Abstract
Waterlogging is one of the major stresses limiting crop production worldwide. The understanding of the mechanisms of plant adaptations to waterlogging stress helps improve plant tolerance to stress. In this study, physiological responses and morpho-anatomical adaptations of seven different barley genotypes were investigated under waterlogging stress. The results showed that the waterlogging-tolerant varieties (TX9425, Yerong, TF58) showed less reduction in plant height, SPAD (soil–plant analyses development analyses) value, tillers, shoot and root biomasses than did the waterlogging-sensitive varieties (Franklin, Naso Nijo, TF57). Under waterlogging stress condition, the tolerant genotypes also showed a much larger number of adventitious roots than did the sensitive genotypes. More intercellular spaces and better integrated chloroplast membrane structures were observed in the leaves of the waterlogging-tolerant cultivars, which is likely due to increased ethylene content, decreased ABA content and less accumulation of O2.−. The ability to form new adventitious roots and intercellular spaces in shoots can also be used as selection criteria in breeding barley for waterlogging tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waterlogging, one of the major abiotic stresses affecting crop growth, occurs more frequently, severely, and unpredictably due to global climate change (Jackson and Colmer 2005). Approximately 10% of land area on earth is affected by waterlogging (Setter and Waters 2003). Excessive water decreases oxygen content in soil, thus affecting plant abilities to absorb nutrients (Jackson and Colmer 2005; Zhou et al. 2007). Barley is relatively more sensitive than other cereal crops to waterlogging, which causes a 40–79% irreversible yield loss, depending on the genotype, growth stage and duration of waterlogging (Romina et al. 2014).
One important mechanism in the waterlogging condition is the formation of adventitious roots (ARs), a phenomenon which widely exists in different plant species such as wheat (Malik et al. 2003), Zea nicaraguensis (Mano and Omori 2013), soybean (Kim et al. 2015), tomato (Vidoz et al. 2010) and cucumber (Xu et al. 2017). New adventitious roots facilitate water and nutrient uptake under hypoxia stress (Wei et al. 2013; Sauter 2013). The timing of waterlogging-induced adventitious root emergence is species-specific, depending on developmental stage of the plant, the water temperature and the waterlogging duration (Dawood et al. 2014; Argus et al. 2015). In cucumber, waterlogging-tolerant genotypes were able to form a larger number of ARs whereas almost no ARs were generated in waterlogging-sensitive genotypes (Xu et al. 2017). Major quantitative trait loci (QTL) for waterlogging-triggered adventitious roots have been reported in maize (Mano et al. 2009) and cucumber (Xu et al. 2017).
The formation of aerenchyma is another feature of plant tolerance to waterlogging (Setter and Waters 2003). Aerenchyma consists of intercellular spaces, which capture oxygen and facilitate gas exchange in different plant tissues (Colmer 2003). Under hypoxia, aerenchyma is induced in most cereals, such as maize (Rajhi et al. 2011), wheat (Yamauchi et al. 2014) and soybean (Shimamura et al. 2014). Under waterlogging treatment, waterlogging-tolerant barley genotypes have significantly higher root porosity and a faster aerenchyma development in adventitious roots (Zhang et al. 2015a). In wetland plants, such as rice, aerenchyma forms not only in roots but also in leaf sheaths, leaves and stems (Matsukura et al. 2000).
Plants exposed to waterlogging stress inevitably undergo the production of reactive oxygen species (ROS), which rapidly attack leaf chloroplasts and ultimately lead to leaf chlorosis and senescence (Yordanova et al. 2004). The mesophyll cell is a fundamental component of photosynthesis, playing an important role in photosynthetic capacity (Xu et al. 2006). Chloroplast ultrastructure and thylakoid are disrupted under adverse circumstances in turf grass and Anoectochilus roxburghii, resulting in significant reductions in chlorophyll content and photosynthetic capacity (Xu et al. 2006; Shao et al. 2014). On the other hand, ROS could act as signaling molecules in plant adaptive responses to waterlogging. It has been identified that interactions of plant hormones and ROS could induce lysigenous aerenchyma formation and adventitious root growth in rice (Sauter 2013), maize (Rajhi et al. 2011), and wheat (Yamauchi et al. 2014). Ethylene is believed to be a primary signal that activates physiological and biochemical adaptations in deep-water rice (Visser and Voesenek 2004). Abscisic acid (ABA) is related to the formation of aerenchyma in soybean hypocotyls when flooded (Shimamura et al. 2014), and it could regulate stomatal closure in leaf tissue, further affecting the photosynthesis system (Kim et al. 2015).
