Abstract
In this study, we aimed to elucidate the defense mechanism of Alcea rosea (Linn.) Cavan. and Hydrangea macrophylla (Thunb.) Ser. against the single and compound toxicity of lead (Pb) and zinc (Zn) along with the synergistic effect of ethylenediaminetetraacetic acid (EDTA) in accumulation of metals in these two species. The two plant species were subjected to single metal treatment (Pb 1000 mg kg−1, Zn 600 mg kg−1) and compound metal treatment (Pb 1000 mg kg−1 + Zn 600 mg kg−1) in a greenhouse. Besides, different levels of EDTA were applied (2.5, 5.0, and 10.0 mmol kg−1) with compound metal treatment. Several physiological and biochemical parameters, including plant photosynthetic parameters, enzymatic antioxidant system, accumulation concentration of metals, and subcellular distribution were estimated. The results showed that the antioxidative enzymes, proline, root morphological changes, and metal localization all played important roles in resisting Pb and Zn toxicity. A notable difference was that Zn was concentrated in the roots (58.5%) of H. macrophylla to reduce the damage but in the leaves (38.5%) of A. rosea to promote photosynthesis and resist the toxicity of metals. In addition, Zn reduced the toxicity of Pb to plants by regulating photosynthesis, Pb absorption and Pb distribution in subcells. The biological concentration factors (BCF) and translocation factors (TF) for Pb in two plants were less than 1, indicating that they could be considered as phytostabilizators in Pb-contaminated soils. Moreover, EDTA could enhance the enrichment and transport capacity of Pb and Zn to promote the phytoremediation effect. In summary, both plants have a certain application potential for repairing Pb–Zn-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is an effective heavy metal pollutant. It enters the environment mainly through processes such as mining, metallurgy, manufacturing, and recycling activities (Ramana et al. 2021), while zinc (Zn) is an essential nutrient for plants and a heavy metallic element that pollutes the environment. Metal pollution has become a critical problem that has raised public concern because of the ability of metals to enter the food chain and their high persistence in the environment, particularly Pb (Chen et al. 2014). In plants, excessive amounts of Pb and Zn destroy the cell structure of the blade, causing photosynthetic inhibition, cell membrane lipid oxidation, and subsequent growth reduction (Chen et al. 2019, 2017). However, the effects of metal mixtures on plants are difficult to describe. Most metals exist in coexistence in nature (such as Pb and Zn). Therefore, soil metal pollution is generally a comprehensive pollution caused by the joint participation of various metals (Gao et al. 2019). The observed effects of Pb and Zn are either antagonistic or synergistic (Lanier et al. 2019; Zeng et al. 2020).
It is difficult to remediate metal-contaminated soil (Sheoran et al. 2011). Compared to some physical or chemical repair methods, phytoremediation technology is gradually becoming a promising and cost-effective technology in recent years (He et al. 2020; Kavousi et al. 2021). It can be used to protect the environment by using the natural capacity of green plants to remove contaminants or render toxic compounds harmless (Wang et al. 2018). Nonetheless, metal enrichment and the underlying mechanisms of different plants are often related to factors such as plant species and metal concentrations. Therefore, we tried to improve the extraction efficiency of heavy metals by plants of high biomass or by adding chelating agents, namely plant-chemical combined remediation, and this method has proved to be effective (Shahid et al. 2014).
Phytoremediation research generally focuses on hydrophytes and crops, and studies on ornamental plants are currently lacking. Ornamental plants do not enter the food chain, and the application of ornamental plants for the phytoremediation of contaminated soil has both economic and ecological benefits (Liu et al. 2018). Alcea rosea (Linn.) Cavan. and Hydrangea macrophylla (Thunb.) Ser. are two common ornamental plants. They are all native to China and have attracted more and more people’s attention. Moreover, they also have the advantages of high ornamental value, rapid growth, large biomass, and more importantly, they have a certain degree of resistance and enrichment capacity to heavy metals. So they are good materials for studying phytoremediation (Forte and Mutiti 2017; Huang et al. 2020) .
However, to our knowledge, phytoremediation potential of A. rosea and H. macrophylla under Pb and Zn stress has not been undertaken. To develop phytoremediation technology, it is necessary to study the tolerance and detoxification mechanism of these two plants to Pb and Zn (Dong et al. 2019). Therefore, the objectives of our study were: (i) to elaborate the tolerance mechanism of A. rosea and H. macrophylla to single and compound heavy metals; (ii) to study the effects of EDTA on the accumulation and detoxification of heavy metals in these two plants; and (iii) to evaluate the phytoremediation potential of A. rosea and H. macrophylla for Pb–Zn polluted soil.
Materials and method
Plant culture
The seeds of A. rosea were provided by the College of Landscape Architecture and Arts, Northwest A&F University (34°15′49″N, 108°3′42″E), and were stored in a dark and dry condition of 4℃. The healthy and consistent seeds were selected and immersed in 0.1% sodium hypochlorite (NaClO) solution for 15 min. After being cleaned with distilled water, the seeds were sown in the substrate to germinate, and 2 months later, the uniform and healthy plants were transplanted into flower pots with the experimental soil. The substrate used in this experiment was bought from the Yangling Linke Ecological Engineering Company (Shaanxi, China). The substrate contained organic matter (> 20%) required for plant growth. For H. macrophylla, the one-year-old cuttings that were healthy and growing consistently were chosen, and grown in the greenhouse.
