical analysis
Data were statistically evaluated by one-way analysis of variance using SPSS 15 software, and the mean values of each treatment were compared using a Tukey-test at the P < 0.05 confidence level. Each treatment was conducted with three replicates.
Results
Root elongation assay
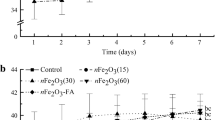
The seed germination test revealed that IONPs and their counterpart bulk suspensions at seven concentrations did not negatively affect root elongation in rice (Fig. 1). The rice seeds exposed to IONPs at 500, 1,000 and 2,000 mg/L had significantly higher root elongation than the control and their counterpart bulk suspensions (P < 0.05). After 5 days, the average root lengths in the presence of nanoparticles at concentrations of 500, 1,000 and 2,000 mg/L were 47, 53 and 48 mm, respectively, representing a 34, 51 and 37 % increase in root elongation. The average root elongation across all treatments with the corresponding bulk materials at concentrations above 250 mg/L was 13 % (±4 %).
Length of rice root grown in nano and bulk iron oxide suspensions for 5 days. Results are shown as the average of triplicate samples. Asterisk symbol denotes significant difference between treatment and control. Letter indicates significant difference between nano and bulk iron oxide (P < 0.05). Error bars indicate standard deviations (SD)
Effect of IONPs on root zone soil pH
In this study, root-mediated changes of pH in the fractionated rhizosphere and non-rhizosphere soils in response to different treatments were evident. It was also found that treatments with iron oxide nanoparticles produced higher pH values than treatments with their bulk counterparts, with an increase of 0.09 and 0.05 pH units following the addition of 500 and 1,000 mg/pot IONPs and an increase of 0.1 and 0.09 pH units following the addition of 500 and 1,000 mg/pot IONPs-Cit, respectively (Fig. 2). The results indicate that the rhizosphere soil tends to adopt an intermediate position between the rhizoplane and the bulk soils. In general, the pH of the rhizoplane soils only increased minimally in the control groups. Interestingly, the addition of IONPs to the pots influenced the pH of rhizoplane soils similar to the control groups, whereas IOBKs slightly acidified the rhizoplane soil pH.
Influence of IONPs on the physiological performance of rice
Table 1 shows the SPAD indices. Neither IONPs nor IONPs-Cit had negative effects on the chlorophyll content within a period of 7 days after treatments began. Furthermore, there was no significant difference between the IONP and IOBK treatments with or without a citric acid coating in regard to the SPAD indices. Iron oxide nanoparticles did not induce an acute inhibition of photosynthesis in rice leaves following the addition of IONP suspensions. No adverse impacts on the CO2 assimilation rate were observed after the application of IOBKs. With the exception of the treatment with citrate-coated bulk materials (IOBKs-Cit) at 500 mg/pot, the CO2 assimilation rate of all treatments decreased slightly but these effects were not statistically significant (Table 2). However, some insignificant negative effects on the CO2 assimilation rate were evident. Notable results were obtained when IONPs-Cit at a concentration of 500 mg/pot were added to the soil. The three parameters of stomatal conductance, intercellular CO2 concentration and transpiration rate were significantly enhanced (P < 0.05) following the treatment, whereas CO2 assimilation rate was slightly impaired.
Growth of rice plants exposed to IONPs
The dry weight of roots, shoots and grains after the application of the different soil treatments are shown in Fig. 3. Solutions used in the treatments containing 500 and 1,000 mg/pot IONPs decreased the shoot dry weight by 12 and 13 % respectively; however, these values were not statistically different from the control and IOBK groups. The grain weight was most affected by the application of IOBKs at a concentration of 1,000 mg/pot and citric acid coated-IOBKs at a concentration of 500 mg/pot where an 18 and 33 % respective loss in grain yield was observed. Overall, no significant difference in the percentage growth inhibition was observed between treatments with the application of IONP and IOBK suspensions (with or without citric acid coating). The only significant decreases were associated with the application of IOBKs-Cit at 500 and 1,000 mg/pot where the shoot dry weight declined by 37 and 29 %, respectively.