In previous work, it was identified that genetic variations for waterlogging tolerance are apparent in barley (Zhou et al. 2007), and there was a positive correlation between aerenchyma in adventitious roots and waterlogging tolerance (Broughton et al. 2015; Zhang et al. 2015b). To further understand the adaptations of barley to waterlogging, we investigated the differences in physiological responses, ultrastructure changes (particularly, above-ground tissues) and molecular mechanisms among the seven barley genotypes differing in waterlogging tolerance.
Materials and methods
Plant materials and treatments
Five barley (Hordeum vulgare L.) varieties (TX9425, Franklin, Yerong, YYXT, Naso Nijo) and two breeding lines (TF57 and TF58) derived from a double-haploid (DH) population (TX9425/Franklin) were used for morpho-anatomical, physiological and molecular analyses in the present study. The DH population (TX9425/Franklin), consisting of 75 lines, was selected to identify the correlation between waterlogging tolerance and adventitious root parameters. The seeds of all the lines were sown in plastic pots (22 cm in height and 25 cm in diameter) filled with a mixture of peat (Pindstrup Mosebrug A/S, Denmark) and vermiculite in a 3:1 ration. The plants were grown in a greenhouse with a temperature of 22 ± 2 °C/day and 18 ± 2 °C/night. The water in tanks was maintained at 2–3 cm above soil surface through water valve to control water flow during waterlogging period. The control plants remained well-watered (60–70% soil moisture content, as measured by weighing). The waterlogging tolerance ability was scored based on leaf chlorosis and plant survival on a scale of 0 (all dead) to 10 (no damage). A score of 0–5 indicated sensitivity to waterlogging, while a score of 6–10 indicated tolerance to waterlogging (Fig. S1) (Zhou 2012). The experiment was carried out with three replications, and each replicate contained nine seedlings.
Measurement of morphological characteristics
Twenty-one days after the treatments, the shoots and roots were collected and carefully washed with distilled water for further analysis. The chlorophyll content on the youngest fully expanded leaf was measured just prior to final harvest with a SPAD-520 chlorophyll metre (Konica Minalta, Osaka, Japan). The shoots and roots were dried at 105 °C for 3 h, followed by drying at 70 °C for 3 days, and then the dry weights were recorded.
The roots were carefully rinsed with a hydropneumatic elutriation device (SOLO 417, Kleinmotoren, Sindelfingen, German) and detached from their nodal bases. The adventitious roots were arranged and floated on shallow water in a glass tray (30 cm × 30 cm), then scanned using an Epson Expression 1680 scanner (Seiko Epson Corp, Japan). The lengths, diameters, surface areas and root volumes were analysed using a WinRHIZO root analyzer system (WinRHIZO version 2007b, Regent Instruments Canada, Montreal, Canada) (Zhang et al. 2009). The parameters related to the adventitious roots were measured with three replications, and three plants were selected for each replicate.
Determination of superoxide radical (O2 ·−), ATP, ethylene and endogenous ABA content
Fresh leaves and roots (0.5 g each) were rinsed thoroughly with distilled water. The crude enzymatic extracts of each line were prepared in 0.05 M phosphate buffer (pH 7.8) after grinding with a pestle and milling to powder in liquid nitrogen. The homogenate was filtered through four layers of muslin cloth and centrifuged at 12000×g for 10 min at 4 °C. The final supernatants were used for physiological and biochemical assays.
O2·− was determined according to Jiang and Zhang (2002) with some modifications. Briefly, 1 ml supernatant was mixed with 0.9 ml 65 mM potassium phosphate buffer and 0.1 ml 10 mM hydroxylamine hydrochloride and then incubated at 25 °C for 24 h. The samples were further incubated at 25 °C for 20 min after adding sulfanilamide and α-naphthylamine. The same volume (2 ml) of butanol was then added to each sample before centrifugation at 1500×g for 5 min. The absorbances of the samples were measured with a spectrometer (Model 721, Shanghai Mapada Instruments, Shanghai, China) at 530 nm.