Experimental design
The soil was collected from topsoil (0–15 cm) of farmland near the experiment base, and the properties were as follows: pH, 7.44; total nitrogen, 112 mg kg−1; available phosphorus, 7.80 mg kg−1; and available potassium, 219 mg kg−1. The collected soil was air-dried in a dry and ventilated place, completely dehydrated and crushed, and sieved with a 4-mm screen to remove other impurities. The soil was artificially contaminated with Pb (Pb(NO3)2) and Zn (ZnSO4·7H2O), and then placed in the shade for 30 days (Zeng et al. 2020). All treatments in this experiment were as follows: (1) control (CK); (2) 1000 mg kg−1 Pb applied as Pb(NO3)2 (Pb1000); (3) 600 mg kg−1 Zn applied as ZnSO4·7H2O(Zn600); and (4) 1000 mg kg−1 Pb(NO3)2 + 600 mg kg−1 ZnSO4·7H2O (Pb1000 + Zn600). To examine the specific mediated effects of EDTA on Pb–Zn combined treated plants, we designed those experiment as follows: (5) 2.5 mmol kg−1 EDTA + Pb1000 + Zn600 (EDTA2.5); (6) 5 mmol kg−1 EDTA + Pb1000 + Zn600 (EDTA5); and (7)10 mmol kg−1 EDTA + Pb1000 + Zn600 (EDTA10).
The treated soil was placed in the pots cleaned in advance, each contained 6.0 kg of artificially contaminated soil. A healthy plant was planted in each pot, and each treatment was repeated three times. The experiment was conducted from 07/11/2020 to 27/12/2020, harvest was carried out after 50 days of continuous growth, and EDTA was added 10 days before harvest (Qureshi et al. 2020). The average temperature of the greenhouse was maintained at 20 °C to 25 °C, with natural light irradiation at 10/14 h (day/night) photoperiod. Tap water was applied every two days to replenish lost water and controlled to approximately 70% of the soil field capacity (Yang et al. 2020).
Measurement of growth parameters
After the experiment, plant height and root length of each treatment were measured and recorded immediately. All roots need to be thoroughly washed with distilled water and deionized water. After that, the plant was divided into roots, stems and leaves, drained of water, sterilized at 105 °C for 30 min, and then dried at 70 °C for constant weight (Li et al. 2020). Dry biomass was determined and the fresh samples of the plants were stored at -80 °C for further physiological analysis.
Determination of photosynthetic pigments
The photosynthetic pigments was measured according to Gao (2006). 0.2 g of the fresh leaves were collected and placed in glass tubes. Then, 25 mL of 95% ethanol was added to fresh samples and kept for 24 h under dark conditions for complete extraction. After that, absorbance of the extract was recorded at 663, 646, and 440 nm, respectively.
Assessment of lipid peroxidation and proline content
Lipid peroxidation was measured using fresh samples and indicated in terms of malondialdehyde (MDA) content as described by Li (2000). 0.5 g fresh leaf samples were homogenized with 10 mL of 10% trichloroacetic acid (TCA) and centrifuged at 4000 rpm for 10 min. Then, 2 mL of supernatant was added to 2 mL of 0.6% thiobarbituric acid (TBA), incubated at 95 °C for 15 min, quickly cooled in ice-bath, and centrifuged again. The absorbance of supernatant was recorded at 450, 532 and 600 nm, respectively.
Proline (Pro) concentration was estimated using acid ninhydrin solution as described by method of Alia and Saradhi (1991). Fresh leaves (0.5 g) were homogenized in 10 mL of 3% aqueous solution of sulfosalicylic acid. 2 mL of the supernatant, 2 mL of glacial acetic acid, and 2 mL of ninhydrin acid reagent were placed in a test tube at 100 °C for 30 min. After cooling down, the reaction was extracted with 4-mL toluene and centrifuged at a speed of 3000 rpm for 5 min. The supernatant was aspirated to determine the absorbance at 520 nm.
Extraction and assay of antioxidant enzymes
0.5 g fresh leaves were put into a precooled mortar with 0.05 M phosphate buffers (pH 7.8), and then the homogenates were centrifuged at 10,000 rpm for 20 min at 4 °C. The supernatant was collected for measuring the activities of antioxidant enzymes.
Superoxide dismutase (SOD) activity was determined by photochemical method according to Beauchamp and Fridovich (1971). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.8), 75 mM nitroblue tetrazolium (NBT), 13 mM methionine, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 2 mM riboflavin, and appropriate amount of enzyme extract. Test tubes were shaken and placed below a light source of 4000 Lx. Then, the absorbance of the extract was read at 560 nm immediately. Unit of SOD was defined as the amount of enzyme required to generate 50% inhibition of the NBT reduction.
The activity of peroxidase (POD) was measured according to method of Meng et al. (2007). The reaction medium consisted of 25 mM guaiacol, 25 mM H2O2, and 0.1 mL enzyme extract. Then, the absorption was determined spectrophotometrically at a wavelength of 470 nm. One unit of POD activity was calculated as an absorbance change of 0.01 units min−1.
Catalase (CAT) activity was determined by decomposition of H2O2 using the method of Aebi (1984). Then, 0.5 mL enzyme extract and 20 mM H2O2 were added to reaction mixture, and the activity was measured spectrophotometrically by evaluating the absorbance at 240 nm. One unit of CAT activity was defined as an absorbance change of 0.01 units min−1.
