Abstract
Background and aims
Plant adaptation to waterlogged conditions requires a set of morphological and physiological/biochemical changes. The formation of aerenchyma is one of the most crucial adaptive traits for waterlogging tolerance. Enzymatic scavenging may also potentially contribute to waterlogging tolerance by providing detoxification of reactive oxygen species (ROS).
Methods
Changes of root porosity (as an indicator of aerenchyma formation) and activities in leaves of four major antioxidant enzymes, γ-amino butyric acid (GABA) and lactic acid contents in roots were evaluated in six barley genotypes contrasting in waterlogging tolerance.
Results
Soil waterlogging caused significant increases in adventitious root porosity in all genotypes. Waterlogging-tolerant genotypes showed not only significantly higher adventitious root porosity than sensitive genotypes but also much faster development of aerenchyma. The greatest difference in adventitious root porosity among genotypes was observed after 7 days of waterlogging treatment. At the same time, antioxidant enzyme activities in leaves, GABA and lactic acid contents in roots did not correlate with waterlogging tolerance.
Conclusions
A faster formation of aerenchyma in adventitious roots is one of the key factors for waterlogging tolerance in barley. This protocol is recommended to be applied in future studies to identify molecular markers linked to this trait using appropriate mapping populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excess water and poor soil drainage constraints are estimated to adversely affect approximately 10 % of the global land area (Setter and Waters 2003). Dramatic floods occur in all continents and result in an estimated annual damage of crops exceeding 60 billion euros (www.dartmouth.edu/~floods/Archives/2005sum.htm). With the exception of rice, most crops are sensitive to waterlogging and show significant decline in yield when grown in flooded soils. Barley (Hordeum vulgare L.) is one of the most sensitive crops, with 20–25 % yield losses being reported under waterlogging conditions in the field (Setter et al. 1999; de San et al. 2014). One of the main factors influencing plant growth under waterlogging conditions is oxygen deprivation of the roots. Oxygen deprivation reduces ATP levels in plants, causing other physiological and biochemical problems (Bailey-Serres and Voesenek 2008; Colmer 2003b). Common symptoms of waterlogging injury include reduced shoot nitrogen content, leaf area, biomass, shoot growth, root growth, and chlorophyll content (Malik et al. 2001; Pang et al. 2004; Zhou et al. 1997).
While application of fertilisers to either soil or foliage (Pang et al. 2007b; Zhou et al. 1997) was shown to improve crop growth and yields under waterlogging conditions, development of waterlogging-tolerant genotypes is the most effective and economical approach to improve production under stress conditions. However, little progress has been made in breeding barley genotypes for waterlogging tolerance due to the low heritability and highly variable waterlogging conditions (Collaku and Harrison 2005; Zhou 2010). Field-based experiments rather than lab-based physiological traits were mostly used to screen waterlogging-tolerant genotypes by breeders (Khabaz-Saberi et al. 2005). Because of the complexity of waterlogging tolerance and variation in field conditions, it might not be effective to make direct selection for waterlogging tolerance in the field. Understanding the mechanisms of waterlogging tolerance makes it possible for plant breeders to target individual physiological traits and pyramid different tolerance-related traits to generate barley pre-breeding material with enhanced waterlogging tolerance. To achieve this, it is essential to identify physiological traits which are correlated with waterlogging tolerance.
Different mechanisms are involved in plant tolerance to waterlogging stress. High root porosity resulting from the formation of aerenchyma is effective in avoiding adverse effects caused by waterlogging in cereal crops (Setter and Waters 2003), as internal oxygen supply to roots is enhanced (Colmer 2003b). Root porosity, which is the percentage of gas volume per root volume, is widely used as an indicator of aerenchyma formation reviewed by Colmer (2003b). Aerenchyma provides an internal system of gas-filled spaces to improve the diffusion of oxygen (Armstrong 1979; Evans 2003). In waterlogged plants, oxygen supply in roots depends mainly on the oxygen transportation from shoots through aerenchyma (Armstrong 1979). The increased concentration of oxygen leads to root aerobic respiration, resulting in increased energy in roots (Drew et al. 1985). Lysigenous aerenchyma is normally induced under hypoxia conditions among many species, including barley (Barrett-Lennard 2003). The higher degree of aerenchyma formation is the main mechanism contributing to the better waterlogging tolerance in rice than other dryland cereals (Bailey-Serres and Voesenek 2008).
Plant responses to oxygen deprivation also involve the formation of reactive oxygen species (ROS) such as superoxide radical (O2 ·−), hydroxyl radical (OH·), hydroperoxyl radical (HO2 ·) and hydrogen peroxide (H2O2) (Bailey-Serres and Chang 2005; Blokhina et al. 2003), which are harmful to cellular metabolism (Shabala et al. 2014). At the same time, some ROS are used as signalling molecules in plant adaptive responses to the range of abiotic and biotic stresses (Baxter et al. 2014). Waterlogging is not an exception, and considerable evidence is accumulated that ROS production, by either a plasma membrane (PM) NAD(P)H oxidase and/or mitochondria, regulates plant adaptive responses to oxygen deprivation (Bailey-Serres and Chang 2005). To deal with oxidative stress, plants use different enzymatic and non-enzymatic mechanisms to scavenge overproduced ROS. Major antioxidant (AO) enzymes include superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidases (APX). SOD is controlling the dismutation of O2 ·− to H2O2, which is a stable molecule that can be either used for signalling purposes or be further scavenged to water by APX, POD or CAT. Non-enzymatic antioxidants include ascorbic acid (AA), glutathione and phenolic compounds (Blokhina et al. 2003). Due to above potentially harmful role of ROS hyperaccumulation in stressed tissues, plants with higher AO activities are generally considered to be more tolerant to a broad range of stresses, including waterlogging (Yordanova et al. 2004; Zhang et al. 2007).
Deprivation of O2 triggers cessation of the tricarboxylic acid cycle and oxidative phosphorylation, and the production of ATP is shifted from the mitochondrial electron transport chain to alcoholic fermentation (Lee et al. 2014). Even though energy yield from anaerobic fermentation is 18-fold lower than the yield from aerobic respiration (only 2 vs 36 mol of ATP per mole of glucose), this metabolic pathway supports glycolysis by re-oxidizing NADH to NAD+ and allows the production of ATP required for the survival of plant cells. The above alcoholic fermentation includes two components: ethanol fermentation (catalysed by pyruvate decarboxylase and alcohol dehydrogenase) and lactate fermentation (catalysed by lactate dehydrogenase) (Takahashi et al. 2014). Alcoholic fermentation is also accompanied by increased biosynthesis of alanine, γ-aminobutyric acid (GABA), succinate and malate (Gibbs and Greenway 2003; Bailey-Serres and Voesenek 2008; Kreuzwieser et al. 2009). Root carbohydrate reserves seem to be important for waterlogging tolerance since the starch in roots is rapidly used up to maintain the function of roots (Sauter 2013). The up-regulation of α-amylases in the leaf is able to break down starch to sugars to produce more ATP (Voesenek and Bailey-Serres 2015). In addition, carbohydrate transporters are triggered to transport carbohydrates from shoot to root to maintain the higher level of energy (Voesenek and Bailey-Serres 2015; Voesenek and Bailey-Serres 2013). Relatively waterlogging-tolerant oak species showed more effective carbohydrates transporting and higher concentration of carbohydrates in roots (Ferner et al. 2012).