The contents of ATP, ethylene and ABA were determined according to the enzyme linked immunosorbent assay method (Crozier et al. 1986). Purified plant ATP, ABA and ethylene antibodies were used to coat microtiter plate wells, making solid-phase antibodies. Each well contained 40 µl phosphate buffer mixed with 10 µl sample solution, and was kept at 37 °C for 30 min. Next, 50 µl conjugate reagent with horse radish peroxidase (HRP) label was added to each well and washed completely. The plate was kept at 37 °C for a further 30 min and washed five times with washing buffer. The colour-developing agent TMB (3,3′,5,5′-tetramethylbenzidine) was then added to the well, and the samples were stored at 37 °C for 10 min. The reactions were terminated by the addition of a sulfuric acid solution, and the absorbances were measured at a wavelength of 450 nm. The concentrations of ATP, ABA and ethylene in the samples were determined by comparing the OD of the samples to the standard curve (Chen et al. 2017).
Tissue anatomy
Twenty-one days after the treatments, the first fully expanded leaf below the shoot apex and leaf sheath were collected and cut into 0.5 cm segments, then immediately immersed in 0.1 M glutaraldehyde-phosphate buffer fixative (pH 7.2) at 4 °C for at least 3 h. The samples were washed with 0.1 M phosphate buffer fixative (pH 7.2) three times and then fixed with 0.5% OsO4 for 3 h. Subsequently, the samples were dehydrated in a graded ethanol series of 20, 40, 60, 80, 90, 95, and 100% (15 min each). Ethanol was replaced with propyleneoxide, and the tissues were infiltrated and embedded in SPI low-viscosity Spurr’s resin (Zheng and Wang 2011). Sections of 1 µm thickness were cut with a glass knife on a Leica RM 2255 microtome (Leica, Bensheim, Germany), stained with 0.5% methyl violet for 10 min, and photographed under an Olympus BX41 (Olympus, Hamburg, Germany). For leaf mesophyll cell ultrastructure measurement, the samples were cut into 60 nm slices. After stained with uranyl acetate and lead citrate, the samples were observed with the transmission electron microscope (JEM-1230 EX, JEOL, Tokyo, Japan).
Quantitative real-time PCR
Total RNA of leaf and root were isolated using a standard Trizol reagent (Invitrogen, Grand Island, NY, USA). cDNA was generated from the RNA with M-MLV reverse transcriptase (Takara, Tokyo, Japan). Three genes xyloglucan endotransglycosylase (XET), respiratory burst oxidases (RBOHD) and pyruvate decarboxylase (PDC) related to waterlogging were assessed by quantitative real-time PCR (qRT-PCR) (Rajhi et al. 2011; Wang et al. 2016; Zhang et al. 2015b). Specific primers were designed according to the homologous gene sequences using the Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA). The primers were listed in Supplementary Table S1. The amplification of actin was used as an internal control to normalize all data. Reaction was carried out in 20 µl containing 10 mM Tris–HCl (pH 8.5), 50 mM KCl, 2 mM MgCl2, 0.4 µl DMSO, 200 mM dNTPs, 10 pmol/µl specific PCR primers, 1 U Taq DNA polymerase, 0.5 µl SYBR GREEN Ifluorescence dye. qRT-PCR was performed in clear tubes using an Applied Biosystems ViiATM7 Real-Time PCR System (Lifetech, Thermot-Fisher, Waltham, USA): 94 °C for 5 min, followed by 40 cycles at 94 °C for 30 s, 58 °C for 30 s, 72 °C for 30 s, and a final extension of 72 °C for 5 min. Ct values were determined by the Applied Biosystems ViiA™ 7 software with default settings. Differences between the Ct values of target gene and Actin were calculated as ∆Ct = Ct target gene − Ct Actin , and the relative expression levels of target genes were determined as 2−∆Ct. For each sample, PCR was performed with three replicates. The average values of 2−∆Ct were used to determine difference in gene expression.