Pb and Zn concentrations in different plant tissues and soil
The metal concentration in soil and plant tissues was determined according to the method of Muro et al. (2020) with appropriate modifications. Dry samples were ground into a uniform powder and screened through a 2-mm sieve. 0.2 g of plant samples and 0.1 g of soil samples were each weighed accurately and were added to a microwave digestion tank with 6 mL of concentrated HNO3, and digestion was carried out in a microwave digestion instrument (MA165-001, Italy). After the digestion was completed, the digestion solution was transferred to a 50-mL volumetric flask in batches in the rinsing digestion tank, and the volume was fixed with ultra-pure water. After shaking, the concentration of metal was determined by flame atomic absorption spectrometer (PinaACIIE, American PE900). The chemical reagents used in this study were all super pure, all digestion and heavy metal analysis were repeated three times, and correction analysis was performed with blank reagents. The standard reference plant material (GBW-07603) and soil material (GBW-07405) obtained from the National Center for Standard Materials (Beijing, China) were used to validate the accuracy of metal determination. The recoveries of Pb and Zn in GBW-07604 were 102.3% and 94.0%, respectively, while those of Pb and Zn in GBW-07401 were 102.1% and 95.1%.
Biological concentration factors (BCF) and translocation factors (TF)
Biological concentration factors (BCF) and translocation factors (TF) are used to evaluate the phytoextraction ability of the two plants for Pb and Zn:
Separation of Pb and Zn subcellular fractions
To study the effects of single metal and compound metal on the distribution of subcellular in plants, subcellular distribution analysis of plants were conducted. The subcellular fraction in plants was separated according to the method of Weigel and Jager (1980) with appropriate modifications. Briefly, 0.03 g of roots, stems and leaves were accurately weighed and each mixed with 20 mL of precooled 50 mM Tris–HCl buffer (pH 7.5, containing 0.25 M sucrose and 1.0 mM dichloro-diphenyl-trichloroethane). The homogenate was centrifugated under 6700 rpm for 10 min, and the sediment part was the cell wall fraction (F1). The supernatant was centrifuged at 14,000 rpm for 45 min, the sediment was the cell membrane and organelle parts fraction (F2), while the supernatant was soluble cell component fraction (F3). All of the steps were completed at 4℃. The F1, F2 and F3 were placed in the oven to dry at 70℃. After drying, they were digested with microwave digestion instrument. The content of metals was determined by flame atomic absorption spectrometer.
Statistical analysis
Data were presented as three independently repeated mean ± SD. The results were statistically analyzed using ANOVA at a significance level of p < 0.05, with SPSS version 26.0 (IBM SPSS, Armonk, NY, USA). LSD test was used to detect differences between treatments at a significance level of p < 0.05. All diagrams were drawn using Origin 2018 (OriginLab, Northampton, MA, USA).
Results
Plant growth
As for the height of A. rosea, application of Zn at 600 mg kg−1 resulted significantly higher (p < 0.05) than the control, while Pb applied either alone at 1000 mg kg−1 or in combination with Zn at 600 mg kg−1 had no significant effect on the plant height compared to the control. The plant height was decreased under all metal treatments of H.macrophylla. As compared with Pb treatment alone, the height of H.macrophylla was increased by adding Zn. EDTA application had different effects on the heights of the two plants. With the addition of EDTA, the height of A. rosea increased, but the different concentrations of EDTA had no significant effect. Conversely, the height of H.macrophylla decreased and reached the minimum value at EDTA10.
For A. rosea, root length increased under all treatments except with the Pb1000 treatment and combined treatment. (The root length was smaller than that of the control in the both cases.) Meanwhile, the root length of H. macrophylla decreased in the single Pb treatment compared with the control, and the situation was improved after Zn was added. EDTA inhibited the root growth, and reached the lowest value at 10 mg kg−1 of EDTA.
As shown in Table 1, root biomass of A. rosea was significantly increased under Pb1000 (P < 0.05), stem biomass was increased under almost all treatments, and leaf biomass was significantly increased under combined treatments (P < 0.05). On the contrary, the biomass of stem and leaf of H. macrophylla was almost inhibited under Pb1000 and combined treatment. The certain concentration of EDTA could significantly increase the biomass of stem and leaf of A. rosea (P < 0.05), while the biomass of stem and leaf of H. macrophylla was significantly inhibited under all EDTA treatments, and all reached the minimum value at EDTA10.
Photosynthetic pigments
The content of photosynthetic pigments in the leaves of A. rosea and H. macrophylla is shown in Fig. 1a,b. It can be observed that the chlorophyll a and chlorophyll b content of A. rosea was generally higher than the pigment content of H. macrophylla. The EDTA treatments showed considerable effects on chlorophyll a and chlorophyll b concentrations of H. macrophylla relative to the control group (Fig. 1b). In addition, Pb–Zn stress (Pb1000 + Zn600) resulted in significant negative impacts on the chlorophyll a content of A. rosea (Fig. 1a). With single Pb exposure, the carotenoid content of A. rosea and H. macrophylla was significantly (p < 0.05) higher than that of the control group, whereas there were no significant differences with single Zn exposure. The highest content of pigment content (0.93 mg g−1 chlorophyll a, 0.46 mg g−1 chlorophyll b and 0.16 mg g−1 carotene) for A. rosea was observed in the Pb1000 group. The lowest chlorophyll a and b contents were observed with the addition of EDTA5.
