Abstract
In this study, we investigated the possible role of hemin in alleviating zinc (Zn), lead (Pb) and chromium (Cr) toxicity in rice seedlings grown hydroponically by analyzing the morphological and physiological parameters. Our results showed that exposure of rice seedlings to excess Zn, Pb or Cr could cause severe leaf chlorosis, inhibit photosynthetic activity and consequently suppress plant growth. The concentration of O2 •− and H2O2 significantly increased and the activities of antioxidative enzymes decreased in roots of rice seedlings under metal exposure. The combined treatments (hemin + ZnSO4, hemin + Pb(NO3)2 and hemin + K2Cr2O7), on the other hand, significantly enhanced the photosynthesis- and plant growth-related parameters compared with their corresponding heavy-metal-stress alone. Combined treatments dramatically stimulated the activities of superoxide dismutase (SOD), ascorbic peroxidase (APX) and glutathione reductase (GR) as well as the concentrations of ascorbic acid (AsA) and glutathione (GSH) as compared with the metal- stress alone. The concentrations of reactive oxygen species (ROS, e.g. O2 •− and H2O2) were significantly reduced in the metal plus hemin treatments. Hemin addition also reduced metal accumulation in rice seedlings especially in root tissues. These findings suggest that hemin-elevated levels of antioxidants, activities of antioxidative enzymes and hemin-reduced accumulation of heavy-metal could confer resistance against Zn, Pb, and Cr stress in rice seedlings, resulting in improved pigments accumulation, photosynthetic attributes and plant growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution has become one of the major environmental problems (Liu et al. 2014), especially for the agriculture section due to highly anthropogenic activities, including mining (Oliveira et al. 2012), electroplating (Singh et al. 2002) and leather tanning (Anwaar et al. 2014) plus the lacking of proper management toward wastes from these industries (Rafique et al. 2010). Among various kinds of heavy metals, zinc (Zn), as the second abundant transition metal and essential micro-nutrient (Broadley et al. 2007), is required below 5 μg g−1 for plant growth and normal metabolism (Ait et al. 2004). However, at a higher level, Zn will prevent plant growth and be toxic for important cellular processes in plantlets (Azooz et al. 2011). Lead (Pb) and chromium (Cr) are heavy metals that are ubiquitous in environments; they are non-essential and highly toxic for plants, animals and humans even at a low level (Zia et al. 2011; Shafaqat et al. 2015). These three types of heavy metals have some commons in their negative effects on plants. They could delay plant growth (Andrade et al. 2009; Xiong et al. 2014; Rodriguez et al. 2012), cause chlorosis in leaves (Wang et al. 2009; Shahid et al. 2014a; Ali et al. 2013), and damage roots (Lingua et al. 2008; Pourrut et al. 2011; Gill et al. 2014).
As is well-known, the excess production of reactive oxygen species (ROS) is the most common and initial biochemical process in plants exposed to heavy-metal stress (Xu et al. 2011). The outburst of ROS will further break down the redox homeostasis in cells and finally result in oxidative stress to the whole plantlets (Shahid et al. 2014b; Mishra et al. 2006). To scavenge these over-produced ROS and avoid oxidative stress, plants have developed an effective antioxidative system, which is operating through activating activities of various antioxidative enzymes e.g., superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR) and promoting accumulations of antioxidants (GSH and AsA) at the same time (Mitter et al. 2002; Dai et al. 2015; Hossain et al. 2012). However, under severe stressful conditions, the antioxidative capacity may not be sufficient to resist the oxidative damage induced by the excessive production of ROS (Gill et al. 2015; Farooq et al. 2013). It may cause a wide range of damages to nucleic acids, photosynthetic pigments, proteins in plant cells and inhibit the activities of antioxidative enzymes, decline the accumulations of antioxidants, and finally result in cell death (Morina et al. 2010; Lin and Aarts 2012). Except for the oxidative stress, another toxic effect induced by heavy-metal is competed with essential elements to bind with sulphydryl groups of proteins, which finally resulted in disruption and denaturation of functional proteins (Sharma and Dietz 2009). So plants also have developed numerous ways to maintain cellular metal homeostasis (Clemens 2001), such as restricted metal uptake, active efflux, sequestration into vacuole and chelated by metal-binding peptide or proteins (Hall 2002; Verbruggen et al. 2009).
Hemin (ferroprotoporphyrin IX), a compound derivative of heme, is revealed as a potent inducer of heme oxygenase 1 (HO-1) (Li et al. 2015) for it can act as heme which greatly enhances HO1 activity by up-regulating its mRNA abundance and protein level. These responses finally assure hemin to exert protective effects in an HO1-dependent manner (Xuan et al. 2008; Xie et al. 2011). The powerful cytoprotective function of hemin against various abiotic stresses has been widely reported in plants. For example, hemin is associated with ammonium tolerance by regulating antioxidant defense in Oryza sativa (Xie et al. 2015); hemin is involved in the amelioration of aluminium- and cadmium-induced oxidative stress in Medicago sativa (Cui et al. 2012, 2013); hemin contributes to wheat salinity acclimation by regulating ROS homeostasis (Xie et al. 2011) and so on. Based on these reports, here we hypothesize that hemin may act as an effective and efficient additive that can alleviate heavy-metal toxicity in plants. Thus the aim of this study is to investigate: (1) whether hemin application can attenuate adverse effects of heavy metals on plant growth and development; (2) whether this attenuation effect can be applied to diverse metals; (3) why and how hemin can attenuate the heavy-metal toxicity in plants.