Waterlogging also caused a remarkable increase in GABA accumulation (Kreuzwieser et al. 2009). The formation of GABA was mainly induced with increase of cytosolic H+ and Ca2+ concentration (Ratcliffe 1997). In general, GABA had a signalling role to induce a cascade of genes that improve plants’ adapt to waterlogging (Shabala et al. 2014).
Most of the physiological traits associated with waterlogging tolerance are not easy to assess by high-throughput methods, and hence, this limits the ability to utilise these assays/approaches by breeders. In order to effectively pyramid different tolerance-related traits to improve waterlogging tolerance in a breeding program, it is crucial to identify appropriate quantitative trait loci (QTL) for key traits (including aerenchyma and ROS detoxification) and, thus, appropriate molecular markers closely linked to these traits. For this purpose, efficient screening protocols to accurately phenotype these traits have to be developed. In this study, six barley genotypes differing in waterlogging tolerance were used to address differences in hypothesised key traits. We report that waterlogging-tolerant genotypes had significantly higher adventitious root porosity and developed aerenchyma much faster compared with intolerant (sensitive) genotypes. By contrast, antioxidant enzyme activities in leaves, GABA and lactic acid contents in roots did not show any clear correlation with waterlogging tolerance. It is suggested that quantifying root porosity after 7 days of waterlogging may be used as an assay to help identify molecular markers linked to aerenchyma development in barley and to fine map the specific loci conferring this important trait for waterlogging tolerance.
Materials and methods
Experiment 1: waterlogging tolerance, aerenchyma formation, adventitious root porosity and antioxidant enzyme activities of barley in waterlogged brown sodosol soil
Plant genotypes and waterlogging treatment
Six barley (Hordeum vulgare L.) genotypes were used in this experiment. This included five cultivated barley genotypes (Yerong, Franklin, YSM1, Naso Nijo and Gairdner), and one wild barley (TAM407227). Seeds were obtained from the Australian Winter Cereal Collection or China through a joint project with Chinese researchers on barley germplasm research. Waterlogging tolerance of the genotypes was evaluated in 50-L round bins filled with a brown sodosol soil as previously described (Zhou 2011). Texture, pH and electrical conductivity (EC) of brown sodosol soil at different depths are given in Table 1. Waterlogging treatment started at a three-leaf stage and lasted for 6 weeks. Each replication was repeated three times in the glasshouse from August to October, 2013. Waterlogging tolerance score was based on the plant survival and leaf chlorosis and quantified on a 0 (all dead) to 10 (no visual symptoms) scale after 9-week waterlogging treatment (see Zhou 2011 for details).
Plant growth measurements
After 7-day waterlogging treatment, one plant from each replication was sampled. The following parameters were measured: longest adventitious root length, adventitious root number, shoot dry weight and root dry weight.
Root porosity
Root porosity of all genotypes was measured at 0 (just before waterlogging treatment), 1, 3, 5, 7, 14, 21, 28, 35 and 42 days after waterlogging treatment. Measurement of root porosity was based on the buoyancy of the adventitious roots before and after vacuum infiltration (Raskin 1983), using equations modified by Thomson et al. (1990). Adventitious roots of plants were dug out from soils and carefully washed with water. Approximately 0.3 to 0.4 g (fresh weight) of each sample was used for measurements.
Aerenchyma formation
Adventitious roots were sampled from TAM407227, Yerong, Franklin and Naso Nijo at day 0 (before waterlogging treatment) and at day 7 (7 days after waterlogging treatment). About 2-cm-long root segments were taken from the mature zone, approximately 6 cm from the root apex. Cross sections were cut by free-hand with razor blades (Pang et al. 2004) and observed under a bright field light microscope (Olympus BX41). Based on digital images (Olympus DP20), root aerenchyma area and total root cross-sectional area were measured using the public domain UTHSCSA ImageTOOL program (http://compdent.uthscsa.edu/).
Antioxidant enzyme activities
Antioxidant enzyme activities were measured at 7 and 14 days after waterlogging treatment. Fresh fully expanded green leaves (0.5 g) were sampled and homogenised using a mortar and pestle under chilling conditions with 5 mL of 50 mM phosphate buffer, pH 7.8, containing 0.1 mM EDTA and 2 % PVP. The homogenates were centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatants were used for enzyme assays with a spectrophotometer (Genesys10S UV-VIS). Protein concentrations in the extracts were measured at 595 nm (Bradford 1976).
SOD activity was measured with the photochemical nitroblue tetrazolium (NBT) method (Beyer and Fridovich 1987). The 3-mL reaction mixture contained 2 mL solution A (0.05 M pH 7.8 phosphate buffer with 112.5 μM NBT, 19.5 mM methionine, 0.15 mM EDTA), 0.95 mL solution B (0.05 M pH 7.8 phosphate buffer with 60 μM riboflavin) and 0.05 mL enzyme sample solution (tissue extract). The absorbance was recorded at 560 nm after 10-min reaction in a light incubator. One unit of SOD was defined as the amount of enzyme that inhibited 50 % of NBT photo reduction (U mg−1 protein).
CAT activity was assayed by the decrease of absorbance at 240 nm resulting from the decomposition of H2O2 (Aebi and Packer 1984). The 3.1-mL reaction mixture contained 1.5 mL of 0.2 M phosphate buffer (pH 7.8) including 1 % PVP, 1 mL H2O, 0.4 mL 0.1 M H2O2 and 0.2 mL of the tissue extract. One unit of CAT was defined as 0.01 decrease of absorbance at 240 nm per milligram protein per minute (U mg−1 protein min−1).
APX activity was measured by the reduction of absorbance at 290 nm as a consequence of ascorbic acid oxidation induced by enzymes (Nakano and Asada 1981). The 3-mL reaction mixture contained 2.5 mL of 0.05 M phosphate buffer (pH 7.0) including 0.1 mM EDTA, 0.2 mL 5 mM ascorbic acid, 0.2 mL 0.01 M H2O2 and 0.1 mL of the tissue extract. One unit of APX was defined as the amount of enzyme that oxidised ascorbic acid per milligram of protein per minute (μmol mg−1 protein min−1).
POD activity was assayed with the increase of absorbance at 470 nm due to the guaiacol oxidation induced by enzymes (Chance and Maehly 1955). The 3.1-mL reaction mixture contained 2.55 mL of 0.1 M phosphate buffer (pH 7.0) including 0.1 mM EDTA, 0.2 mL 1 % guriacol, 0.3 mL 0.01 M H2O2 and 0.05 mL of the tissue extract. One unit of POD was defined as 0.01 increase of absorbance at 470 nm per milligram protein per minute (U mg−1 protein min−1).
GABA contents
GABA contents in roots of plants were measured at 7 days after waterlogging treatment described by Bai et al. (2009). Roots (200 mg) were sampled and homogenised with 3 mL 4 % acetic acid. The homogenate was deposited for 1 h for sufficient extraction of GABA. Three millilitres of ethanol was further added to the samples, and then centrifuged at 12,000g for 20 min. The supernatant was collected and used for the measurement of GABA content.