Photosynthetic pigments (a,b), malondialdehyde (MDA) content (c,d), and proline (Pro) content (e,f) changes in leaves of Alcea rosea and Hydrangea macrophylla under different treatments. Error bars represent standard deviation. The letters indicate the significant differences among different treatments at p < 0.05. CK, control; Pb1000, 1000 mg kg−1 Pb(NO3)2; Zn600, 600 mg kg−1 ZnSO4·7H2O; Pb1000 + Zn600, 1000 mg kg−1 Pb(NO3)2 + 600 mg kg−1 ZnSO4·7H2O; EDTA2.5, 2.5 mmol kg−1 EDTA + 1000 mg kg−1 Pb(NO3)2 + 600 mg kg−1 ZnSO4·7H2O; EDTA5, 5 mmol kg−1 EDTA + 1000 mg kg−1 Pb(NO3)2 + 600 mg kg−1 ZnSO4·7H2O; EDTA10, 10 mmol kg−1 EDTA + 1000 mg kg−1 Pb(NO3)2 + 600 mg kg−1 ZnSO4·7H2O
Malondialdehyde and proline contents
Exposure to Pb or Zn alone significantly (p < 0.05) increased the malondialdehyde (MDA) content in the leaves of A. rosea and H. macrophylla (Fig. 1c,d). The MDA content was lower in plants exposed to both metals than in those exposed to Pb alone. Meanwhile, a noticeable increase in MDA contents was present in plants grown in EDTA groups compared to control group. In A. rosea, the greatest increase (18.39 umol g−1) in MDA level occurred for plants treated with EDTA5, whereas the MDA level upon treatment with EDTA10 was slightly lower (8.23 umol g−1) than EDTA2.5, as shown in Fig. 1c.
The proline (Pro) content in the leaves of A. rosea and H. macrophylla after treatment with CK, Pb1000, Zn600, and Pb1000 + Zn600 showed the same trends (Fig. 1e,f). The Pro content increased with single metal exposure, whereas it decreased with Pb and Zn exposure together. In addition, Pro content reduced significantly (P < 0.05) in a concentration-dependent manner after the addition of EDTA for A. rosea, and the highest Pro content (2059.46 ug g−1) appeared in the EDTA2.5 group. There were no significant differences of Pro content under the EDTA2.5 and EDTA5 for H. macrophylla.
Antioxidant enzyme activities
The elevated activities of antioxidant enzymes (SOD, POD, and CAT) were recovered in plants treated with different amounts of metals compared to the activities in the control (Fig. 2). The addition of EDTA in A. rosea induced a significant increase in SOD activity (Fig. 2a). This variable in EDTA10 A. rosea reached the maximum amount of 4.27–4.44 U g−1 h−1, and in Zn600, A. rosea reached the minimum amount of 2.42–2.44 U g−1 h−1. There were no significant differences in SOD activity between the single metal treatments of H. macrophylla, but the activity decreased with treatment of the mixture of Pb and Zn (Fig. 2b). Similarly, Pb or Zn stress significantly (p < 0.05) increased CAT activity in both species compared with the control (Fig. 2e,f). In contrast, the combined treatment caused a significant (p < 0.05) decrease in CAT activity in both species compared to a single metal. It can be seen that the highest CAT activity (6.56 U g−1 h−1) of A. rosea appeared in Pb1000 group, while appeared in Zn600 group for H. macrophylla (4.83 U g−1 h−1). Overall, the Pb and Zn mixtures appeared to be less toxic than either metal alone. The activity of POD in A. rosea was lower than that in H. macrophylla under different treatments (Fig. 2c,d). For H. macrophylla, the activity of POD increased in a EDTA concentration-dependent manner, and it reached the maximum amount of 133.60 U g−1 h−1. Compared with the control group, the POD activity of the Pb1000 and Zn600 groups increased significantly (p < 0.05), while there was no significant difference from that of the control group in the Pb1000 + Zn600 group for A. rosea.
Activities of SOD (a,b), POD (c,d), and CAT (e,f) changes in leaves of Alcea rosea and Hydrangea macrophylla under different treatments. Error bars represent standard deviation. The letters indicate the significant differences among different treatments at p < 0.05. CK, control; Pb1000, 1000 mg kg-1 Pb(NO3)2; Zn600, 600 mg kg-1 ZnSO4·7H2O; Pb1000 + Zn600, 1000 mg kg-1 Pb(NO3)2 + 600 mg kg-1 ZnSO4·7H2O; EDTA2.5, 2.5 mmol kg-1 EDTA + 1000 mg kg-1 Pb(NO3)2 + 600 mg kg-1 ZnSO4·7H2O; EDTA5, 5 mmol kg-1 EDTA + 1000 mg kg-1 Pb(NO3)2 + 600 mg kg-1 ZnSO4·7H2O; EDTA10, 10 mmol kg-1 EDTA + 1000 mg kg-1 Pb(NO3)2 + 600 mg kg-1 ZnSO4·7H2O
Pb and Zn metal accumulation
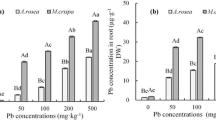
As shown in Table 2, the absorption of Pb and Zn by the two plants was different. Under single Pb 1000 treatment, the Pb concentrations of A. rosea and H. macrophylla were in the order of root > leaf > stem. However, with the addition of EDTA, this turned into root > stem > leaf (except for the different cases of the H. macrophylla treated with EDTA10). Under the treatment of Zn 600, the Zn content of A. rosea was leaf > root > stem, and that of H. macrophylla was root > stem > leaf, and turned into root > leaf > stem after the addition of EDTA (except for different conditions in the treatment of EDTA10). Under the treatment of Pb1000 + Zn600, A. rosea’s absorption of Pb and Zn decreased, while H. macrophylla’s absorption of Pb decreased, but its absorption of Zn increased. Under EDTA treatment, the concentrations of Pb and Zn in different parts of H.macrophylla almost increased, which was the same as Pb in A. rosea. However, under EDTA treatment, the concentration of Zn in the stems and leaves of A. rosea decreased (compared with the combined treatment).