Discussion
The effect of iron oxide nanoparticles or bulk powder at concentrations of 0, 50, 100, 250, 500, 1,000 and 2,000 mg/L on rice root elongation was determined. It was observed that root elongation was significantly (P < 0.05) enhanced by exposing plants to IONP concentrations above 500 mg/L and the enhancement was positively correlated with the increasing concentration of the test suspensions. The effect of IOBKs on rice root elongation was not statistically significant at concentrations above 500 mg/L. This indicates that the effect is nanospecific. A possible underlying mechanism could be that the small particle size of IONPs (6 nm) may lead to a higher bioavailability of iron to seeds. This may result in greater penetration of IONPs into the seed coat as well as greater uptake by seed radicals due to the faster dissolution kinetics of IONPs than IOBKs (Dehner et al. 2010; Kraemer 2004). In this study, no statistical differences were observed between the control and IOBK treatments at all concentration levels, except for the 250 mg/L treatment; this value was not statistically different from the corresponding IONP value. This result is consistent with the findings of a previous study regarding the phytotoxicity of IONPs on root elongation in soybean and lettuce (Alidoust and Isoda 2013; Wang et al. 2012). The latter authors observed an enhanced root growth in lettuce exposed to IONPs but they did not compare their results with the corresponding bulk materials.
The effects of soil amendment of IONPs, with or without a citric acid coating, on various photosynthetic parameters of rice plants were investigated in pot experiments that ran for 14 weeks. This is the first study in which the photosynthetic effects of IONPs and IOBKs and their coated forms have been investigated through soil amendment. Exposure to IONPs and IONPs-Cit at concentrations of 500 and 1,000 mg/pot had generated no negative effects on chlorophyll content by the final day of SPAD measurements (7 days) when compared to the control group. This indicates that soil amendment of IONPs does not generate an acute reduction of the photosynthetic pigments by the inhibition of chlorophyll biosynthesis or the inactivation of photosystem II in rice plants. Generally, exposure to nano iron oxide containing Fe2+ and Fe0 leads to concerns of phytotoxicity due to oxidative stress. Further monitoring revealed that the CO2 assimilation rate in all treatments decreased with time and the decline (maximum 42.5 %) was less pronounced for IONPs (Table 3). The impairment of the CO2 assimilation rate was also found to be insignificant following exposure to IOBKs-Cit at a concentration of 1,000 mg/pot and IOBKs at a concentration of 500 mg/pot, indicating that the effect on CO2 assimilation rate was not due to the nanosized preparation of iron oxide. The reduction in rice photosynthesis could be attributed to stomatal limitations, as evidenced by the lower stomatal conductance (g s). At the pericycle (the cylinder of the parenchyma, which is located between the endodermis and phloem in rice roots), iron is effluxed into the xylem, and moves toward the shoot through the transpiration stream. In this study, the stomatal closure led to a reduced transpiration rate especially after the amendment of IOBKs-Cit at a concentration of 1,000 mg/pot, which might be associated with a defense mechanism of rice plants to minimize excessive iron uptake (Table 3).