Lactic acid contents
Lactic acid contents in roots of plants were measured at 7 days after waterlogging treatment as described by Xia and Saglio (1992). Roots (200 mg) were sampled and homogenised in 10 % perchloric acid and neutralised with KOH. Samples were centrifuged at 12,000g for 20 min, and the supernatant was collected and used for the measurement of lactic content.
UPLC-MS/MS analysis of lactic acid and GABA
Samples were analysed using a Waters Acquity H-Class UPLC instrument coupled to a Waters Xevo triple quadrupole mass spectrometer. A Waters Acquity UPLC BEH Amide column (2.1 mm × 150 mm × 1.7 μm) was used. The mobile phase consisted of two solvents: 95 % (v/v) acetonitrile in water with the addition of 0.1 % (v/v) formic acid and 0.075 % (v/v) ammonium hydroxide (solvent A) and 2 % (v/v) acetonitrile in water with the addition of 0.2 % (v/v) formic acid and 0.1 % (v/v) ammonium hydroxide (solvent B). The UPLC program was 100 % solvent A held for 4 min, then to 86 % solvent A: 14 % solvent B at 12 min, held for 0.5 min, and this was followed by re-equilibration to starting conditions for 5 min. The flow rate was 0.50 mL min−1; the column was held at 60 °C; and the sample compartment was at 6 °C. Lactic acid extracts were analysed as the neat solution, while GABA extracts were diluted 50 times with laboratory water. Injection volume was 2 μL. Approximate retention times were 4.7 min for lactic acid and 11.9 min for GABA.
The mass spectrometer was operated in positive and negative ion electrospray modes with a needle voltage of 2.7 kV. The ion source temperature was 130 °C; the desolvation gas was N2 at 950 L h−1; the cone gas flow was 100 L h−1; and the desolvation temperature was 400 °C. Quantitative data was collected in selected ion recording (SIR) mode monitoring (m/z) 89.1 [M-H]− (cone voltage 22 V) for lactic acid and (m/z) 104.1 [M+H]+ (Cone voltage 15 V) for GABA. Analyte identifications were confirmed by simultaneous multiple reaction monitoring (MRM) analysis using the following precursor to product transitions: (m/z) 89.1 [M-H]− to (m/z) 43.0 [M-H]− for lactic acid and (m/z) 104.1 [M+H]+ to (m/z) 87.0 [M+H]+ for GABA. Cone voltages were as described above, with collision energies of 10 V for both analytes.
Quantitation was undertaken by external calibration curves within the ranges 0.25 to 10 μg mL−1 (lactic acid) and 0.05 to 1.0 μg mL−1 (GABA). Sample matrix suppression was assessed by sample extract spike recovery at 2 μg mL−1 (lactic acid) and 0.5 μg mL−1 (GABA).
Experiment 2: adventitious root porosity and antioxidant enzyme activities of barley in aerated commercial potting mixture
As shown in Table 1, the texture of brown sodosol soil was fine sandy loam to 27-cm depth of soil, and heavy clay from 27- to 150-cm depth. The brown sodosol soil was normally waterlogged with slow drainage when observed during the winter growing season at the Cressy Research Station, Tasmania, Australia. This soil also showed poor drainage in the tanks, so an alternative was needed to ensure growth in aerobic root zones. Therefore, in order to maintain the aerobic conditions in a substrate, six barley (Hordeum vulgare L.) genotypes, the same as described in experiment 1, were sown and grown in 50-L bins, filled with a pine bark/loam-based potting mix with premixed slow release fertiliser.
Plant growth measurements
Growth parameters were also recorded when barley plants were grown in aerated potting mix. Longest adventitious root length, adventitious root number, shoot dry weights, and root dry weights were measured 7 days after stress onset, from barley plants being at three-leaf stage (e.g., for the same period of time and plant age as described for experiment 1).
Root porosity
Root porosity of all genotypes in aerobic conditions was measured at 7, 14, 21, 28, 35 and 42 days after three-leaf stage of barley, the same period of time and method described in experiment 1.
Antioxidant enzyme activities
Antioxidant enzyme activities in leaves of plants with roots in aerobic conditions were measured at 7 and 14 days after three-leaf stage of barley, the same period of time and method described in experiment 1.
GABA and lactic acid contents in roots
GABA lactic acid contents in roots of plants with roots in aerobic conditions were measured at 7 days after three-leaf stage of barley, the same period of time and method described in experiment 1.
Statistical analyses
ANOVA was used to examine the differences of plant growth parameters in different genotypes, under aerobic conditions and waterlogging treatment. Root porosity was also analysed by two-factorial (genotypes and days of measurement) ANOVA. The Student’s t test was also used to examine the difference of plant growth parameters and root porosity between aerobic conditions and waterlogging treatment.
Results
Waterlogging tolerance and plant growth of the six genotypes
The waterlogging tolerance of all genotypes used in this study was ranked based on a combined score of plant healthiness using a score from 0 to 10 (0 = plant is totally dead; 10 = no visual symptoms of stress) (Zhou 2011) after 9-week waterlogging treatment. Genotypes showed significant difference in waterlogging tolerance. TAM407227 (9.5) and Yerong (8.0) were the most tolerant, followed by YSM1 (6.5) and Gairdner (5.0). Franklin (1.5) and Naso Nijo (1.0) were the most waterlogging-susceptible genotypes. The difference in two contrasting genotypes after 6-week waterlogging is illustrated in Fig. S1. TAM407227 showed very good tolerance to waterlogging, which kept a high growth rate under waterlogging conditions. The results are consistent with those previously reported that Yerong was tolerant while both Franklin and Naso Nijo were very sensitive (Zhou 2011; Zhou et al. 2012; Zhou et al. 2007).
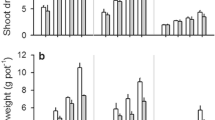
In aerated conditions, there was no significant difference in root parameters (longest adventitous root length, adventitous root number, and root dry weights) among six genotypes. When plants were grown in aerated potting mix, Naso Nijo had considerably higher shoot dry weight than the other genotypes (Table 2).
Waterlogging greatly reduced (P < 0.05) the length of the longest adventitous root, shoot dry weights and root dry weight among all these six genotypes (Table 2). After 7 days of waterlogging, there was no significant difference in terms of either shoot (0.11 g on average) or root dry weights (0.07 g on average). Waterlogging-tolerant genotypes TAM407227 and Yerong had a much higher ability of maintaining adventitious roots length than the other four genotyps after 7-day waterlogging (Table 2). Waterlogging also had a significant impact on adventitous root number (P < 0.01). After 7-day waterlogging, the number of adventitious roots increased in Naso Nijo; decreased in Yerong, TAM407227 and Gairdner; and did not show any significant (P < 0.05) change in Franklin and YSM1 (Table 2). None of the measured plant growth parameters was significantly correlated with waterlogging tolerance and aerenchyma formation (at P < 0.05).