Until now, the major reports on protective effects of hemin against heavy-metal stress have been focused on a few plant species, such as Arabidopsis (Han et al. 2014), M. sativa (Han et al. 2008; Cui et al. 2012; Jin et al. 2013) and soybean (Noriega et al. 2012). Furthermore, types of heavy-metal were limited to aluminum (Lin et al. 2013; Cui et al. 2013) and cadmium (Cui et al. 2012; Han et al. 2014). However, studies are still lacking on the beneficial effects of hemin on rice seedlings exposed to heavy metal stress. Rice (O. sativa L.), as one of the most important cereal crops, is widely cultivated across the world and considered a staple food in many Asian countries, such as India, China and Japan (Sasaki and Burr 2000; Mostofa et al. 2015). Therefore, rice was chosen as our experimental material in the present study. In order to test whether hemin has a wide applicability to mitigate toxicities of diverse heavy metals, we selected three metals (i.e. Zn, Pb and Cr) to analyze the influences of hemin addition on plant growth, photosynthetic ability and antioxidant activities of rice seedlings grown hydroponically with these metals.
Materials and methods
Plant material, growth conditions and treatments
Seeds of rice (O. sativa L.) were surface-sterilized with 1 % (v/v) sodium hypochlorite solution for 20 min and soaked in distilled water at room temperature for 24 h. They were then germinated at 20–25 °C in a plastic disk and covered by moisture gauze to maintain water till white buds emerged. After germination, they were sown on plastic nets floating on distilled water in 600 ml plastic beakers and kept in the dark at 25 ± 2 °C. Each plastic beaker contained about 50 rice seedlings. After 2 days, uniformly germinated seeds were transplanted to a growth chamber (Ningbo Sai Fu Instrument Co., Ltd., China) with a light density of 100 µmol m−2 s−1 and 14 h photoperiod. The day/night temperatures were set at 25/22 °C, and the relative humidity was kept as 80 %. The seedlings were cultured in 1/4-strength Kimura B nutrient solution for the 1 week and than transferred into a half-strength nutrient solution. The nutrient solution was renewed every 2 days and the pH of solution was adjusted to 5.5 ± 0.1 by adding 1 M NaOH or HCl. On the 12th day after transplanting into the growth chamber, uniform-sized seedlings were selected to be exposed to various forms of heavy-metal stress (Zn, Pb, and Cr). Based on our preliminary experiments, the growth of rice seedlings was significantly inhibited at 100 μM ZnSO4, 100 μM Pb(NO3)2 or 80 μM K2Cr2O7, while addition of 1 μM hemin showed optimum alleviative effects on Zn-induced toxic symptoms, and inclusion of 5 μM hemin could effectively relieve Pb or Cr toxicity. Therefore, 12 different treatments were established as follows: control; hemin (1 μM hemin); Zn (100 μM Zn SO4); hemin + Zn (1 μM hemin + 100 μM ZnSO4); control; hemin (5 μM hemin); Pb (100 μM Pb(NO3)2); hemin + Pb (5 μM hemin + 100 μM Pb(NO3)2); control; hemin (5 μM hemin); Cr (80 μM K2Cr2O7); hemin + Cr (5 μM hemin + 80 μM K2Cr2O7). The roots of rice seedlings were harvested at 12 h after treatment to determine various biochemical parameters, and the whole seedlings were harvested on the 7th day after treatment to measure growth parameters and physiological indexes. All experiments were arranged in a complete randomized block design with each treatment being replicated three times.
Measurement of growth parameters
On the 7th day after treatment with heavy metal and/or hemin, seedlings were harvested and various growth parameters including plant height, root length, and fresh weight of roots and shoots were measured immediately. For determination of dried weight, roots were firstly submerged in 0.1 μM EDTA solution for 30 min to remove elements adhered to the roots surface, and then washed with distilled water repeatedly and dried out with absorbent paper. Afterwards all samples were oven-dried for 30 min at 105 °C, and then at 85 °C until they reached a constant weight.
Determination of chlorophyll concentration and photosynthetic parameters
The chlorophyll concentration was measured according to the method of Knudson et al. (1977) with slight modifications. Briefly, fresh leaf samples (0.1 g) were accurately weighed and extracted in 95 % ethanol overnight in the dark, and then the concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b) and total carotenoids were determined by reading the absorbance at 665, 649 and 470 nm on a spectrophotometer (PGENERAL, Beijing, China), respectively.
The net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci) and transpiration rate (Tr) of the second fully expanded leaf were determined using a portable photosynthesis system (Li-6400, LICOR, Lincoln, NE, USA). Measurements were done in the growth chamber between 09:00 and 11:30 am before harvest. During the measurement, the photosynthetically active radiation was set at 800 μmol m−2 s−1, the ambient CO2 concentration was 400 μmol CO2 mol−1 and the temperature was 29 ± 0.5 °C.
Determination of O2 •− and H2O2 concentration
The concentration of O2 •− was measured by monitoring the absorbance at 530 nm during nitrite formation from hydroxylamine hydrochloride in the presence of O2 •− according to the procedure of Jiang and Zhang (2001).