This result is consistent with Pereira et al. (2013) who found that exposure to high levels of microsized iron (9 mM) resulted in an increase in the internal CO2 concentration and reduced stomatal closure and transpiration rate in rice plants under hydroponic culture. It was found that the growth rates of rice after soil amendments of IONPs decreased slightly but the decreases were not statistically significant. Stunted growth of roots and grains following the treatments with IOBKs-Cit, regardless of the iron oxide concentration, was also evident. It was observed that the rice growth parameters were less inhibited when nano iron oxide coated with citric acid was amended. It was assumed that the lower pH of root zone soils resulted in excess uptake of iron, which in turn stunted the growth of rice. Interestingly, this phenomenon did not occur when IONPs-Cit were applied. Both the solubility of iron oxide and the dissolution rate govern the supply of soluble Fe to plant roots. The dissolution of iron by complexing agents such as citric acid is widely used to estimate plant-available iron. In this study, citric acid was utilized as a coating agent to simulate the actual synthesis and surface engineering conditions of iron oxide nanoparticles in bioengineering applications. Due to the high surface area to volume ratio of IONPs, there is a tendency for the particles to merge into one another until one large particle remains. To avoid this, IONPs are coated with different types of polymers and small molecules in solution to ensure colloidal stability. The coatings often serve multiple purposes: they allow for better water solubility and hydrophilicity (Jun et al. 2007), the attachment of various functional probes (Jun et al. 2007; Bulte and Kraitchman 2004), promote the formation of monodisperse particles (Laurent et al. 2008) and stabilize the magnetite core against agglomeration (Arvizo et al. 2007). For example, the surface of IONPs can be stabilized in an aqueous dispersion by the adsorption of citric acid. The acid can be adsorbed on the surface of IONPs by coordinating via one or two of the carboxylate functionalities. Bee et al. (1995) reported that after adding increasing amounts of citrate ions the diameter of citrate-coated nanoparticles decreased from 8 to 3 nm. Fauconnier et al. (1996) further demonstrated that the co-adsorption of citric acid on γFe2O3 particles resulted in colloidal solutions that were stable within a pH range of 3.5–11. Therefore, after stabilization, IONPs can remain in their monodispersed form with minimal formation of aggregates. The dissolution of hematite with citric acid was investigated by Zhang et al. (1985) and the maximum dissolution was found at about pH 4–5. The iron released by 20 mmol citric acid solutions at 25 and 60 °C was calculated to be approximately 1 and 2.65 μg Fe/g iron oxide, respectively. The close similarity of the study by Zhang et al. (1985) and this study can be used to indicate that Fe released by citric acid in the current work was 15 μg (for 1,000 mg Fe2O3 suspensions) and 7.5 μg (for 500 mg Fe2O3 suspensions), which may be directly available to plants. It is commonly agreed that the solubility product constant of particles <1 μm in diameter increases with decreasing particle size (Trolard and Tardy 1987). This shows that the dissolution of IONPs with citric acid may even favor Fe detachment when compared to the corresponding IOBKs. Therefore, higher concentrations of iron detached from the surface of IONPs by citric acid would be expected during the coating process compared to the corresponding IOBKs. The concentration of water extractable iron recorded after the direct addition of IONPs-Cit to the test Andosol supports this hypothesis (Fig. 4). From a toxicological perspective, excess iron can produce stunted growth of roots, shoots and finally lower grain yields in rice. Under anaerobic conditions, insoluble Fe(III) is reduced to the more soluble Fe(II), which is readily taken up by rice roots leading to severe yield losses in sensitive rice varieties (Becker and Asch 2005). The focal point of these chemical reactions is soil in the root zone where heavy loadings of ferric iron may be reduced into ferrous iron with the help of iron-reducing bacteria. In these circumstances, if the loading of ferric iron exceeds the level of oxidative protection of the rice plant via aerenchymatic air transport, ferrous iron intoxication is inevitable (Jackson and Armstrong 2009; Colmer and Voesenek 2009). However, when the effects of exposure to IONPs-Cit were evaluated, the larger release of iron induced by this treatment could not be the reason for iron toxicity and stunted growth because IOBKs-Cit diminished the shoot dry weight to a larger extent (Fig. 3). This phenomenon may be attributed to three different processes:
-
1.
Changes in the pH of the root zone soils following the amendment of nanosized and microsized iron oxides were evident, therefore the decrease in the pH of root zone soils in the IOBKs-Cit treatment could have enhanced the solubility of iron in the rhizocylinder fraction (the soil adhering to the root surface in a layer <1 mm thick, Hoffmann and Barber 1971) resulting in a greater iron uptake by roots (Marschner et al. 1989), which may induce iron toxicity and stunt growth. The appearance of bronzing symptoms in rice leaves following the IOBKs-Cit treatment was observed and is a typical visual symptom associated with iron toxicity in rice plants. The bronzing symptoms developed in older leaves with the occurrence of brown spots on the leaf base.
-
2.