Aerenchyma formation in experiment 1
Before waterlogging treatment (day 0), a small proportion of aerenchyma was found in two waterlogging-tolerant genotypes, TAM407227 (1.8 ± 0.1 %) (Fig. 1a) and Yerong (4.4 ± 0.2 %) (Fig. 1b), but no aerenchyma was found in the roots of Franklin (Fig. 1c) and Naso Nijo (Fig. 1d). Seven days after waterlogging treatment, aerenchyma was formed in all genotypes (Figs. 1e–h). However, the percentage of aerenchyma differed considerably, with waterlogging-tolerant genotypes TAM407227 (20.0 ± 2.2 %) and Yerong (11.7 ± 1.5 %) (Fig. 1e, f) showing a much higher percentage of aerenchyma than waterlogging-susceptible genotypes Franklin (2.6 ± 0.2 %) and Naso Nijo (4.5 ± 0.6 %) (Fig. 1g, h).
Light micrographs of cross section of adventitious roots growing in brown sodosol soil. Under aerobic conditions (day 0, just before waterlogging treatment), TAM407227 (a) and Yerong (b) had a small proportion of aerenchyma, while Franklin (c) and Naso Nijo (d) lacked aerenchyma. After 7-day waterlogging, TAM407227 (e) and Yerong (f) had a larger proportion of aerenchyma than Franklin (g) and Naso Nijo (h). Bar = 100 μm
Root porosity of plants in experiment 1
There was no significant difference in the root porosity on day 0 (before waterlogging treatment) among six genotypes. After waterlogging treatments, root porosity of all the genotypes increased steadily and quickly from day 1 to day 14 (Fig. 2). On average, the percentage of root porosity increased from about 7.2 % at day 0 to 8.1 % at day 3, 10.8 % at day 7 and 17.7 % at day 14. There were significant differences in root porosity among the six genotypes in different days of measurement, and the interactions between genotypes and days of measurement were also significant (P < 0.01). The root porosity of two waterlogging-tolerant genotypes, TAM407227 and Yerong, increased to approximately 14 % at 7 days after waterlogging. In contrast, the most sensitive genotypes, Franklin and Naso Nijo, showed a slower increase of root porosity, with only a slightly higher percentage of root porosity (7 % at day 7) than the control (6 % at day 0). Both YSM1 and Gairdner showed an intermediate increase in root porosity within the first 7 days of waterlogging treatment, which is consistent with their medium waterlogging tolerance. Significant increases (P < 0.01) in root porosity from day 7 to day 14 were found in all six genotypes, from 10.8 to 17.7 % on average (Fig. 2).
Adventitious root porosity of six different barley genotypes after 0, 1, 3, 5, 7, 14, 21, 28, 35 and 42 days of waterlogging in a brown sodosol soil. Waterlogging was started at the three-leaf stage of barley. Values are the means ± standard deviations of three replicates. Each replicate represents roots from three single plants growing in different tanks
Figure 3 compares kinetics of aerenchyma development at early stages of waterlogging stress between contrasting (sensitive—Franklin and Naso Nijo; tolerant—TAM407227 and Yerong) genotypes. As can be seen, the slope of the curve is drastically different between tolerant (ascending; closed symbols in Fig. 3) and sensitive (flat; open symbols in Fig. 3) varieties. At later stages, however, the rate of aerenchyma changes is about the same between sensitive and tolerant varieties. Taken together, this suggested that the difference in percentage of aerenchyma comes from the time when the process has started (sooner in tolerant varieties). This is further supported by Table S1 showing statistical information on root porosity as a function of time.
All genotypes showed a decrease in root porosity from day 14 to day 42 (Figs. 2 and 3). At most stages, waterlogging-tolerant genotypes (TAM407227 and Yerong) had higher root porosity than sensitive genotypes (Franklin and Naso Nijo) (Fig. 2). The biggest differences between tolerant genotypes and sensitive genotypes were found 7 days after waterlogging treatment, with both waterlogging-tolerant and intermediate genotypes having a significant increase and waterlogging-sensitive genotypes showing a little change in the percentage of root porosity.
Root porosity of plants in experiment 2
When grown in aerobic potting mix, the adventitious root porosity was relatively consistent in a period of 6 weeks for all the genotypes, ranging from 3 to 5 % (Fig. S2), confirming the above observations that no aerenchyma or little aerenchyma was formed when waterlogging stress was not applied. Root porosity for all the genotypes on day 0, in non-waterlogged brown sodosol soil (7.2 ± 0.8 %), were significantly higher (P < 0.01) than the root porosity of all the genotypes in aerated potting mixture (4.0 ± 0.5 %). There was no significant difference in root porosity among the selected genotypes when grown in the drained brown sodosol soil before waterlogging started (P = 0.23) or when in the aerobic potting mix at the same growth stage (P = 0.40).
Antioxidant enzyme activity
Waterlogging stress showed significant effects on antioxidant enzyme activity in leaves (Fig. 4a). SOD activity of waterlogging-tolerant genotypes TAM407227, Yerong and YSM1 decreased and those of waterlogging-sensitive genotypes, Franklin, Naso Nijo and Gairdner increased after 7 days of waterlogging treatment. Except for Gairdner, the 14-day waterlogging treatment showed the opposite trend in the changes of SOD activities, with slight increases in TAM407227 and Yerong, a significant increase in YSM1 but significant decreases in both Franklin and Naso Nijo.
Antioxidant enzyme activities, including SOD (a), CAT (b), APX (c) and POD (d), under aerated conditions (potting mixture, experiment 2) and waterlogging stress (brown sodosol soil, experiment 1) after 7- and 14-day waterlogging treatment which started at three-leaf stage of barley. Values are the means ± standard deviations of three replicates. Each replicate represents only green leaves from three single plants growing in different tanks
Seven days of waterlogging treatment caused a significant decrease in CAT activity in leaves of all genotypes except TAM407227 (Fig. 4b). Similarly, 14-day waterlogging treatment changed the pattern of CAT activity. Yerong, YSM1, Gairdner and Naso Nijo showed significant increase in CAT activity, while TAM407227 had a significant decrease in CAT activity after 14 days of waterlogging treatment.
Waterlogging treatment showed no effects on APX activity in leaves of both Yerong and TAM407227, but significantly increased APX activity in YSM1 (Fig. 4c). Opposite effects of 7- and 14-day waterlogging treatments on APX activity were found for Gairdner and Naso Nijo (Fig. 4c). The most significant change in APX activity was found in YSM1 with the activity being four times higher than that of control after 14-day waterlogging treatment.
A significant increase in POD activity was only found in leaves of TAM407227 after 7 days of treatment, but the activity was much lower than the controls after 14 days of waterlogging (Fig. 4d). Waterlogging treatment caused significant increases in POD activity of YSM1, Franklin and Naso Nijo (Fig. 4d).
GABA contents in roots
GABA contents in waterlogging-tolerant genotypes (TAM407227 and Yerong) and waterlogging-sensitive genotypes (Franklin and Naso Nijo) increased dramatically after 7-day waterlogging treatment (Fig. 5). No significant changes in GABA contents were found in both YSM1 and Gairdner.
GABA contents under aerated conditions (potting mixture, experiment 2) and waterlogging stress (brown sodosol soil, experiment 1) after 7-day waterlogging treatment which started at three-leaf stage. Values are the means ± standard deviations of three replicates. Each replicate represents roots from three single plants growing in different tanks
Lactic acid contents in roots
Seven days after waterlogging treatment, lactic acid contents in roots increased significantly in YSM1 and Franklin (Fig. 6). In contrast, lower lactic acid contents were found in Yerong and Gairdner while lactic acid contents in TAM407227 and Naso Nijo changed little after waterlogging treatment.