The BCF of A. rosea and H. macrophylla for Pb were less than 1. The BCF and TF of two plants for Pb and Zn in the combined treatment were almost unchanged from those of single metal treatment (Table 3). With the increase in EDTA concentration, the BCF of Pb and Zn in A. rosea and H. macrophylla showed a trend of increasing and then decreasing. The change of TF value showed a completely opposite trend, which decreased and then increased.
Pb and Zn subcellular distribution
As shown in Fig. 3, most of the Pb in A. rosea cells was distributed in the cell wall and organelle fractions in the Zn600 treatment. Under the combined treatment, the Pb content in the cell wall fraction of A. rosea root cells increased, while that in other fractions decreased. The Pb content in the roots and leaves of H. macrophylla was mainly distributed in the cell wall and soluble fractions of the cells. The Pb content in the stem was mainly distributed in the organelle and cell wall fractions. Under the combined treatment, the Pb content in the organelle fraction and cell wall fractions in the roots and stems decreased, while that in the soluble fraction of the cells increased significantly.
The distribution of Zn in the cells of the two plants was significantly different from that of Pb. Zn content in A. rosea was mainly distributed in the soluble and organelle fractions. Under combined treatment, only Zn content in the organelle fraction in roots decreased, while that in other organelle fractions showed little change. The Zn content in the roots and leaves of H. macrophylla was mainly distributed in the organelle fraction. Under compound treatment, the Zn content in the root organelle fraction decreased. The Zn content in the organelle fraction of the leaves and stems increased.
Discussion
Plant growth
Roots are the only carrier for the connection between plants and soil and the only way for plants to obtain material and energy in the soil, which largely determines the overall growth of plants (Usharani and Vasudevan 2017) . Lead is toxic to plant growth, while Zn is an essential micronutrient for enzymatic activation that can cause toxicity in excess amounts (Shen et al. 2019). The excessive absorption of Pb from the soil by roots inhibits the development of lateral roots and reduces root growth rate, thus impeding the development of plant roots and affecting overall plant height and biomass (Rizvi and Khan 2017) . In addition, the inhibition of root development is a resistance mechanism in plants under Pb stress. The obvious reduction in root growth may due to the decrease in cell division, which leads to an increase in the cell wall thickness of roots when exposed to metals (Batool et al. 2015). However, the results of our study showed that biomass of A. rosea increased with single Pb exposure, which may be due to the fact that A. rosea is a Pb-tolerant plant. This positive effect on the tolerant plants was explained by the phenomenon of hormesis (Peco et al. 2020), which was similar to Solanum nigrum L. in previous studies (Xu and Wang 2014) . What’s more, the root length of A. rosea increased under Zn stress, which may be because Zn is essential and beneficial to plant growth, or Zn stress in this study stimulated the root growth of A. rosea and caused a hormesis response. The same phenomenon were observed in other plants (Xu and Wang 2014). Zn can reduce the toxic effects of metals in plants because it is a component or auxiliary component of many important enzymes in plants, which can effectively synergize or antagonize the absorption and change the absorption intensity of metals in plants to reduce Pb toxicity (Tabelin et al. 2018). In this study, the damage to plant morphology, including plant height and root growth, under Pb–Zn combined treatment was significantly (p < 0.05) less than that under Pb alone treatment for H. macrophylla. It appears that H. macrophylla is more sensitive to Pb toxicity, and the addition of Zn may help to reduce the negative effects.
The mechanism of action of EDTA differed in the root systems of the two plant species. EDTA increased the absorption of metals by promoting root development in A. rosea. Combined with Table 3, EDTA treatment significantly increased the absorption of Zn by A. rosea, which led to the growth of A. rosea’s root. The growth of the root promoted more contact between the root and the soil, which in turn allowed more metal absorption. However, the root length of H. macrophylla decreased because EDTA may damage root cell membranes due to the chelation of Zn and Ca that stabilize the membrane, thereby destroying the physiological barriers of the roots, resulting in the excessive uptake of metals, consequently reducing plant growth (Glińska et al. 2014).
Photosynthetic pigments, MDA, and Pro contents
Metals cause damage to the photosynthetic system of leaves, which is a key toxic mechanism. The content of chlorophyll a in the leaves of H. macrophylla decreased under single Zn stress, indicating that metals can combine with an active gene, such as protein -SH, or replace trace elements, such as iron (Fe), zinc (Zn), copper (Cu), or magnesium, thereby directly destroying chlorophyll and affecting chlorophyll synthesis. There were no significant differences of chlorophyll a content with single Pb exposure and composite stress, indicating that the damage were not enough to inhibit chlorophyll biosynthesis. In contrast, the chlorophyll content of A. rosea slightly increased. It may be the compensatory growth caused by the interaction of multiple factors such as temperature to maintain the structure and function of the chloroplast, which was consistent with the research conclusions of He (2020). At the same time, carotenoids could act as antioxidants to scavenge active oxygen (Qureshi et al. 2020). The increased carotenoid content could prevent the peroxidation of membrane lipids and maintain the stability of the membrane structure. The chlorophyll a content decreased with the application of EDTA for H. macrophylla. This may be because EDTA increased the fluidity of metals in the soil, resulting in an increase in toxicity to plants and consequently decreasing the chlorophyll content in the plant. Another possible reason may be that EDTA threatened the normal growth of plants, affecting the absorption and conversion of nutrients and moisture by the plant roots, eventually leading to the normal synthesis of chlorophyll in the plant.