Statistical analysis
The data analyses were performed with SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Two-factorial (genotypes and waterlogging treatment) ANOVAs were used to examine the differences in the plant growth parameters among the different genotypes, under control and waterlogging treatments. Student’s t test was used to assess the statistical significance of the treatment differences. Correlations between waterlogging tolerance and plant traits in 75 lines of the DH population were calculated via Spearman’s rank correlation coefficient using Microsoft Excel. Comparisons with P values less than 0.05 were considered significantly different.
Results
Plant growth and adventitious roots formation
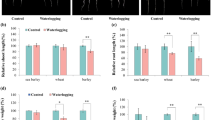
As shown in Fig. 1, seven barley genotypes, except for TF58, used in this study showed significant differential growth between the control and waterlogging treatments. The plant height, SPAD value, tiller development in Naso Nijo and TF57 all showed dramatic reductions under waterlogging, while those characteristics were less changed in TX9425 and TF58. In comparison with the control, waterlogging caused significant reductions in the shoot dry weight in Naso Nijo, YYXT and TF57. For the root dry weight, seven barley genotypes, except for TF58, showed a marked decrease, with a greater effect in Franklin, Naso Nijo and TF57. The morphological parameters of the adventitious roots of the seven barley genotypes under the two treatments are shown in Fig. 2. In comparison with control, all genotypes except Franklin had better total AR length, surface area, diameter, volume and number. The AR number of TX9425, Yerong and TF58 increased 3.0-, 3.8- and 3.4-fold, respectively, compared with the control, while no significant differences were observed in Franklin. In the DH population, there were also significant differences in plant growth and adventitious root formation among barley genotypes under different treatments, and the interactions between genotypes and waterlogging treatment were also significant (P < 0.01) (Table S2). Table S3 shows the correlations between waterlogging tolerance score and all the other traits in the DH population under waterlogging stress. The waterlogging tolerance was significantly correlated with the SPAD value, shoot dry weight and root dry weight. Significant correlations between waterlogging tolerance and AR parameters were also observed among 75 DH lines (P < 0.01) (Table S3).
Difference in anatomical structure among the barley genotypes
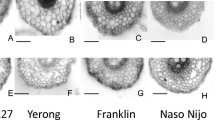
The barley leaf anatomy was of a typical monocotyledonous type, consisting of epidermal cells, mesophyll cells and vascular tissue (Fig. S2). Upper and lower epidermal cells lined up tightly and constituted a single layer. The mesophyll showed irregular shapes and contained many chloroplasts. The mesophyll is the main location for photosynthesis. The control and the tolerant genotypes TX9425, Yerong, YYXT and TF58 had more inter cellular spaces among mesophyll cells than did the other, sensitive genotypes (Fig. S2). Under waterlogging conditions, larger areas of aerenchyma were detected in the tolerant genotypes compared with the control, and there were no significant changes in aerenchyma of the sensitive genotypes.
Leaf sheath of barley is composed of upper and lower epidermis tissues, two layers of mesophyll cells and vascular tissue (Fig. S3). In the control, large aerenchyma could be found in the middle of mesophyll cells, and under waterlogging stress, aerenchyma was more significant due to the degradation of lower mesophyll cells. There were no significant differences in leaf sheath anatomical structure among genotypes under either the control or waterlogging conditions.
The chloroplast ultrastructure of mesophyll cells in the seven barley genotypes were investigated after waterlogging treatment (Fig. 3). Under normal conditions, no obvious differences were found in chloroplasts (CH)/thylakoid (Th) of the mesophyll cells among seven genotypes. However, under waterlogging stress, an increase in osmiophilic plastoglobuli was observed in all the genotypes. There were no significant differences in the ultrastructure of the chloroplasts of TX9425, Yerong and TF58 lines compared with the control, while the chloroplasts of YYXT, Franklin and Naso Nijo became round and partially damaged, with blurred grana lamellae. Furthermore, waterlogging stress induced deterioration of the chloroplast ultrastructure was more severe in the sensitive genotype TF57, with the chloroplast membrane becoming disrupted (Fig. 3).