Lactic acid contents under aerated conditions (potting mixture, experiment 2) and waterlogging stress (brown sodosol soil, experiment 1) after 7-day waterlogging treatment which started at three-leaf stage. Values are the means ± standard deviations of three replicates. Each replicate represents roots from three single plants growing in different tanks
Correlations between waterlogging tolerance and physiological traits
The percentage of root porosity at different stages of waterlogging stress showed significant correlations with waterlogging tolerance. The highest correlation between waterlogging tolerance and root porosity was found 7 days after waterlogging treatment (R 2 = 0.91, P < 0.01) (Table S2). Thus, the percentage of root porosity after 7 days of waterlogging treatment can be the best indication for waterlogging tolerance of a variety.
Waterlogging treatment showed significant effects (P < 0.01) on the activity of different antioxidant enzymes in leaves. However, no clear correlation between waterlogging stress tolerance and activity of major enzymatic antioxidants in leaves was observed. The best putative fit was observed between waterlogging tolerance and SOD activities (r = 0.63 after 7 days of waterlogging and r = 0.61 after 14 days of waterlogging). However, none of these correlations were statistically significant (P < 0.05). Moreover, the correlation between SOD activity and waterlogging tolerance was negative, suggesting that, as a very best, elevated SOD levels may be used as stress markers but not as traits conferring waterlogging tolerance in barley.
Varieties showed different responses to waterlogging treatment in accumulating both GABA and lactic acid, but no significant correlation (at P < 0.05) between waterlogging tolerance and GABA or lactic acid contents was found. The relatively higher correlation coefficient (r = 0.67) between waterlogging tolerance and GABA contents after 7-day waterlogging treatment indicated that the relatively waterlogging-tolerant genotypes might have lower levels of GABA contents in roots.
Discussion
Waterlogging tolerance is associated with faster aerenchyma formation
Oxygen diffusion in water is 104 times slower than the diffusion in air (Armstrong 1979). Therefore, roots surrounded by water have very limited oxygen uptake and ATP production is greatly decreased with the oxygen deprivation, resulting in the lack of energy in waterlogged plants (Colmer and Voesenek 2009). Root aerenchyma is a special tissue with gas spaces, forming an internal system to improve the diffusion and thus concentration of oxygen within roots when in waterlogged soil (Armstrong 1979; Colmer 2003b). Increased oxygen concentration in roots leads to higher respiration rates, generating increased energy (ATP) in roots, improving nutrient uptake (Colmer and Greenway 2011) and plant survival under waterlogging conditions (Armstrong and Armstrong 1999; Colmer and Voesenek 2009).
Wetland species, such as rice, are able to form constitutive aerenchyma. The constitutive root porosity in rice can be 20–30 %, increasing to more than 40 % in waterlogged soils (Colmer 2003a; Steffens et al. 2010). The wild relative of barley Hordeum marinum and some wild relatives of maize can also form constitutive root aerenchyma under well-aerated conditions. This ability is expected to be a valuable waterlogging tolerance trait for environments with transient waterlogging since plants with developed aerenchyma adapt to waterlogged soils quickly (Malik et al. 2009; Mano and Omori 2008). In our experiments, neither cultivated barley genotypes nor their wild relative TAM407227 formed a significant amount of constitutive aerenchyma in aerobic conditions. All the genotypes had a low percentage of root porosity and no significant differences were found among genotypes. Our results were slightly different from the report by Broughton et al. (2015), who found not only higher percentage of root porosity but significant differences among barley genotypes when subjected to hydroponic aerated solutions. It is possible that the aerated hydroponic solution generated slight hypoxic conditions (or perhaps increased root ethylene), causing the increase of root porosity in waterlogging-tolerant genotypes.
Higher percentage of aerenchyma can also be induced in roots of many plants by waterlogging stress. Waterlogging-tolerant species, such as the wild relative of barley H. marinum (Garthwaite et al. 2003) have significantly higher root porosity than the susceptible species under waterlogging conditions. The linkage between root porosity and waterlogging tolerance were also found in wheat (Mcdonald et al. 2001), maize (Mano and Omori 2013), soybean (Shimamura et al. 2010) and forage legumes (Gibberd et al. 1999; Teakle et al. 2011). In our experiments, waterlogging-tolerant barley genotypes had significantly higher root porosity than susceptible genotypes under waterlogging treatment. The tolerant genotypes also had a faster increase of root porosity with accelerated aerenchyma development under waterlogging treatment. In legumes, the faster aerenchyma formation is associated with the recovery of N metabolism in roots (Thomas et al. 2005) and improves the internal oxygen transport from shoot to waterlogged roots, enhancing an increased concentration of oxygen in the root zone (Shimamura et al. 2010; Teakle et al. 2011). The waterlogging-tolerant legume Melilotus siculus (Teakle et al. 2011) and waterlogging-tolerant soybean genotypes (Shimamura et al. 2010; Thomas et al. 2005) were able to form aerenchyma rapidly, reaching more than 20 % of porosity after 7 days waterlogging treatment. In contrast, less than 10 % of root porosity was detected in the relatively less waterlogging-tolerant wheat (Yamauchi et al. 2014) and canola (Voesenek et al. 1999) after 7 days of waterlogging treatment. In our experiments, two waterlogging-tolerant genotypes, TAM407227 and Yerong, started to form aerenchyma within 7 days of waterlogging stress with the root porosity showing a significant increase (from 6 to 14 %) at that time. In contrast, two sensitive genotypes, Franklin and Naso Nijo, showed only a slight increase in the percentage of root porosity (from 6 to 7 %) at 7 days after waterlogging. Therefore, fast aerenchyma formation is likely a key mechanism in tolerant barley genotypes under waterlogging stress.
The percentage of root porosity of almost all genotypes used in this study reached the highest level at 14 days after waterlogging and declined afterwards. The low and inconsistent root porosity may be caused by damage to the root system after prolonged waterlogging (more than 14 days). Prolonged waterlogging was reported to induce microelement toxicities, such as Mn2+ and Fe2+ (Shabala 2011) or toxic secondary metabolites (Pang et al. 2007a). These toxicities affect root nutrient uptake and membrane transport activities (Pang et al. 2007a), resulting in the disturbance of signalling systems in waterlogged plants (Voesenek and Sasidharan 2013) and leading to root system damage.
Waterlogging influence on plants growth
Waterlogging significantly decreased plant growth and development. After 21 days of waterlogging treatment, growth parameters, including longest adventitious root length, shoot dry weight, and root dry weight were reduced to 20–80 % of the same growth parameters in aerated conditions (Broughton et al. 2015; Garthwaite et al. 2003; Mcdonald et al. 2001; Pang et al. 2004). After 7 days of waterlogging treatment, the longest adventitious root length, shoot dry weight and root dry weight of all the six genotypes also decreased by 40–95 % of the same growth parameters in aerated conditions. Adventitious root number of some Hordeum and Triticeae crops increased or decreased in anoxia treatment, compared with the adventitious root number of crops in aerated conditions (Garthwaite et al. 2003; Mcdonald et al. 2001). Similar results are reported in our experiment. Under waterlogging stress the adventitious root number increased in Naso Nijo but decreased in three other barley genotypes. However, none of the measured growth parameters were significantly correlated with waterlogging tolerance and aerenchyma formation. Close correlations between root aerenchyma formation and leaf injury, root dry mass and root length were reported in legume Lotus japonicas (Striker et al. 2014). However, these growth parameters cannot be used as the fast selection criteria to screen waterlogging tolerance in barley.