Metal stress promotes lipid peroxidation by generating free radicals (Li et al. 2017). The MDA content increases, to a certain extent, with the increase in free radical concentrations, such as those of -OH and O2−, and the toxic effect of metals on plants. Our study showed that the MDA contents in the leaves of H. macrophylla and A. rosea under all treatments significantly (p < 0.05) increased compared with those in the control, indicating that cell injury and degree of oxidation of the plasma membrane were further intensified. In this study, the Pro content in the plant blades increased under single metal stress and decreased under composite stress. This indicated that Pro content in the blade decreased under composite stress, during which the osmotic pressure and moisture in the cells were adjusted by soluble sugars and proteins in the plant. Pro was synthesized under single stress or with the addition of EDTA and played a role in resistance to metals (Barceló and Poschenrieder 1990).
In short, it is clear that A. rosea has a more selective response depending on the type of treatment. A. rosea appears to have a greater ability to tolerate metals, and modifying some biological properties to support these treatments. But in the case of H. macrophylla, it basically increased all these variables (MDA and Pro content), which may indicate that in almost all treatments, H. macrophylla is having some negative effect.
SOD, POD, and CAT activities
To scavenge excessive active oxygen under adverse conditions, plants have evolved a set of complex antioxidant defense mechanisms (Hossain et al. 2012; Zhang et al. 2012). Under metal stress, SOD is used as the first line of defense in the enzymatic antioxidant system, and O2− is removed and transformed into H2O2. H2O2 produced by SOD is then further converted to H2O and O2 by CAT and POD (Irfan et al. 2014). Therefore, CAT and POD are the catalysts that decompose H2O2 produced by SOD. In our study, the results showed that SOD activity in H. macrophylla significantly (p < 0.05) improved under a single metal stress or with EDTA addition, which was related to the large amount of H2O2 produced in H. macrophylla. However, opposite results were found in A. rosea against Pb or Zn, with strong inhibition of SOD activity in the leaves, which indicated that antioxidant enzymes behaved differently in different plants. Actually, antioxidant protection system of plants was a complex system regulated by multiple parties. In addition to antioxidants, other substances such as carotenoids and proline could also play a role in resisting ROS damage. Similar results (SOD activity decreased and POD activity increased) were reported in other Pb-tolerant plants, such as Eclipta prostrata (L.) L. (Chandrasekhar and Ray 2019) and Coronopus didymus (L.) J. E. Smith (Sidhu and Batish 2016) . The POD and CAT activities of the two plant species under metal stress or with the addition of EDTA improved to varying degrees, which showed that they could remove excessive active oxygen, compared with the activities in the control. Meanwhile, the damage to plants under composite stress was less than that under a single metal stress. In general, antioxidant enzymes, including SOD, POD, and CAT, participate in the detoxification of Pb or Zn, thus providing beneficial effects for the improvement of plant growth.
Pb and Zn metal accumulation
The total content of Zn concentrations in both plant species under almost all treatments were higher than those of Pb in the study, although the amount of Pb in soil was much higher than that of Zn. (The elements contained in the plants originally was considered.) Adsorption in the solid phase of soil profoundly affects the mobility and unstable parts of metals in soil (Liu et al. 2019), and the metal affinity of soil is of the order Pb > Cu > nickel ≥ cadmium ≈ Zn (Basta and Tabatabai 1992). Zn is more mobile than Pb in the soil and an essential element for plants, thus is more readily absorbed by plants. Pb was toxic to both species of plants, but the preferential accumulation of Pb in the roots indicated their stress tolerance strategies (Marques et al. 2017). The difference in Zn distribution in the two plants also indicated the difference in Zn tolerance mechanisms between the two plants. Zn was concentrated in the roots of H. macrophylla to reduce damage, whereas it was concentrated in the leaves of A. rosea to promote photosynthesis and produce large amounts of nutrients that resist damage.
In this study, the concentrations of Pb decreased in all parts of the two plant species under Pb + Zn treatment, indicating that Zn decreased Pb uptake and accumulation in tissues, which is also a mechanism by which Zn reduces Pb toxicity in plants. In other words, Pb absorption was reduced when it occurred at the same time as that of Zn, indicating competition between the two metals (Srivastava et al. 2014). This phenomenon can be explained based on the findings of previous studies. Zn and Pb compete for metal transporters in roots, such as Zn/Fe-regulated transporter-like protein (ZIP) and Fe-regulated transporter (IRT) (Liu et al. 2017), thus controlling their transfer from the roots to the aboveground parts of plants and inhibiting the transmembrane transport of Pb (Wang et al. 2020). In addition, Zn stress promotes the lignification of cell walls, which further prevents the metal from entering the roots (Lin and Aarts, 2012). Simultaneously, the presence of Pb promoted Zn absorption by H. macrophylla, and the same phenomenon has been observed in Ricinus communis L. (Yi 2018). The synergistic transport effect of Pb on Zn may lead to greater toxicity of Zn to H. macrophylla than of Pb.
EDTA promoted the absorption and transport of Pb by A. rosea and H. macrophylla. At a concentration of 5 mmol kg−1, EDTA had the best effect on enhancing the ability of the two plants to accumulate metals, but it reduced the dry weight of the plant to a certain extent, especially for H. macrophylla. The current study showed that 10 mmol kg−1 of EDTA decreased Pb accumulation in the roots of the studied plants, especially in H. macrophylla. This reduction may be due to the side effects of EDTA on root growth (Shahid et al. 2014). EDTA elevated Pb and Zn concentrations in plants as well as the BCF and TF for the two metals, which was in accordance with the results of previous studies (Kumar et al. 2017; Kalyvas et al. 2018). EDTA can mobilize As, Pb and Zn in metal-polluted soils. Meers et al. (2005) studied Zn mobilization in metal-contaminated soil with EDTA and found that its mobility increased. EDTA have the ability to increase Pb desorption from the soil solid phase to the soil solution, which enhances the availability of Pb in the soil and increases Pb mobility in plants (Kumar et al. 2017). In the presence of EDTA, the metal–EDTA complex affects the pathway of almost all metals into plant roots (Garcia et al. 2017). In addition, physical damage (e.g., damage from metals and chelating agents) increases the absorption of metal–EDTA by the plant roots (Jiang et al. 2019). Therefore, with the increase in uptake of metals in plants, damage to plants can also occur.