The root-induced reduction of iron would be expected to shift the dissolution/precipitation equilibria of iron oxide to promote dissolution. In waterlogged soil, metal toxicity can occur due to the ambient reducing conditions of the bulk soil and the increased solubility of metal (Fe and Mn, in particular) oxides. However, the leakage of oxygen from the roots provides a substantial supply of oxygen for rice root respiration, thereby reoxidation can occur in the root zone soil, which can result in the coating of root surfaces with oxidized Fe and the precipitation of Fe oxide on the root surface (Hinsinger 2001). The Fe plaque formed on the surface of the roots of rice plants can act as a sink for essential nutrients through adsorption and/or co-precipitation onto Fe oxides, resulting in a decline of the growth rate. Massive accumulations of Fe plaque were observed on the root surfaces of plants exposed to IOBKs-Cit treatments compared to those exposed to IONPs-Cit.
-
3.
A further reason for this phenomenon could be that nanoparticles in the soil might behave differently from their bulk counterparts, thus affecting their transport potential (Nowack and Bucheli 2007). The interaction of nano or microsized iron oxides with soil particles is an important phenomenon when the solubility and availability of iron in soil solution is of concern. Due to the large surface area to mass ratio of nanosized particles, they have a greater potential to be adsorbed onto soil colloids than larger particles of the same material. Figure 4 shows the concentration of water extractable iron after the addition of IONPs or IOBKs and their citrate-coated forms. Water extraction was undertaken because this fraction is the most available iron pool for absorption in rice. In the test Andosol, a larger concentration of water extractable iron was observed after the addition of IOBKs than IONPs, indicating excessive adsorption of IONPs onto soil colloids. Although higher levels of water extractable iron were generated from citrate-treated IONPs, in the pot experiments (long growth period) a faster re-adsorption of iron to soil colloids than for citrate-treated IOBKs would be expected mainly due to the large surface area of IONPs. Further studies should be undertaken to investigate the relationship of IONPs with different sizes on the pH of the rhizoplane soils and iron uptake, and their effects on the agronomic traits of rice plants.
Conclusion
This study evaluated the phytotoxicity potential of γ-Fe2O3 nanoparticles (IONPs) in terms of root elongation and the physiological performance of rice plants. Enhanced root elongation of rice plants exposed to the γ-Fe2O3 nanoparticles was observed, whereas similar results were not apparent for their bulk counterpart (IOBKs) and control groups. Gas exchange monitoring revealed that the CO2 assimilation rate in IOBK and IONP-treated pots decreased with time and the decline was less pronounced for IONPs. Amendment of IONPs coated with 20 mmol citric acid significantly diminished the CO2 assimilation rate and the decrease was almost similar to their bulk counterpart. However, maximum shoot growth inhibition was associated with the application of citric acid coated-IOBKs. It is concluded that the massive Fe plaques, which accumulated on the root surfaces of IOBKs-Cit treated rice plant compared to IONPs-Cit treated plants, were responsible for the remarkable reduction in shoot growth.
References
Alidoust D, Isoda A (2013), Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol Plant. doi: 10.1007/s11738-013-1369-8
Alidoust D, Suzuki S, Matsumura S, Yoshida M (2012) Chemical speciation of heavy metals in the fractionated rhizosphere soils of sunflower cultivated in a humic Andosol. Commun Soil Sci Plant Anal 43(17):2314–2322
Arvizo RR, De M, Rotello VM (2007) Proteins and nanoparticles: covalent and noncovalent conjugates. In: Mirkin CA, Niemeyer CM (eds) Nanobiotechnology II: more concepts and applications. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Barrena E, Casals E, Colon J, Fon Xt, Sanchez A, Puntes V (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75:850–857
Becker M, Asch F (2005) Iron toxicity in rice-conditions and management concepts. J Plant Nutr Soil Sci 168:558–573