The levels of H2O2 were determined following the procedure of Hossain et al. (2010). Briefly, fresh root samples (0.5 g) were firstly extracted with 5 ml 50 mM sodium phosphate buffer (pH 7.8) and centrifuged at 12,000 rpm for 15 min under 4 °C. The yellow color was developed after reaction of 3 ml extracted solution with 1 ml 0.1 % TiCl4 (in 20 % H2SO4) for 10 min at room temperature. The absorbance was then measured at 410 nm.
Determination of anti-oxidant enzyme activity
For extraction of antioxidant enzymes, fresh root tissues (0.5 g) were homogenized in 4 ml 50 mM phosphate buffer (pH 7.8) on the ice, and then the extracted samples were centrifuged at 12,000 rpm for 20 min under 4 °C. The supernatants were collected in 5 ml centrifuge tubes and used for further determination of the activities of various antioxidant enzymes.
The activity of SOD was measured according to Zhou et al. (1997). Each 3 ml reaction mixture contained 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 100 μM EDTA, 75 μM NBT, 2 μM riboflavin (added last). The mixture reacted with 40 μl crude extract of roots for 20 min at a light destiny of 80 mol m−2 s−1. One unit of SOD activity is the amount of enzyme required to cause 50 % inhibition of the reduction rate of NBT as monitored at 560 nm.
The APX activity in roots was measured following the method of Nakano et al. (1980). The reaction solution contained 50 mM phosphate buffer (pH 7.0) with 2 mM EDTA, 5 mM AsA, 20 mM H2O2 and 0.1 ml crude extract. The APX activity was calculated by recording the decrease in absorbance at 290 nm within 40 s as a result of AsA oxidation.
GR activity was assayed by the method of Wheeler et al. (1990) with minor modifications. Briefly, 2 ml assay mixture contained 40 mM Hepes buffer (pH 7.8), 10 mM GSSG, 2.4 mM NAPDH and 0.1 ml crude extract. The GR activity was calculated by measuring the decline in absorbance at 340 nm for GSSG-dependent oxidation of NAPDH.
Total protein concentration in the extracts was measured by the method of Coomassie blue staining described by Bradford (1976). Briefly, 2.9 ml Coomassie brilliant blue G-250 solution was mixed with 0.1 ml crude extract for 2 min. The concentration of total soluble protein was calculated by recording the absorbance at 595 nm.
Determination of non-enzymatic antioxidants
Reduced ascorbic acid (AsA) was determined by the method of Zhang et al. (1996) with some modifications. Briefly, fresh root samples (0.5 g) of each treatment were homogenized in 5 ml trichloroacetic acid (TCA) and then centrifuged at 12,000 rpm for 20 min under 4 °C. Then 0.3 ml supernatant was mixed with 0.75 ml 150 mM phosphate buffer (pH 7.4) containing 5 mM EDTA, and 0.6 ml 10 % (v/v) TCA, 0.6 ml 44 % (v/v) o-phosphoric acid, 0.6 ml 0.5 % (w/v) red phenanthroline (BP) in absolute ethanol, and 0.15 ml 0.3 % (w/v) FeCl3 were added in order. The reaction mixtures were then incubated at 40 °C for 40 min, and their absorbances at 525 nm were finally detected. A standard curve with ascorbate was used.
Reduced glutathion (GSH) was estimated following the method described by Moron et al. (1979). Briefly, fresh root samples (0.5 g) were homogenated in 5 ml 5 % (v/v) TCA and immediately centrifuged at 12,000 rpm for 20 min under 4 °C. Then 0.1 ml supernatant was mixed with 0.9 ml 150 mM phosphate buffer (pH 7.8), and 2 ml 1 mM DNTB was added and mixed thoroughly to react. The reaction was terminated after 10 min. Finally the absorbance at 412 nm was recorded.
Determination of heavy-metal concentration
On the 7th day after treatment with heavy metal and/or hemin, seedlings were harvested to measure the heavy metal concentrations in rice seedlings according to the reported methods (Kováčik et al. 2014) with certain modification. The intact plants were washed with distilled water and then submerged in 0.1 μM Na2EDTA for 30 min to remove elements adhere to the roots surface. After that, plants were washed repeatedly with distilled water and finally dried out with absorbent paper. The parts of plants shoots and roots were separated, and all of them were oven-dried at 80 °C for approximately 48 h and ground into power. Each sample (0.1 g) was digest in the mixture of concentrated HNO3 and water (2 + 8 ml) using microwave decomposition (Milestone, Ethos T, USA) at 200 °C over 30 min. The digest solutions were washed with distilled water in 25 ml flasks. Heavy-metal concentration was then determined through ICP-MS (Optima 2100DV, PerkinElmer, Waltham, MA, USA).
Statistical analysis
All presented values are means of three independent experiments with each treatment being replicated three times. The data were subjected to one-way analysis of variance (ANOVA) and different letters indicate significant differences between treatments at p < 0.05, according to Duncan’s multiple range test using SPSS 20.0 for Windows Statistical Software Package (SPSS. Chicago, IL, USA).