Waterlogging tolerance is not correlated to antioxidant enzyme activities in the leaves
Activities of antioxidant enzymes under waterlogging conditions have been studied in various plant species but gained rather controversial results (Table 3). While activities of some AO enzymes are indeed increased under stress conditions, others are unchanged, or even decreased (Table 3). Even in the same species, the results vary between different experiments. For example, in maize, SOD, CAT and APX activity in leaves increased under waterlogging conditions in one experiment (Tang et al. 2010), but decreased under waterlogging conditions in another experiment (Yan et al. 1996). The inconsistency of antioxidant enzyme activities is mainly due to the fact that ROS production and enzyme activities are highly unstable and time-dependent (Fan et al. 2014). In barley leaves, waterlogging stress caused decreased SOD activity in one experiment (Yordanova et al. 2004), but increased SOD activity in another experiment (Zhang et al. 2007). Consistent with this, our experiments showed no correlation between different enzyme activities and waterlogging tolerance. Since excessive accumulation of metal ions in plants is a possible factor in triggering ROS production (Shabala et al. 2014), selecting waterlogging-tolerant genotypes can partially be achieved through plant tolerance to toxic Mn2+ and Fe2+ which often is increased in waterlogged soils (Khabaz-Saberi et al. 2005). This is consistent with our recent study with the demonstration of a significant correlation between Mn2+ tolerance and waterlogging tolerance in barley (Huang et al. 2014). In the light of above, we believe that using activity of enzymatic AO as biochemical markers will not be able to discover QTLs conferring waterlogging stress tolerance in barley.
While this work was focused predominantly on aerenchyma and AO enzymes, other physiological traits need to be considered in future experiments and, specifically, those important to mitigate damaging effects of energy crisis and cell acidosis under hypoxic conditions.
Cytosolic pH decreases sharply in response to anoxia, typically from 7.2 to 6.7–6.8 pH units within minutes or even seconds (Ratcliffe 1997; Felle 2005). This cytosolic pH decrease is believed to represent the optimal conditions for metabolism under suboptimal oxygen supply (Ratcliffe 1997) and was postulated to act as an intracellular messenger to mediate the activation of the H+-ATPase (Reggiani et al. 1992). Thus, finding QTLs associated with such cell acidosis and regulation of H+-ATPase may be a useful strategy for breeders, especially in the light of the crucial role of H+-ATPase in membrane potential maintenance and control over plant membrane transport activity (see Shabala et al. 2014 for most recent review). Another important target may be hypoxia-induced shifts in cell metabolism and, specifically, interplay between carbohydrate concentrations, alcoholic fermentation, and GABA production (Jaeger et al. 2009; Shabala 2011). GABA content increases dramatically in waterlogged plants (Bailey-Serres and Voesenek 2008). It was shown that differences in GABA conversion into alanine (which might result in an accumulation of phytotoxic levels of intermediates) was a crucial factor differentiating waterlogging stress tolerance among Fraxinus species from different ecophysiological habitats (Jaeger et al. 2009). Also important is the transport of sugar to the root system (Drew 1997). All these traits should be considered as potential biochemical targets in breeding programs.
Waterlogging tolerance is not related to GABA and lactic acid contents in roots
GABA is formed with glutamate decarboxylase as a catalyser, with cytosolc H+ and Ca2+ activating the glutamic acid precursor process (Ratcliffe 1997; Shabala et al. 2014). The GABA is considered a mechanism adapting to oxygen deprivation (Drew 1997; Kreuzwieser et al. 2009). The increase in GABA contents under waterlogging conditions was reported in both trees (Kreuzwieser et al. 2002) and Lotus japonicus (Rocha et al. 2010). Similar with the report in trees (Kreuzwieser et al. 2002), we found that GABA could accumulate in both waterlogging-tolerant and waterlogging-sensitive genotypes in barley. Therefore, GABA accumulation under waterlogging stress is unlikely to be a mechanism for differential waterlogging tolerance in barley.
A lack of oxygen in waterlogged roots induces the anaerobic mode of the plants, such as alcoholic and lactic acid fermentation. However, anaerobic respirations were relatively inefficient for energy production and the overproduction of lactic acid can also cause cell death in roots (Drew 1997; Shabala 2011). A correlation between waterlogging tolerance and higher lactic acid fermentation was reported in Limonium (Rivoal and Hanson 1993). In addition, lactic acid efflux also plays an important role for waterlogging tolerance in maize (Xia and Saglio 1992). However, in our study, no associations were found between waterlogging tolerance lactic acid contents in barley.
In conclusion, waterlogging-tolerant genotypes of barley showed not only significantly higher adventitious root porosity than susceptible genotypes but, more importantly, a faster increase of root porosity resulting from faster development of aerenchyma. The percentage of root porosity after 7 days of waterlogging treatment showed the greatest differences among genotypes. In barley, the changes in antioxidant enzyme activities in leaves, GABA and lactic acid contents in roots under waterlogging conditions do not appear to be targets when thinking of selection criteria for waterlogging tolerance in barley.