In contrast to the above results, the Zn concentration, BCF, and TF values of A. rosea decreased with EDTA addition. We have also observed this phenomenon in previous studies. The TF value of Zn decreased sharply after the addition of EDTA poplar cultivation (Lingua et al. 2014). This maybe because A. rosea can absorb high amounts of Zn under normal soil conditions, and the addition of EDTA damaged root physiology (Glińska et al. 2014), reduced plant vitality and absorption capacity, and resulted in a decrease in Zn absorption. Without the addition of EDTA, the BCF and TF values for both Pb and Zn in H. macrophylla were less than 1, indicating that it had characteristics of Pb and Zn exclusion. A. rosea excludes Pb but not Zn. One of the important characteristics for measuring hyperaccumulators is that both BCF and TF values need to be greater than one. Obviously, the two plants does not meet the hyperaccumulator criteria, but they could be good phytostabilizators in Pb-contaminated soils. The phytostabilization is the technique that is used to reduce the mobility and bioavailability of pollutants in the environment, thus preventing their migration to groundwater or their entry into the food chain. Moreover, adding EDTA plays an important role in increasing accumulation of heavy metals and phytoremediation effect. With the addition of EDTA, the BCF and TF values in the two plants were increased, especially in the presence of EDTA 5 mmol kg−1. This indicated that both plants had great potential to accumulate heavy metals, so they could be appropriate candidates for repairing Pb–Zn-contaminated soil by phytoremediation.
Pb and Zn subcellular distribution
In the root of both plant species, Pb was partly enriched in the cell wall fraction and Zn in the soluble fraction, which reflected the resistance mechanism of the two plants to different metals. The precipitation of the cell wall was more important in Pb resistance, while the localization of soluble fraction (mainly vacuoles) was more important in resisting Zn. These two processes form the mechanism of plant resistance to metals at the subcellular level. Plants can fix metals in areas of low biological activity to reduce the impact of metals on normal life activities (Zhang et al. 2019). Because cell walls contain large amounts of pectin, polysaccharides, and proteins, Pb is heavily bound to the cell wall to protect protoplasts from damage (Zeng et al. 2020). Most of the Zn can penetrate the cell wall and enter the protoplast, where it is separated into vacuoles (Lasat et al. 1998). The soluble fraction composed mostly of vacuoles is rich in thiopeptides and organic acids, which can form organic coordination compounds by complexing with metal ions, thereby reducing metal ion activity and immobilizing metal ions, so that it reduces toxicity (Xin et al. 2018). Under the combined treatment, the Pb contents in the organelle fraction in root of A. rosea and H. macrophylla decreased, which may be a reason why Zn reduced Pb poisoning to a certain extent.
Conclusion
This study demonstrated that A. rosea and H. macrophylla had strong resistance to Pb and Zn pollution. Root system and photosynthetic pigments played the critical role in the detoxification of Pb and Zn. Their MDA, Pro contents, POD, and CAT activities increased, which could more effectively deal with the toxic effects of metals. The deposition of cell walls and the ventralization of vacuoles could not be ignored. Pb mainly accumulated in roots to reduced toxicity. However, Zn content was mainly concentrated in the leaves (38.5%) of A. rosea and in the roots (58.5%) of H. macrophylla. The BCF and TF values for Pb in two plants were less than 1. In addition, Zn reduced the toxicity of Pb to plants by regulating photosynthesis, Pb absorption, and distribution in subcells.