Superoxide radical (O2 ·−) and ATP content
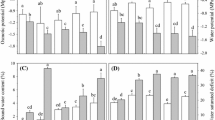
As shown in Fig. 4, after waterlogging treatment for 21 days, the contents of O2·− in the leaves of TX9425, Yerong, YYXT, Franklin, Naso Nijo and TF57 were 9.2, 7.9, 27.8, 28.25, 27.3 and 20.4%, respectively, which were higher than in the control, while no significant difference was found in TF58 (Fig. 4a). In the roots, waterlogging caused an increase in O2·− contents in TX9425, Yerong and TF58 (22.0, 4.7 and 19.6%, respectively) but a decrease in Franklin, Naso Nijo and TF57 (15.0, 24.1 and 17.2%, respectively). No significant differences between the waterlogged and control treatments were found for O2·− contents in YYXT (Fig. 4b). In the leaves of waterlogged plants, the ATP contents in YYXT, Franklin, Naso Nijo and TF57 were 13.8, 27.6, 9.8 and 25.3%, respectively, which were lower than in the control, while no significant differences in the ATP contents were found in TX9425, Yerong and TF58 (Fig. 4c). In the roots, compared to the control, the ATP contents were 41.0, 21.8, 26.4 and 34.0% lower in Franklin, Naso Nijo, YYXT and TF57, respectively, and 19.4% higher in Yerong. No significant differences between the waterlogged and control treatments were observed in TX9425 or TF58 (Fig. 4d).
Ethylene and ABA content
The ethylene and ABA contents in the leaves and roots were measured after 3 weeks of waterlogging treatment (Fig. 5). The ethylene contents increased in all genotypes under waterlogging stress. The tolerant genotypes had more ethylene accumulations than did the sensitive ones. Under waterlogging conditions, the ethylene content in the leaves increased by 26.2, 31.3 and 33.3% in TX9425, Yerong and TF58, respectively, while no significant changes were found in Franklin and TF57 (Fig. 5a). A similar trend was found for the ethylene content in the roots (Fig. 5b). In contrast, the ABA contents were significantly decreased in all genotypes under waterlogging stress in both leaves (Fig. 5c) and roots (Fig. 5d), with the tolerant genotypes showing greater decreases compared with the control.
Molecular response to waterlogging stress
As shown in Fig. S4A and as compared with the control, waterlogging treatment caused a decrease in the transcript levels of XET expression in the leaves for all the genotypes. In contrast, the expression levels of XET in roots increased significantly in waterlogged plants of all genotypes, except for YYXT and Franklin (Fig. S4B). The expression levels of RBOHD significantly increased in the leaves of TX9425, Naso Nijo, Yerong and TF58 but were significantly reduced in the other genotypes (Fig. S4C). TX9425, Yerong, and TF58 also showed dramatic increases in RBOHD expression in the roots, while TF57 showed a significant decrease in RBOHD expression (Fig. S4D). The transcription levels of PDC were significantly up-regulated in Yerong but significantly down-regulated in Naso Nijo (Fig. S4E). In roots, the expression of PDC was significantly up-regulated in all genotypes, with that in the tolerant genotypes TX9425, Yerong and TF58 being more pronounced (Fig. S4F).
Discussion
More adventitious roots in waterlogging-tolerant genotypes
ARs on the soil surface could absorb oxygen directly from the air, facilitate nutrient uptake during hypoxia stress, and play important roles in adaptation to waterlogging stress (Colmer and Voesenek 2009). The linkage between ARs and waterlogging tolerance has been stated in maize (Mano et al. 2009), cucumber (Xu et al. 2017). In this experiment, the number of ARs in the waterlogging-tolerant genotypes showed a significant increase after 21 days of waterlogging stress. In contrast, two sensitive genotypes, Franklin and TF57, showed only slight increases (Fig. 2). Therefore, AR formation could be a key mechanism in tolerant cultivars under waterlogging stress, the idea confirmed in a DH population with a correlation of r = 0.44 (P < 0.01) between ARs number and waterlogging tolerance (Table S3). It is suggested that ARs formation may be used as the criterion to identify waterlogging tolerance in barley.