References
Aebi H, Packer L (1984) Methods in enzymology. Academic, New York
Arbona V, Hossain Z, López-Climent MF, Pérez-Clemente RM, Gómez-Cadenas A (2008) Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol Plant 132:452–466. doi:10.1111/j.1399-3054.2007.01029.x
Armstrong W (1979) Aeration in higher plants, vol 7. Advances in Botanical Research, London
Armstrong J, Armstrong W (1999) Phragmites die-back: toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytol 142:201–217. doi:10.1046/j.1469-8137.1999.00395.x
Bai Q, Chai M, Gu Z, Cao X, Li Y, Liu K (2009) Effects of components in culture medium on glutamate decarboxylase activity and γ-aminobutyric acid accumulation in foxtail millet (Setaria italica L.) during germination. Food Chem 116:152–157. doi:10.1016/j.foodchem.2009.02.022
Bailey-Serres J, Chang R (2005) Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann Bot 96:507–518. doi:10.1093/aob/mci206
Bailey-Serres J, Voesenek LA (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339. doi:10.1146/annurev.arplant.59.032607.092752
Barrett-Lennard EG (2003) The interaction between waterlogging and salinity in higher plants: causes, consequences and implications. Plant Soil 253:35–54
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240. doi:10.1093/jxb/ert375
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem 161:559–566. doi:10.1016/0003-2697(87)90489-1
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Broughton S, Zhou GF, Teakle LN, Matsuda R, Zhou MX, O’Leary AR, Colmer DT, Li CD (2015) Waterlogging tolerance is associated with root porosity in barley (Hordeum vulgare L.). Mol Breed 35:27. doi:10.1007/s11032-015-0243-3
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Collaku A, Harrison SA (2005) Heritability of waterlogging tolerance in wheat. Crop Sci 45:722–727
Colmer TD (2003a) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann Bot 91:301–309
Colmer TD (2003b) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Greenway H (2011) Ion transport in seminal and adventitious roots of cereals during O2 deficiency. J Exp Bot 62:39–57. doi:10.1093/jxb/erq271
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Damanik R, Maziah M, Ismail M, Ahmad S, Zain A (2010) Responses of the antioxidative enzymes in Malaysian rice (Oryza sativa L.) cultivars under submergence condition. Acta Physiol Plant 32:739–747. doi:10.1007/s11738-009-0456-3
de San P, Celedonio R, Abeledo LG, Miralles D (2014) Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 378:265–277. doi:10.1007/s11104-014-2028-6
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Biol 48:223–250
Drew MC, Saglio PH, Pradet A (1985) Larger adenylate energy charge and ATP/ADP ratios in aerenchymatous roots of Zea mays in anaerobic media as a consequence of improved internal oxygen transport. Planta 165:51–58. doi:10.1007/bf00392211
Evans DE (2003) Aerenchyma formation. New Phytol 161:35–49
Fan Y, Zhu M, Shabala S, Li CD, Johnson P, Zhou MX (2014) Antioxidant activity in salt-stressed barley leaves: evaluating time- and age-dependence and suitability for the use as a biochemical marker in breeding programs. J Agron Crop Sci 200:261–272. doi:10.1111/jac.12068
Felle HH (2005) pH regulation in anoxic plants. Ann Bot 96:519–532. doi:10.1093/aob/mci207
Ferner E, Rennenberg H, Kreuzwieser J (2012) Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol 32:135–145. doi:10.1093/treephys/tps009
Garthwaite AJ, von Bothmer R, Colmer TD (2003) Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Funct Plant Biol 30:875–889
Gibberd MR, Colmer TD, Cocks PS (1999) Root porosity and oxygen movement in waterlogging-tolerant Trifolium tomentosum and -intolerant Trifolium glomeratum. Plant Cell Environ 22:1161–1168. doi:10.1046/j.1365-3040.1999.00472.x
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:999–1036
Hossain Z, Lopez-Climent MF, Arbona V, Perez-Clemente RM, Gomez-Cadenas A (2009) Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J Plant Physiol 166:1391–1404. doi:10.1016/j.jplph.2009.02.012
Huang X, Shabala S, Shabala L, Rengel Z, Wu X, Zhang G, Zhou M (2014) Linking waterlogging tolerance with Mn2+ toxicity: a case study for barley. Plant Biol. doi:10.1111/plb.12188
Jaeger C, Gessler A, Biller S, Rennenberg H, Kreuzwieser J (2009) Differences in C metabolism of ash species and provenances as a consequence of root oxygen deprivation by waterlogging. J Exp Bot. doi:10.1093/jxb/erp268
Khabaz-Saberi H, Setter TL, Waters I (2005) Waterlogging induces high to toxic concentrations of iron, aluminum, and manganese in wheat varieties on acidic soil. J Plant Nutr 29:899–911. doi:10.1080/01904160600649161
Kreuzwieser J, Fürniss S, Rennenberg H (2002) Impact of waterlogging on the N-metabolism of flood tolerant and non-tolerant tree species. Plant Cell Environ 25:1039–1049. doi:10.1046/j.1365-3040.2002.00886.x
Kreuzwieser J, Hauberg J, Howell KA, Carroll A, Rennenberg H, Millar AH, Whelan J (2009) Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiol 149:461–473
Kumutha D, Ezhilmathi K, Sairam RK, Srivastava GC, Deshmukh PS, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biol Plant 53:75–84. doi:10.1007/s10535-009-0011-5
Lee K-W, Chen PW, Yu S-M (2014) Metabolic adaptation to sugar/O2 deficiency for anaerobic germination and seedling growth in rice. Plant Cell Environ 37:2234–2244. doi:10.1111/pce.12311
Malik AI, Colmer TD, Lambers H, Schortemeyer M (2001) Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Aust J Plant Physiol 28:1121–1131
Malik AI, English JP, Colmer TD (2009) Tolerance of Hordeum marinum accessions to O2 deficiency, salinity and these stresses combined. Ann Bot 103:237–248. doi:10.1093/aob/mcn142
Mano Y, Omori F (2008) Verification of QTL controlling root aerenchyma formation in a maize × teosinte “Zea nicaraguensis” advanced backcross population. Breed Sci 58:217–223
Mano Y, Omori F (2013) Relationship between constitutive root aerenchyma formation and flooding tolerance in Zea nicaraguensis. Plant Soil 370:1–14. doi:10.1007/s11104-013-1641-0
Mcdonald MP, Galwey NW, Colmer TD (2001) Waterlogging tolerance in the tribe Triticeae: the adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell Environ 24:585–596
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pang J, Zhou M, Mendham N, Shabala S (2004) Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust J Agric Res 55:895–906. doi:10.1071/ar03097
Pang J, Cuin T, Shabala L, Zhou M, Mendham N, Shabala S (2007a) Effect of secondary metabolites associated with anaerobic soil conditions on ion fluxes and electrophysiology in barley roots. Plant Physiol 145:266–276. doi:10.1104/pp. 107.102624
Pang J, Ross J, Zhou M, Mendham N, Shabala S (2007b) Amelioration of detrimental effects of waterlogging by foliar nutrient sprays in barley. Funct Plant Biol 34:221–227. doi:10.1071/fp06158
Raskin I (1983) A method for measuring leaf volume, density, thickness, and internal gas volume. HortScience 18:698–699
Ratcliffe RG (1997) In vivo NMR studies of the metabolic response of plant tissues to anoxia. Ann Bot 79:39–48
Reggiani R, Zaina S, Bertani A (1992) Plasmalemma ATPase in rice coleoptiles; stimulation by putrescine and polyamines. Phytochemistry 31:417–419. doi:10.1016/0031-9422(92)90009-F
Rivoal J, Hanson AD (1993) Evidence for a large and sustained glycolytic flux to lactate in anoxic roots of some members of the halophytic genus Limonium. Plant Physiol 101:553–560
Rocha M, Licausi F, Araújo WL, Nunes-Nesi A, Sodek L, Fernie AR, van Dongen JT (2010) Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol 152:1501–1513