Results
Effect of different metals and/or hemin treatments on plant growth
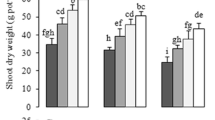
As shown in Table 1 and Fig. 1, stresses caused by Zn2+, Pb2+ and Cr6+ exhibited detrimental effects on plant growth and biomass accumulations as compared to the controls, in which inhibition of root elongation was the most visible character. Zn2+-, Pb2+- or Cr6+-stress reduced the root length by 27, 19 and 21 %, respectively, while no significant differences in shoot height were found between the control and heavy-metal treatments. Both fresh and dry weights of aboveground and underground were markedly decreased by heavy-metal-treatment. Nevertheless, the toxic effects of heavy metals on both plant growth and biomass accumulation in rice seedlings were strikingly mitigated by hemin application (Fig. 1). Higher plant height, root length, fresh weight and dry weight of shoot and root were observed in combined treatments as compared with sole heavy metal exposure.
Effects of hemin, heavy-metal (Zn/Pb/Cr) and their combinations on the growth of rice seedlings grown hydroponically. 12 days-old seedlings were treated with nutrient solution only (Con), 1 μM or 5 μM hemin (H 1 or H 5), 100 μM ZnSO4 (Zn), 100 μM PbNO3 (Pb) or 80 μM K2Cr2O7 (Cr), respectively, or in the combinative treatments, 1 μM hemin + 100 μM ZnSO4 (Zn + H1), 5 μM hemin + 100 μM PbNO3 (Pb + H5), 5 μM hemin + 80 μM K2Cr2O7 (Cr + H5) for 7 days. Photographs were then taken
Effect of different metal and/or hemin treatments on chlorophyll concentration and photosynthetic parameters
Effect of heavy metals (Zn2+ or Pb2+ or Cr6+) toxicity on chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoids values in roots of rice seedlings with or without supplementation of hemin is illustrated in Table 2. All these chlorophyll concentrations exhibited significantly reduction after exposure to metal-stress for 7 days as compared with control. However, hemin application remarked recovered all these chlorophyll concentrations when compared with their corresponding metal-treated seedlings.
Toxic Zn2+ or Pb2+ or Cr6+ contained in the growth media significantly inhibited the photosynthetic parameters viz. Pn, Gs, Ci and Tr of rice seedlings (Table 2). However, co-treatment of hemin with excess Zn2+ or Pb2+ or Cr6+ could effectively alleviate their adverse effects; the recoveries of hemin mainly expressed on elevating Pn by 35, 58 and 21 %, respectively, as compared with their corresponding heavy-metal-stress control.
Effect of different metals and/or hemin treatments on ROS accumulation
After 12 h exposure, levels of O2 •− and H2O2 in roots of rice seedlings were significantly elevated by heavy metals (Zn2+ or Pb2+ or Cr6+). As compared with the controls, O2 •− concentrations were respectively increased by 23, 31, 105 % (Fig. 2a), and the concentration of H2O2 were respectively enhanced by 48, 63 and 53 % (Fig. 2b). While hemin application, could remarkably inhibited heavy-metal-induced oxidative damage to rice seedlings by lowering O2 •− and H2O2 levels. As shown in Fig. 2a and b, compared with corresponding heavy-metal stress, O2 •− and H2O2 concentrations were decreased by 30 and 31 % in the Zn plus hemin treatment, and by 22 and 16 % in the Pb plus hemin treatment, and by 23 and 25 % in the Cr plus hemin treatment.
Effects of hemin, heavy-metal (Zn/Pb/Cr) and their combinations on the accumulation of O2 •− (a) and H2O2 (b) in roots of rice seedlings grown hydroponically. 12-days-old seedlings were treated with nutrient solution only (control), 1 μM or 5 μM hemin (hemin 1 or hemin 5), 100 μM ZnSO4 (Zn 100), 100 μM PbNO3 (Pb 100) or 80 μM K2Cr2O7 (Cr 80), respectively, or in the combinative treatments, 1 μM hemin + 100 μM ZnSO4 (hemin 1 + Zn 100), 5 μM hemin + 100 μM PbNO3 (hemin 5 + Pb 100), 5 μM hemin + 80 μM K2Cr2O7 (hemin 5 + Cr 80) for 12 h. Data represented as mean ± SD. Different letters on vertical bars indicate differences at P ≤ 0.05
Effect of different metals and/or hemin treatments on antioxidant enzyme activity and antioxidant concentrations
Modification in antioxidant enzymes (SOD, APX and GR) of rice roots under various treatments of heavy metal and hemin as alone or in combination is described in Fig. 3. Control group showed the higher values of antioxidant enzymes, and under sole heavy-metal stress or combinative treatment, all three tested antioxidant enzymes showed similar variation trend, i.e., both three kinds of toxic metals (Zn2+ or Pb2+ or Cr6+) possessed strikingly inhibition effects on these enzymes; while exogenous hemin application significantly recovered activities of all these three antioxidant enzymes.