Anatomical responses to waterlogging in barley shoots
Aerenchyma formation in ARs has been suggested as a reliable method for screening waterlogging tolerance in maize and barley (Yamauchi et al. 2014; Mano and Omori 2008; Broughton et al. 2015; Zhang et al. 2016). The formation of aerenchyma in shoots has also been reported as a main anatomical characteristic that is observed in aquatic and wetland plants under flooding (Jung et al. 2008). In the present study, the waterlogging-tolerant barley cultivars TX9425, Yerong, TF58 and YYXT showed more intercellular spaces in the leaves than in the waterlogging-sensitive cultivars under both well-drained and waterlogged conditions (Fig. S2). Well-developed systems of intercellular spaces in the mesophyll increase photosynthesis, because rapid gas exchange is facilitated (Colmer and Pedersen 2008). Leaf sheath aerenchyma was developed in the middle parenchymal cells in the control, which was enhanced under waterlogging conditions (Fig. S3). This phenomenon is consistent with rice leaf sheath aerenchyma formation, which is usually located between vascular bundles (Matsukura et al. 2000; Parlanti et al. 2011). The formation of leaf sheath aerenchyma could be related to the time of waterlogging treatment; hence, it is necessary to try time-gradient waterlogging treatments in future research.
Chloroplasts are easily damaged when plants are exposed to abiotic stresses, such as salinity (Arias-Moreno et al. 2017), drought (Chaves et al. 2009) and waterlogging (Ren et al. 2016). In this research, the chloroplasts of the sensitive genotypes showed significantly more damage, as membranes were deliquescent and grana lamellae were blurred (Fig. 3). The destruction of the chloroplasts could be caused by the accumulation of O2·−, further leading to declines in chlorophyll and ATP contents (Figs. 1, 4). Thus, waterlogging-stress induced leaf senescence might be caused by reduced stability of the membrane system structures of chloroplasts. Similarly, it has also been observed that, under waterlogging conditions, impairments in the mesophyll cell ultrastructure result in a decrease in leaf photosynthetic capacity and a yield reduction in wheat (Zheng et al. 2009) and summer maize (Ren et al. 2016).
A possible mechanism of barley adjustment to waterlogging stress
It is well known that plant hormones and ROS play important roles in responses to various abiotic stresses (Steffens 2014; Wang et al. 2017; Zhang et al. 2015a). In the present work, ethylene content significantly increased in the leaves and roots of the waterlogging tolerant genotypes (TX9425, Yerong, TF58) compared to the control, while ABA significantly decreased (Fig. 5). Ethylene accumulation induces ROS, which facilitates ARs primordial emergence and lysigenous aerenchyma formation (Steffens and Rasmussen 2016; Sauter 2013). ABA negatively regulates ARs emergence in an antagonistic interaction (Benschop et al. 2005). In rice, the interaction between ethylene and ROS has been confirmed as a major signal that mediates salinity, chromium, and flooding stress responses (Steffens et al. 2011). Therefore, our results suggest that interactions among ethylene, ABA and ROS are integrated to promote the formations of ARs and aerenchyma under waterlogging stress.
In our study, aerenchyma formation- and energy metabolism-related genes (XET, RBOHD, PDC) showed significant up-regulation in the roots under waterlogging stress, and the tolerant genotypes showed higher expressions than did the sensitive genotypes (Fig. S4). It has been reported that the expression of these genes plays a crucial role in adaptations to waterlogging treatment (Rajhi et al. 2011; Wang et al. 2016; Zhang et al. 2015b). Based on the present research, a potential model of barley waterlogging tolerance is proposed (Fig. 6). Under waterlogging conditions, the expression of 1-amino cyclopropane 1-carboxylic acid oxidase (ACO) was strongly up-regulated in the roots (unpublished data), leading to a significantly increased ethylene accumulation. Ethylene diffuses from roots to leaves via the intercellular spaces in the leaf sheath and induces up-regulation of RBOHD, which triggers the generation of ROS. The coordination of ethylene and ROS induces epidermal and cortical programmed cell death. The up-regulation of XET leads to the formation of ARs and aerenchyma. Ethylene and ROS also enhance the expression of genes involved in ethanol fermentation pathways, such as PDC, under waterlogging stress, resulting in more ATP production.
In conclusion, waterlogging-tolerant genotypes had a significantly more ARs and greater proportion of aerenchyma in the shoots than did the sensitive genotypes. The mutual coordination between the shoots (sources of oxygen and carbon) and roots (sources of water and mineral nutrients) is an important mechanism of barley in response to waterlogging. The formations of ARs and aerenchyma in shoots could be due to the interactions of ethylene, ABA and ROS as well as the expression of corresponding genes. Thus, our results facilitate a comprehensive understanding of the mechanism of waterlogging tolerance in barley and the breeding of barley varieties for waterlogging tolerance.