Sairam RK, Srivastava GC (2001) Water stress tolerance of wheat (Triticum aestivum L.): variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J Agron Crop Sci 186:63–70. doi:10.1046/j.1439-037x.2001.00461.x
Sairam RK, Kumutha D, Ezhilmathi K, Chinnusamy V, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol Plant 53:493–504. doi:10.1007/s10535-009-0090-3
Sauter M (2013) Root responses to flooding. Curr Opin Plant Biol 16:282–286. doi:10.1016/j.pbi.2013.03.013
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253:1–34
Setter TL, Burgess P, Water I, Kuo J (1999) Genetic diversity of barley and wheat for waterlogging tolerance in Western Australia. In: Proceeding 9th Australian Barley Technical Symposium, Melbourne, Australia, 1999. Hindawi Publishing Corporation, Egypt
Shabala S (2011) Physiological and cellular aspects of phytotoxicity tolerance in plants: the role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol 190:289–298. doi:10.1111/j.1469-8137.2010.03575.x
Shabala S, Shabala L, Barcelo J, Poschenrieder C (2014) Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ 37:2216–2233. doi:10.1111/pce.12339
Shimamura S, Yamamoto R, Nakamura T, Shimada S, Komatsu S (2010) Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Ann Bot 106:277–284. doi:10.1093/aob/mcq123
Steffens B, Geske T, Sauter M (2010) Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol 190:369–378. doi:10.1111/j.1469-8137.2010.03496.x
Striker GG, Casas C, Manzur ME, Ploschuk RA, Casal JJ (2014) Phenomic networks reveal largely independent root and shoot adjustment in waterlogged plants of Lotus japonicus. Plant Cell Environ 37:2278–2293. doi:10.1111/pce.12268
Takahashi H, Greenway H, Matsumura H, Tsutsumi N, Nakazono M (2014) Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water. Ann Bot 113:851–859. doi:10.1093/aob/mct305
Tan W, Liu J, Dai T, Jing Q, Cao W, Jiang D (2008) Alterations in photosynthesis and antioxidant enzyme activity in winter wheat subjected to post-anthesis water-logging. Photosynthetica 46:21–27. doi:10.1007/s11099-008-0005-0
Tang B, Xu S-z, Zou X-l, Zheng Y-l, Qiu F-z (2010) Changes of antioxidative enzymes and lipid peroxidation in leaves and roots of waterlogging-tolerant and waterlogging-sensitive maize genotypes at seedling stage. Agricult Sci China 9:651–661. doi:10.1016/S1671-2927(09)60140-1
Teakle NL, Armstrong J, Barrett-Lennard EG, Colmer TD (2011) Aerenchymatous phellem in hypocotyl and roots enables O2 transport in Melilotus siculus. New Phytol 190:340–350. doi:10.1111/j.1469-8137.2011.03655.x
Thomas AL, Guerreiro SMC, Sodek L (2005) Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Ann Bot 96:1191–1198. doi:10.1093/aob/mci272
Thomson CJ, Armstrong W, Waters I, Greenway H (1990) Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant Cell Environ 13:395–403
Ushimaru T, Kanematsu S, Shibasaka M, Tsuji H (1999) Effect of hypoxia on the antioxidative enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiol Plant 107:181–187. doi:10.1034/j.1399-3054.1999.100205.x
Ushimaru T, Kanematsu S, Katayama M, Tsuji H (2001) Antioxidative enzymes in seedlings of Nelumbo nucifera germinated under water. Physiol Plant 112:39–46. doi:10.1034/j.1399-3054.2001.1120106.x
Voesenek L, Bailey-Serres J (2013) Flooding tolerance: O2 sensing and survival strategies. Curr Opin Plant Biol 16:647–653. doi:10.1016/j.pbi.2013.06.008
Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206:57–73. doi:10.1111/nph.13209
Voesenek LACJ, Sasidharan R (2013) Ethylene—and oxygen signalling—drive plant survival during flooding. Plant Biol 15:426–435. doi:10.1111/plb.12014
Voesenek LACJ, Armstrong WO, Gemann GM, Colmer TD, McDonald MP (1999) A lack of aerenchyma and high rates of radial oxygen loss from the root base contribute to the waterlogging intolerance of Brassica napus. Funct Plant Biol 26:87–93. doi:10.1071/PP98086
Wang K, Jiang Y (2007) Antioxidant responses of creeping bentgrass roots to waterlogging. Crop Sci 47:232–238. doi:10.2135/cropsci2006.07.0498
Xia J-H, Saglio PH (1992) Lactic acid efflux as a mechanism of hypoxic acclimation of maize root tips to anoxia. Plant Physiol 100:40–46
Yamauchi T et al (2014) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65:261–273. doi:10.1093/jxb/ert371
Yan B, Dai Q, Liu X, Huang S, Wang Z (1996) Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179:261–268. doi:10.1007/bf00009336
Yordanova RY, Christov KN, Popova LP (2004) Antioxidative enzymes in barley plants subjected to soil flooding. Environ Exp Bot 51:93–101. doi:10.1016/S0098-8472(03)00063-7
Yu Q, Rengel Z (1999) Waterlogging influences plant growth and activities of superoxide dismutases in narrow-leafed lupin and transgenic tobacco plants. J Plant Physiol 155:431–438. doi:10.1016/S0176-1617(99)80127-8
Zhang G, Tanakamaru K, Abe J, Morita S (2007) Influence of waterlogging on some anti-oxidative enzymatic activities of two barley genotypes differing in anoxia tolerance. Acta Physiol Plant 29:171–176. doi:10.1007/s11738-006-0022-1
Zhou M (2010) Improvement of plant waterlogging tolerance. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer, Heidelberg, pp 267–285. doi:10.1007/978-3-642-10305-6_13
Zhou M (2011) Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breed 130:203–208. doi:10.1111/j.1439-0523.2010.01792.x
Zhou W, Zhao D, Lin X (1997) Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L.). J Plant Growth Reg 16:47–53
Zhou MX, Li HB, Mendham NJ (2007) Combining ability of waterlogging tolerance in barley. Crop Sci 47:278–284. doi:10.2135/cropsci2006.02.0065
Zhou M, Johnson P, Zhou G, Li C, Lance R (2012) Quantitative trait loci for waterlogging tolerance in a barley cross of Franklin × YuYaoXiangTian Erleng and the relationship between waterlogging and salinity tolerance. Crop Sci 52:2082. doi:10.2135/cropsci2012.01.0008
Acknowledgments
This work was supported by the Australian Research Council Linkage grant (project LP120200516) and the Grains Research and Development Corporation (GRDC) of Australia. We are also grateful to Dr Lukasz Kotula for his technical help in root porosity measurement and Professor Tim Colmer of The University of Western Australia for providing many useful suggestions on a draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Guillermo Santa Maria.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Summary of two-factorial (days of measurement and genotypes) ANOVA analysis of changes in root porosity in brown sodosol soil. (DOC 36 kb)
Table S2
Correlation coefficients between waterlogging tolerance score and adventitious root porosity of waterlogging across 42 days waterlogging stress among six barley genotypes. Waterlogging tolerance score is based on plant healthiness score after 9 weeks waterlogging treatment in brown sodosol soil. (DOC 34 kb)
Figure S1
The appearance of two contrasting genotypes after 6 weeks waterlogging growing in brown sodosol soil: TAM407227 (waterlogging tolerance score = 9.5) and Franklin (waterlogging tolerance score = 1.5) (PDF 103 kb)
Figure S2
Adventitious root porosity of six different barley genotypes in aerobic conditions (potting mixture) after 7, 14, 21, 28, 35, 42 days of three-leaf stage growing barley, the same period of growing stages with measuring adventitious root porosity under waterlogging conditions (Fig. 3). Values are the means ± standard deviations of 3 replicates. Each replicate represents only green leaves from 3 single plants growing in different tanks. (PDF 111 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Shabala, S., Koutoulis, A. et al. Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant Soil 394, 355–372 (2015). https://doi.org/10.1007/s11104-015-2536-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2536-z