Effects of hemin, heavy-metal (Zn/Pb/Cr) and their combinations on the activity of SOD (a), APX (b) and GR (c) in roots of rice seedlings grown hydroponically. 12-days-old seedlings were treated with nutrient solution only (control), 1 μM or 5 μM hemin (hemin 1 or hemin 5), 100 μM ZnSO4 (Zn 100), 100 μM PbNO3 (Pb 100) or 80 μM K2Cr2O7 (Cr 80), respectively, or in the combinative treatments, 1 μM hemin + 100 μM ZnSO4 (hemin 1 + Zn 100), 5 μM hemin + 100 μM PbNO3 (hemin 5 + Pb 100), 5 μM hemin + 80 μM K2Cr2O7 (hemin 5 + Cr 80) for 12 h. Data represented as mean ± SD. Different letters on vertical bars indicate differences at P ≤ 0.05
As shown in Fig. 4a and b, heavy metal stress stimulated the accumulation of reduced AsA and GSH in rice roots compared with controls, i.e., under Zn2+ or Pb2+ or Cr6+ stress, the concentrations of AsA were, respectively, increased by 34, 13 and 16 %, and the concentrations of GSH were elevated about 1.17, 0.85, 0.52 times orderly. While hemin application further increased the levels of reduced AsA and GSH, where hemin + ZnSO4, hemin + Pb(NO3)2, hemin + K2Cr2O7 elevated the concentrations of AsA by 9.8 , 20.8 and 17.6 %, respectively; and these combinative groups orderly enhanced the concentrations of GSH by 22 , 21 and 25 %, respectively, as compared with corresponding sole heavy-metal group.
Effects of hemin, heavy-metal (Zn/Pb/Cr) and their combinations on the concentration of AsA (a) and GSH (b) in roots of rice seedlings grown hydroponically. 12 days-old seedlings were treated with nutrient solution only (control), 1 μM or 5 μM hemin (hemin 1 or hemin 5), 100 μM ZnSO4 (Zn 100), 100 μM PbNO3 (Pb 100) or 80 μM K2Cr2O7 (Cr 80), respectively, or in the combinative treatments, 1 μM hemin + 100 μM ZnSO4 (hemin 1 + Zn 100), 5 μM hemin + 100 μM PbNO3 (hemin 5 + Pb 100), 5 μM hemin + 80 μM K2Cr2O7 (hemin 5 + Cr 80) for 12 h. Data represented as mean ± SD. Different letters on vertical bars indicate differences at P ≤ 0.05
Effect of different metals and/or hemin treatments on metal uptake and accumulation
Excess heavy-metal (Zn100 or Pb100 or Cr80) exposure substantially elevated their endogenous contents both in shoots and roots, and rice roots accumulated more metals than shoots (Fig. 5a, b). Specifically, under Zn100 treatment, zinc concentrations in shoots and roots reached as high as 1,352 and 3,283 μg g−1 DW respectively; under Pb100 treatment, lead concentrations in shoots and roots achieved as high as 256 and 5245 μg g−1 DW; and under Cr80 treatment, Chromium concentrations in shoots and roots were 163 and 1556 μg g−1 DW, respectively. However, addition of hemin significantly inhibited the uptake of heavy-metals by roots as compared to sole heavy-metal stress, where 18 , 26 and 13 % reduction were observed in hemin + ZnSO4, hemin + Pb(NO3)2 and hemin + K2Cr2O7 treatments, respectively. While for the metal bioaccumulation in shoots, except for hemin + ZnSO4 treatment, which induced 21 % reduction in Zn accumulation compared with the Zn treatment alone, the other two combinative treatments exerted none inhibiting effect.
Metal (Zn, Pb or Cr) concentration in shoots (a) or roots (b) of rice seedlings grown hydroponically. 12 days-old seedlings were treated with nutrient solution only (control), 1 μM or 5 μM hemin (hemin 1 or hemin 5), 100 μM ZnSO4 (Zn 100), 100 μM PbNO3 (Pb 100) or 80 μM K2Cr2O7 (Cr 80), respectively, or in the combinative treatments, 1 μM hemin + 100 μM ZnSO4 (hemin 1 + Zn 100), 5 μM hemin + 100 μM PbNO3 (hemin 5 + Pb 100), 5 μM hemin + 80 μM K2Cr2O7 (hemin 5 + Cr 80) for 12 h. Data represented as mean ± SD. Different letters on vertical bars indicate differences at P ≤ 0.05
Discussion
Three kinds of heavy metals, i.e., Zn2+, Pb2+ and Cr6+ were selected in the present study to verify heavy-metal toxic effects on morphological, physiological and biochemical properties of rice seedlings and role of hemin in alleviating metal-induced damages. Excess heavy-metal exposure significantly elevated the metal concentrations in tissues (shoots and roots) to toxic level (Fig. 5a, b), which further negatively influenced plant growth and development. These adverse effects have been widely found in many plant species (Liu et al. 2014; Laura et al. 2014; Ali et al. 2015). As reported in the previous studies, we revealed that metal toxicity symptoms primarily expressed on leaf chlorosis (Fig. 1), growth retardation and biomass reduction (Table 1; Fig. 1). And these symptoms might be a result of metal-induced alterations of basic metabolic processes, such as diminished photosynthesis and imbalanced ROS (Hamid et al. 2010; Yadav 2010). The results of this study indeed showed that excess Zn2+ or Pb2+ or Cr6+ markedly retained photosynthetic abilities (Table 2) viz., Pn, Gs,Ci and Tr. Similar results have also been achieved (Gajewska et al. 2006; Hamid et al. 2010). And the suppression of photosynthesis induced by heavy-metal-toxicity has been ascribed to the degradation of chlorophyll (Patsikka et al. 2002). In consistent with previous studies, the current study also found that heavy metals resulted in a striking decrease in chlorophyll concentration (Table 2). As for the possible reasons of the sharply reduced chlorophyll content, especially the concentration of Chl b, may include but not limited to, the inhibited biosynthesis but speeded up decomposition of these photosynthetic pigments (Drazkiewice 1994; Rao et al. 2007).