Bee A, Massart R, Neveu S (1995) Synthesis of very fine maghemite particles. J Magn Magn Mater 149:6–9
Bulte JW, Kraitchman DL (2004) Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 17(7):484–499
Colmer TD, Voesenek L (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Dehner CA, Barton L, Maurice PA, Dubois JL (2010) Size-dependent bioavailability of hematite (α-Fe2O3) nanoparticles to a common aerobic bacterium. Environ Sci Technol 45:977–983
Fauconnier N, Roger J, Pons JN (1996) Adsorption of gluconic and citric acids on maghemite particles in aqueous medium. Prog Colloid Polym Sci 100:212–216
Fox TR, Comerford NB, McFee WW (1990) Phosphorus and aluminium from a spodic horizon mediated by organic acids. Soil Sci Soc Am J 54:1763–1767
Ghodak G, Seo YD, Lee DS (2011) Hazardous phytotoxic nature of cobalt and zinc oxide nanoparticles assessed using Allium cepa. J Hazard Mater 186:952–955
Gobran GR, Clegg S (1996) A conceptual model for nutrient availability in the mineral soil-root system. Can J Soil Sci 76:125–131
He S, Feng Y, Ren H, Zhang Y, Gu N, Lin X (2011) The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J Soils Sed 1:1–10
Hinsinger P (2001) Trace elements in the rhizosphere. Bioavailability of trace elements as related to root-induced chemical changes in the rhizosphere, chapter 2. CRC Press LCC, Boca Raton, pp 25–41
Hoffmann WF, Barber SA (1971) Phosphorus uptake by wheat (Triticum aestivum) as influenced by ion accumulation in the rhizocylinder. Soil Sci 112:256–262
Jackson MB, Armstrong W (2009) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1:274–287
Jun YW, Lee JH, Cheon J (2007) Nanoparticle contrast agents for molecular magnetic resonance imaging. In: Mirkin CA, Niemeyer MC (eds) Nanobiotechnology II: more concepts and applications. Wiley-VCH, Weinheim
Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ASC Nano 3–10:3221–3227
Kotsmar C, Yoon KY, Yu H, Ryoo SY, Barth J, Shao S, Prodanovic M, Milner TE, Bryant SL, Huh C, Johnston KP (2010) Stable citrate-coated iron oxide superparamagnetic nanoclusters at high salinity. Ind Eng Chem Res 49:12435–12443
Kraemer SM (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108(6):2064–2110
Lei Z, Fashui H, Shipeng L, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Marschner H, Treeby M, Römheld V (1989) Role of root-induced changes in the rhizosphere for iron acquisition in higher plants. Z Pflanzenernähr Bodenkd 152:197–204
Nowack B, Bucheli TD (2007) Occurence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
Pereira EG, Olivaa MA, Rosado-Souzaa L, Mendesa GC, Colaresb DS, Stopatoa CH, Almeida AM (2013) Iron excess affects rice photosynthesis through stomatal and non-stomatal limitations. Plant Sci 201–202:81–92
Seeger EM, Baun A, Kästner M, Trapp S (2009) Insignificant acute toxicity of TiO2 nanoparticles to willow trees. J Soils Sediment 9:46–53
Sellers K, Mackay C, Bergeson LL, Clough SR, Hoyt M, Chen J, Henry K, Hamblen J (2009) Nanotechnology and the environment. CRC Press, Boca Raton
Trolard F, Tardy Y (1987) The stabilities of gibbsite, boehmite, aluminous goethite and aluminous hematites in bauxites, ferricretes and laterites as a function of water activity, temperature and particle size. Geochim Cosmochim Acta 51:945–957
US Environmental Protection Agency (1996) Ecological effects test guidelines (OPPTS 580.4200), seed germination/root elongation toxicity test
Wang M, Chen L, Chen S, Ma Y (2012) Alleviation of cadmium-induced root growth inhibition in crop seedlings by nanoparticles. Ecotoxicol Environ Safe 79:18–51
Zhang Y, Kallay N, Matijevic E (1985) Interactions of metal hydrous oxides with chelating agents. VII. Hematite-oxalic and citric acid systems. Langmuir 1:201–206
Acknowledgments
The authors wish to thank Masanori Hanata and Yosuke Miyauchi for their contribution to the laboratory works as well as for their technical supports. Special thanks go to Dr. Masayuki Kawahigashi from Tokyo Metropolitan University for his kind supports in determination of iron using ICP-AES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alidoust, D., Isoda, A. Phytotoxicity assessment of γ-Fe2O3 nanoparticles on root elongation and growth of rice plant. Environ Earth Sci 71, 5173–5182 (2014). https://doi.org/10.1007/s12665-013-2920-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2920-z