References
Argus RE, Colmer TD, Grierson PF (2015) Early physiological flood tolerance is followed by slow post-flooding root recovery in the dryland riparian tree Eucalyptus camaldulensis subsp. refulgens. Plant Cell Environ 38:1189–1199
Arias-Moreno DM, Jiménez-Bremont JF, Maruri-López I, Delgado-Sánchez P (2017) Effects of catalase on chloroplast arrangement in Opuntia streptacantha chlorenchyma cells under salt stress. Sci Rep 7:8656
Benschop JJ, Jackson MB, Gühl K, Vreeburg RA, Croker SJ, Peeters AJ, Voesenek LA (2005) Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J 44:756–768
Broughton S, Zhou GF, Teakle NL, Matsuda R, Zhou MX, O’Leary RA, Colmer T, Li CD (2015) Waterlogging tolerance is associated with root porosity in barley. Mol Breeding 35:27
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen XM, Qiu LL, Guo HP, Wang Y, Yuan HW, Yan DL, Zheng BS (2017) Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz J Bot 40:841–851
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Pedersen O (2008) Oxygen dynamics in submerged rice (Oryza sativa). New Phytol 178:326–334
Colmer TD, Voesenek LACJ. (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Crozier A, Sandberg G, Monteiro AM, Sundberg B (1986) The use of immunological techniques in plant hormone analysis. In: Bopp M (ed) Plant growth substances 1985. Springer, Berlin, pp 13–21
Dawood T, Rieu I, Wolters-Arts M, Derksen EB, Mariani C, Visser EJ (2014) Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants 6:490–552
Jackson MB, Colmer TD (2005) Response and adaptation by plants to flooding stress. Ann Bot 96:501–505
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410
Jung J, Lee SC, Choi HK (2008) Anatomical patterns of aerenchyma in aquatic and wetland plants. J Plant Biol 51:428–439
Kim YH, Hwang SJ, Waqas M, Khan AL, Lee JH, Lee JD, Nguyen T, Lee H IJ (2015) Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front Plant Sci 6:714
Malik AI, Colmer TD, Lambers H, Schortemeyer M (2003) Aerenchyma formation and radial O2 loss along adventitious roots of wheat with only the apical root portion exposed to O2 deficiency. Plant Cell Environ 26:1713–1722
Mano Y, Omori F (2008) Verification of QTL controlling root aerenchyma formation in a maize × teosinte “Zea nicaraguensis” advanced backcross population. Breed Sci 58:217–223
Mano Y, Omori F (2013) Relationship between constitutive root aerenchyma formation and flooding tolerance in Zea nicaraguensis. Plant Soil 370:447–460
Mano Y, Omori F, Loaisiga CH, Bird RMK (2009) QTL mapping of above-ground adventitious roots during flooding in maize × teosinte “Zea nicaraguensis” backcross population. Plant Root 3:3–9
Matsukura C, Kawai M, Toyofuku K, Barrero RA, Uchimiya H, Yamaguchi J (2000) Transverse vein differentiation associated with gas space formation-fate of the middle cell layer in leaf sheath development of rice. Ann Bot 85:19–27
Parlanti S, Kudahettige NP, Lombardi L, Mensuali-Sodi A, Alpi A, Perata P, Pucciariello C (2011) Distinct mechanisms for aerenchyma formation in leaf sheaths of rice genotypes displaying a quiescence or escape strategy for flooding tolerance. Ann Bot 107:1335–1343
Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, Nakazono M (2011) Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 190:351–368
Ren BZ, Zhang JW, Dong ST, Liu P, Zhao B (2016) Effects of waterlogging on leaf mesophyll cell ultrastructure and photosynthetic characteristics of summer maize. PLoS ONE 11:e0161424
Romina P, Abeledo LG, Miralles DJ (2014) Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 378:265–277
Sauter M (2013) Root responses to flooding. Curr Opin Plant Biol 16:282–286
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253:1–34
Shao QS, Wang HZ, Gou HP, Zhou AC, Huang YQ, Sun YL, Li MY (2014) Effects of shade treatment on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS ONE 9:1–10