In addition to the inhibited photosynthesis ability, redox imbalance is another toxic impact from heavy metals on plant (Habiba et al. 2015). Considering that roots were directly exposed to toxic growth media, and oxidative damage, antioxidant mobilization were rapid-response progresses (Yang et al. 2010), oxidative and antioxidative parameters were primarily determined in roots of rice seedlings after 12 h treatment in the current study. In accordance with the previous observations (MacFarlane et al. 2003), higher concentrations of O2 •− and H2O2 were exhibited under heavy-metal (Zn2+ or Pb2+ or Cr6+) toxicity (Fig. 2). As is well known, to cope with these hyper generated ROS, plants already evolved a complex antioxidative system including enzymatic and non-enzymatic antioxidants (Gill and Tuteja 2010; Yadav 2010). Among them, SOD catalyzes the conversion of superoxide union to H2O2, APX and GR belong to the ascorbate–glutathione cycle and also play vital roles in eliminating H2O2 (Apel and Hirt 2004; Hossain et al. 2012). Results in this study showed that excess Zn100 or Pb100 or Cr80 remarkably restricted activities of all these three antioxidant enzymes (Fig. 3), which was in consistent with the reports by Shao et al. (2008). The reduction in antioxidant enzymes might be due to the severe oxidative damage caused by these overproduced ROS (Chen and Murata 2011). Furthermore, antioxidants including reduced AsA and GSH are crucial for minimizing ROS-induced oxidative stress (Gill and Tuteja 2010). Interestingly, higher concentrations of AsA and GSH were observed (Fig. 4), which is contrasting to the results of Mostofa et al. (2015) and Thounaojam et al. (2012). This discrepancy might be attributed to the different plant species, metal ions, concentrations and exposure durations. However, enhanced accumulation of antioxidants was still inadequate to obliterate these overproduced ROS induced by excess heavy metal ions (Fig. 2).
In this study, hemin application dramatically alleviated the phytotoxicities caused by toxic heavy-metal, which is convincingly supported by the higher shoot and root length, heavier fresh and dry weight of aboveground and underground (Table 1; Fig. 1). The promotion of plant growth by hemin was also reported by Fu et al. (2011). Apart from these growth parameters, hemin addition also greatly counteracted the damaging effects from excess metals on pigments accumulation (Table 2). The protective effect of hemin in chlorophyll generation may be correlated with function of HO1 in phytochrome chromophore synthesis (Muramoto et al. 2002). The higher chlorophyll level made the stronger Pn reasonable (Table 2). Moreover, alleviation of metal toxicity by hemin was also related to protecting rice seedlings against oxidative damage resulted from the outburst of ROS in the roots. Strengthened activities of antioxidant enzymes (SOD, APX and GR), elevated levels of AsA and GSH (Fig. 4) finally resulted in lower concentration of O2 •− and H2O2 (Fig. 2) in this present study. Similar protective effect of hemin against oxidative stress also can be observed in soybean and alfalfa which suffered from Al, Cd or UV-B toxicity (Cui et al. 2013; Yannarelli et al. 2006; Han et al. 2008). Therefore, we inferred that hemin might operate through stimulating the productions of antioxidants and activating antioxidative enzymes to reconstruct the ROS-balance and to ameliorate oxidative stress.
Moreover, hemin application also significantly limited metal uptake by roots (Fig. 5b), and inhibiting root metal uptake, which is an important mechanism for plants to detoxify heavy-metal-stress (Hall 2002). This result was in line with the outcome of Cui et al. (2012) who showed that hemin-pre-treatment inhibited Cd uptake of M. sativa roots. These outcomes suggested that alleviated effect of hemin, even partially, might be achieved by blocking metal uptake by roots, thus declined the biotoxicity suffered by plants. As to how can hemin limited absorption of metal, we inferred that it may be due to the decreased activity of respective transporters or due to the composition variation of cell walls, although this need to be further investigated.
In conclusion, hemin is an effective and beneficial additive which could significantly reduce heavy-metal-induced toxicity in rice seedlings. The retardation functions seem to be achieved by the following approaches: (1) up-regulating activities of SOD, APX and GR in roots; (2) promoting accumulation of antioxidants (AsA, GSH) in roots; (3) down-regulating productions of ROS (O2 •− and H2O2) in roots; (4) inhibiting metal accumulation in plants especially in root tissues; (5) finally resulting in longer root length and heavier biomass of underground; (6) counteracting the decrease of chlorophyll concentration, thus maintaining the net photosynthetic rate, and preserving the accumulation of biomass in shoots. Taken together, hemin can exhibit similar mitigative impacts on Zn, Pb and Cr stress.
Abbreviations
- APX:
-
Ascorbat peroxidase
- AsA:
-
Ascorbic acid
- Chl:
-
Chlorophyll
- Ci:
-
Intercellular CO2 content
- Cr:
-
Chromium
- DW:
-
Dry weight
- FW:
-
Fresh weight
- GR:
-
Glutathione reductase
- Gs:
-
Stomatal conductance
- GSH:
-
Glutathione
- H2O2 :
-
Hydrogen peroxide
- HO1:
-
Heme oxygenase 1
- O2 •− :
-
Superoxide radical
- Pb:
-
Lead
- Pn:
-
Net photosynthetic rate
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- Tr:
-
Transpiration rate
- Zn:
-
Zinc
References
Ait NA, Ater M, Sunahara GI et al (2004) Phytotoxicity and bioaccumulation of copper and chromium using barley (Hordeum vulgare L.) in spiked artificial and natural forest soils. Ecotoxicol Environ Saf 57:363–374
Ali S, Farooq MA, Jahangir MM et al (2013) Effect of chromium and nitrogen form on photosynthesis and anti-oxidative system in barley. Biol Plant 57:785–791
Ali S, Chaudhary A, Rizwan M et al (2015) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.) Environ Sci Pollut Res 22:10669–10678
Andrade SAL, Gratão PL, Schiavinato MA et al (2009) Zn uptake, physiological response and stress attenuation in mycorrhizal jack bean growing in soil with increasing Zn concentrations. Chemosphere 75:1363–1370
Anwaar SA, Ali S, Ishaque W et al (2014) Silicon (Si) alleviates cotton (Gossypiumhirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ Sci Pollut Res 22:3441–3450
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Azooz MM, Youssef MM, Al-Omair MA (2011) Comparative evaluation of Zinc and Lead and their synergistic effects on growth and some physiological responses of Hassawi Okra (Hibiscus esculentus) seedlings. Am J Plant Physiol 6:269–282
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Broadley MR, White PJ, Hammond JP et al (2007) Zinc in plants: Tansley review. New Phytol 173:677–702
Chen TH, Murata N (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–786
Cui WT, Li L, Gao ZZ, Wu HH et al (2012) Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J Exp Bot 63:5521–5534
Cui WT, Zhang J, Xuan W et al (2013) Up-regulation of heme oxygenase-1 contributes to the amelioration of aluminum-induced oxidative stress in Medicago sativa. J Plant Physio 170:1328–1336
Dai HP, Shan CJ, Zhao H et al (2015) The difference in antioxidant capacity of four alfalfa cultivars in response to Zn. Ecotox Environ Saf 114:312–317
Drazkiewicz M (1994) Chlorophyllase: occurrence, functions, mechanism of action, effects of external and internal factors (review). Photosynthetica 30(3):321–331
Farooq MA, Ali S, Hameed A et al (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf 96:242–249
Fu GF, Zhang LF, Cui WT et al (2011) Induction of heme oxygenase-1 with β-CD-hemin complex mitigates cadmium-induced oxidative damage in the roots of Medicago sativa. Plant Soil 345:271–285
Gajewska E, Sklodowska M, Slaba M et al (2006) Effect of nickel on antioxidative enzyme activities, praline and chlorophyll contents in wheat shoots. Plant Biol 50:653–659
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gill RA, Hu XQ, Ali B et al (2014) Genotypic variation of the responses to chromium toxicity in four oilseed rape cultivars. Biol Plant 58:539–550
Gill RA, Zang L, Ali B et al (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Habiba U, Ali S, Farid M et al (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res 22:1534–1544
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Hamid N, Bukhari N, Jawaid F (2010) Physiological responses of phaseolus vulgaris to different lead concentrations. Pak J Bot 42(1):239–246
Han Y, Zhang J, Chen X et al (2008) Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol 177:155–166
Han B, Yang Z, Xie YJ et al (2014) Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Mol Plant 7:388–403
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of anti-oxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plant 26:259–272
Hossain MA, Piyatida P, Silva JAT et al (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:1–37
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Jin QJ, Zhu KK, Xie YJ et al (2013) Heme oxygenase-1 is involved in ascorbic acid-induced alleviation of cadmium toxicity in root tissues of Medicago sativa. Plant Soil 366:605–616
Knudson LL, Tibbitts TW, Edwards GE (1977) Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiol 60:606–608
Kováčik J, Babula P, Hedbavny J et al (2014) Hexavalent chromium damages chamomile plants by alteration of antioxidants and its uptake is prevented by calcium. J Hazard Mater 273(3):110–117
Laura M, Gabriella B, Mohammad W et al (2014) Morphological changes induced by heavy metals in dandelion (Taraxacum officinale Web.) growing on mine soils. J Soils Sediments 14:731–743
Li JL, Zhu D, Wang R et al (2015) β-Cyclodextrin-hemin complex-induced lateral root formation in tomato: involvement of nitric oxide and heme oxygenase 1. Plant Cell Rep 34:381–393
Lin YF, Aarts MGM (2012) The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci 69:3187–3206
Lin CY, Hsiao WC, Huang CJ et al (2013) Heme oxygenase-1 induction by the ROS-JNK pathway plays a role in aluminum-induced anemia. J Inorg Biochem 128:221–228
Lingua G, Franchin C, Todeschini V et al (2008) Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environ Pollut 153:137–147
Liu D, Chen JR, Mahmood Q et al (2014) Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environ Sci Pollut Res 21:13615–13624