Shimamura S, Yoshioka T, Yamamoto R, Hiraga S, Nakamura T, Shimada S, Komatsu S (2014) Role of abscisic acid in flood-induced secondary aerenchyma formation in soybean (Glycine max) hypocotyls. Plant Prod Sci 17:131–137
Steffens B (2014) The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front Plant Sci 5:685
Steffens B, Rasmussen A (2016) The physiology of adventitious roots. Plant Physiol 170:603–617
Steffens B, Geske T, Sauter M (2011) Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol 190:369–378
Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P (2010) Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J 63:551–562
Visser EJW, Voesenek LACJ (2004) Acclimation to soil flooding-sensing and signal-transduction. Plant Soil 254:197–214
Wang LH, Li DH, Zhang YX, Yuan G, Yu JY, Sauter X, Zhang XR (2016) Tolerant and susceptible sesame genotypes reveal waterlogging stress response patterns. PLoS ONE 11:e0149912
Wang RF, Dai HX, Shi M, Ahmed IM, Liu WX, Chen ZH, Zhang GP, Wu FB (2017) Genotype-dependent effects of phosphorus supply on physiological and biochemical responses to Al-stress in cultivated and Tibetan wild barley. Plant Growth Regul 82:259–270
Wei WL, Li DH, Wang LH, Wang LH, Ding X, Zhang YX, Gao Y, Zhang XR (2013) Morpho-anatomical and physiological responses to waterlogging of sesame (Sesamum indicum L.). Plant Sci 208:102–111
Xu S, Li JL, Zhang XQ, Wei H, Cui LJ (2006) Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 56:274–285
Xu XW, Ji J, Xu Q, Q XH, Chen XH (2017) Inheritance and quantitative trail loci mapping of adventitious root numbers in cucumber seedlings under waterlogging conditions. Mol Genet Genomics 292:353
Yamauchi T, Watanabe K, Fukazawa A, Mori H, Kawaquchi K, Oyanagi A, Nakazono M (2014) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65:261–273
Yordanova RY, Christov KN, Popova LP (2004) Antioxidative enzymes in barley plants subjected to soil flooding. Environ Exp Bot 51:93–101
Zhang H, Xue YG, Wang ZQ, Yang JC, Zhang JH (2009) Morphological and physiological traits of roots and their relationships with shoot growth in “super’’ rice. Field Crop Res 113:31–40
Zhang XC, Shabala S, Koutoulis A, Shabala L, Johnson P, Hayes D, Nichols DS, Zhou MX (2015a) Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant Soil 394:355–372
Zhang YJ, Song XZ, Yang GZ, Li ZH, Lu HQ, Kong XQ, Eneji AE, Dong HZ (2015b) Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field Crop Res 179:164–172
Zhang M, Jin ZQ, Zhao J, Zhang GP, Wu FB (2015c) Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regul 75:567–574
Zhang XC, Zhou GF, Shabala S, Koutoulis A, Shabala L, Johnson P, Li CD, Zhou MX (2016) Identification of aerenchyma formation related QTL in barley that can be effective in breeding for waterlogging tolerance. Theor Appl Genet 129:1167–1177
Zheng YK, Wang Z (2011) Contrast observation and investigation of wheat endosperm transfer cells and nucellar projection transfer cells. Plant Cell Rep 30:1281–1288
Zheng CF, Jiang D, Liu FL, Dai TB, Jing Q, Cao WX (2009) Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci 176:575–582
Zhou MX (2012) Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breed 130:203–208
Zhou MX, Li HB, Mendham NJ (2007) Combining ability of waterlogging tolerance in barley. Crop Sci 47:278–284
Acknowledgements
This research was funded by the National Natural Science Foundation of China (31571648), the National Barley and Highland Barley Industrial Technology Specially Constructive Foundation of China (CARS-05), the opening project of Jiangsu Key Laboratory of Biochemistry and Biotechnology of Marine Wetland (K2016-03), the funds institute of Agricultural Science in Jiangsu Coastal Areas (YHS201605), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Luan, H., Guo, B., Pan, Y. et al. Morpho-anatomical and physiological responses to waterlogging stress in different barley (Hordeum vulgare L.) genotypes. Plant Growth Regul 85, 399–409 (2018). https://doi.org/10.1007/s10725-018-0401-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-018-0401-9