MacFarlane GR (2003) Chlorophyll a fluorescence as a potential biomarker of zinc stress in the grey mangrove, Avicennia marina. Bull Environ Contam Toxicol 70:90–96
Mishra S, Srivastava S, Tripathi RD et al (2006) Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 65:1027–1039
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Morina F, Jovanovic L, Mojovic M et al (2010) Zinc-induced oxidative stress in verbascum thapsus, is caused by an accumulation of reactive oxygen species and quinhydrone in the cell wall. Physiol Plant 140(3):209–224
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Mostofa MG, Hossain MA, Fujita M, Tran LSP (2015) Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci Rep. doi:10.1038/srep11433
Muramoto T, Tsurui N, Terry MJ et al (2002) Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol 130(4):1958–1966
Nakano Y, Asada K (1980) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Noriega G, Santa CD, Batlle A et al (2012) Heme oxygenase is involved in the protection exerted by jasmonic acid against cadmium stress in soybean roots. J Plant Growth Regul 31:79–89
Oliveira H (2012) Chromium as an environmental pollutant: insights on induced plant toxicity. J Bot 2012:1–8
Patsikka E, Kairavuo M, Šeršen F et al (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129:1359–1367
Pourrut B, Jean S, Silvestre J et al (2011) Lead-induced DNA damage in Vicia faba root cells: potential involvement of oxidative stress. Mutat Res 726:123–128
Rafique U, Ashraf A, Khan AK, Nasreen S, Rashid R et al (2010) Toxic chromium from tanneries pollute water resources and soils of Sialkot (Pakistan). J Chem Soc Pak 32(5):644–649
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98:560–564
Rodriguez E, Santos C, Azevedo R et al (2012) Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol Biochem 53:94–100
Sasaki T, Burr B (2000) International rice genome sequencing project: the effort to completely sequence the rice genome. Curr Opin Plant Biol 3(2):138–142
Shahid M, Dumat C, Pourrut B et al (2014a) Assessing the effect of metal speciation on lead toxicity to Vicia faba pigment contents. J Geochem Explor 144:290–297
Shahid M, Pourrut B, Dumat C et al (2014b) Heavy-metal-induced reactive oxygen species: phytotoxicity and physico-chemical changes in plants. Rev Environ Contam Toxicol 232:1–44
Shao G, Chen M, Wang W et al (2008) The effect of salinity pretreatment on Cd accumulation and Cd-induced stress in BADH-transgenic and nontransgenic rice seedlings. J Plant Growth Regul 27:205–210
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Singh DB, Varma S, Mishra SN (2002) Putrescine effect on nitrate reduc-tase activity, organic nitrogen, protein, and growth in heavy metal and salinity stressed mustard seedlings. Biol Plant 45:605–608
Thounaojam TC, Panda P, Mazumdar P et al (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Wang C, Zhang SH, Wang PF et al (2009) The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 75:1468–1476
Wheeler CR, Salzman JA, Elsayed NM et al (1990) Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem 184:193–199
Xie YJ, Cui WT, Yuan XX et al (2011) Heme oxygenase-1 is associated with wheat salinity acclimation by modulating reactive oxygen species homeostasis. J Integr Plant Biol 53:653–670
Xie YJ, Xu DK, Cui WT et al (2012) Mutation of Arabidopsis HY1 causes UV-C hypersensitivity by impairing carotenoid and flavonoid biosynthesis and the down-regulation of antioxidant defence. J Exp Bot 63:3869–3883
Xie YJ, Mao Y, Xu S et al (2015) Heme-heme oxygenase 1 system is involved in ammonium tolerance by regulating antioxidant defence in Oryza sativa. Plant Cell Environ 38:129–143
Xiong T, Leveque T, Shahid M et al (2014) Lead and cadmium phytoavailability and human bioaccessibility for vegetables exposed to soil or atmospheric pollution by process ultrafine particles. J Environ Qual 43:1593–1600
Xu Y, Shi GX, Ding CX et al (2011) Polyamine metabolism and physiological responses of Potamogeton crispus leaves under lead stress. Russ J Plant Physiol 58:460–466
Xuan W, Zhu FY, Xu S et al (2008) The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol 148:881–893
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Yang G, Miao C, Liang M et al (2010) Improvement of phytoextraction and antioxidative defense in solanum nigrum L. under cadmium stress by application of cadmium-resistant strain and citric acid. J Hazard Mater 181(1–3):771–777
Yannarelli GG, Noriega GO, Batlle A et al (2006) Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta 224:1154–1162
Zhang J, Kirkham MB (1996) Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol 132:361–373
Zhou W, Zhao D, Lin X (1997) Effects of water logging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napes L.) J Plant Growth Regul 16:47–53
Zia MH, Codling EE, Scheckel KG et al (2011) In vitro and in vivo approaches for the measurement of oral bioavailability of lead (Pb) in contaminated soils: a review. Environ Pollut 159:2320–2327
Acknowledgments
This work was financially supported by the national key research and development program (No. 2016YFD0800300) and the National Natural Science Foundation of China (No. 31572169).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Q., Zhang, X., Liu, Y. et al. Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul 81, 253–264 (2017). https://doi.org/10.1007/s10725-016-0202-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-016-0202-y