EDTA promoted root growth in A. rosea to absorb more metals, whereas it induced root damage in H. macrophylla. Meanwhile, it increased proline content and antioxidant enzyme activity of two plants. Although EDTA reduced the dry weight of the plants to a certain extent, especially for H. macrophylla, EDTA could elevate the BCF and TF values to improve the phytoremediation effect. In short, both plants had a certain application potential for repairing Pb–Zn-contaminated soil. Moreover, our results provided helpful reference for further study on the defense mechanism of plants against metals, optimization of phytoremediation.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Alia PP, Saradhi (1991) Proline accumulation under heavy metal stress. J Plant Physiol 138:554–558. https://doi.org/10.1016/s0176-1617(11)80240-3
Basta NT, Tabatabai MA (1992) Effect of cropping systems on adsorption of metals by soils. Soil Sci 153(2):108–114. https://doi.org/10.1097/00010694-199203000-00004
Barceló J, Poschenrieder Ch (1990) Plant water relations as affected by heavy-metal stress-a review. J Plant Nutr 13(1):1–37. https://doi.org/10.1080/01904169009364057
Batool R, Hameed M, Ashraf M, Sajid M, Ahmad A, Fatima S (2015) Physioanatomical responses of plants to heavy metals. In: Oztürk M, Ashraf M, Aksoy A, Ahmad MSA (eds) Phytoremediation for Green Energy. Springer, Netherlands, pp 79–96
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to PAGE. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Chandrasekhar C, Ray JG (2019) Lead accumulation, growth responses and biochemical changes of three plant species exposed to soil amended with different concentrations of lead nitrate. Ecotox Environ Safe 171:26–36. https://doi.org/10.1016/j.ecoenv.2018.12.058
Chen DM, Chen DQ, Xue RR, Long J, Lin XH, Lin YB, Jia LH, Zeng RS, Song YY (2019) Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J Hazard Mater 367:447–455. https://doi.org/10.1016/j.jhazmat.2018.12.111
Chen JR, Zhong B, Shaff M, Guo J, Wang Y, Wu JS, Ye ZQ, He LZ, Liu D (2017) Effect of lead (Pb) on antioxidation system and accumulation ability of Moso bamboo (Phyllostachys pubescens). Ecotox Environ Safe 138:71–77. https://doi.org/10.1016/j.ecoenv.2016.12.020
Chen LH, Gao S, Zhu P, Liu Y, Hu TD, Zhang J (2014) Comparative study of metal resistance and accumulation of lead and zinc in two poplars. Physiol Plant 151(4):390–405. https://doi.org/10.1111/ppl.12120
Dong X, Yang F, Yang S, Yan C (2019) Subcellular distribution and tolerance of cadmium in Canna indica L. Ecotox Environ Safe 185(15):109692. https://doi.org/10.1016/j.ecoenv.2019.109692
Forte J, Mutiti S (2017) Phytoremediation potential of Helianthus annuus and Hydrangea paniculata in copper and lead-contaminated soil. Water Air Soil Pollut 228:77. https://doi.org/10.1007/s11270-017-3249-0
Gao HB, Yu PY, Sun YJ, Jiang LJ (2019) Analysis of carbon metabolism and differential expression of related enzyme genes in castor leaves under Pb and Zn stress. J Plant Physiology 55(4):483–492. https://doi.org/10.13592/j.cnki.ppj.2018.0456
Gao JF (2006) Experimental guidance of plant physiology. Higher Education Press, Beijing, pp 210–211
Garcia S, Zornoza P, Hernndez LE, Esteban E, Carpena RO (2017) Response of Lupinusalbus to Pb-EDTA indicates relatively high tolerance. Toxicol Environ Chem 99(9–10):1378–1388. https://doi.org/10.1080/02772248.2017.1387263
Glinska S, Michlewska S, Grapinska M, Seliger P (2014) The effect of EDTA and EDDS on lead uptake and localization in hydroponically grown Pisumsativum L. Acta Physiol Plant 36(2):399–408. https://doi.org/10.1007/s11738-013-1421-8
He C, Zhao YP, Wang FF, Kokyo O, Zhao ZZ, Wu CL, Zhang XY, Chen XP, Liu XY (2020) Phytoremediation of soil heavy metals (Cd and Zn) by castor seedlings: Tolerance, accumulation and subcellular distribution. Chemosphere 252:126471. https://doi.org/10.1016/j.chemosphere.2020.126471
Huang Y, Zu LH, Zhang M, Yang T, Zhou MZ, Shi C, Shi FC, Zhang WJ (2020) Tolerance and distribution of cadmium in an ornamental species Althaea rosea Cavan. Int J Phytoremediat 22(7):713–724. https://doi.org/10.1080/15226514.2019.1707771
Hossain MA, Fujita M, Jaime A, Silva T, Piyatida P (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:1–37. https://doi.org/10.1155/2012/872875
Irfan M, Ahmad A, Hayat S (2014) Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21:125. https://doi.org/10.1016/j.sjbs.2013.08.001
Jiang MY, Liu SL, Li YF, Li X, Luo ZH, Song HX, Chen QB (2019) EDTA-facilitated toxic tolerance, absorption and translocation and phytoremediation of lead by dwarf bamboos. Ecotox Environ Safe 170:502–512. https://doi.org/10.1016/j.ecoenv.2018.12.020
Kalyvas G, Tsitselis G, Gasparatos D, Massas I (2018) Efficacy of EDTA and olive mill wastewater to enhance As, Pb, and Zn phytoextraction by Pteris vittata L. from a soil heavily polluted by mining activities. Sustainability 10(6):1962. https://doi.org/10.3390/su10061962
Kavousi HR, Karimi MR, Neghab MG (2021) Assessment the copper-induced changes in antioxidant defense mechanisms and copper phytoremediation potential of common mullein (Verbascum thapsus L.). Environ Sci Pollut Res 28(14):18070–18080. https://doi.org/10.1007/s11356-020-11903-9
Kumar B, Smita K, Cumbal FL (2017) Plant mediated detoxification of mercury and lead. Arab J Chem 102:S2335–S2342. https://doi.org/10.1016/j.arabjc.2013.08.010
Lanier C, Bernard F, Dumez S, Dransart JL, Lemire S, Vandenbulcke F, Nesslany F, Platel A, Devred I, Hayet A, Cuny D, Deram A (2019) Combined toxic effects and DNA damage to two plant species exposed to binary metal mixtures (Cd/Pb). Ecotox Environ Safe 167:278–287. https://doi.org/10.1016/j.ecoenv.2018.10.010
Lasat MM, Baker A, Kochian LV (1998) Altered Zn compartmentation in the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspicaerulescens. Plant Physiol 118(3):875–883. https://doi.org/10.1104/pp.118.3.875
Li F, Qiu Y, Xu X, Yang F, Wang Z, Feng J, Wang J (2020) EDTA-enhanced phytoremediation of heavy metals from sludge soil by Italian ryegrass (Lolium perenne L.). Ecotox Environ Safe 191:110185. https://doi.org/10.1016/j.ecoenv.2020.110185
Li HS (2000) Principles and techniques of plant physiological biochemical experiment. Higher Education Press, Beijing
Lingua G, Todeschini V, Grimaldi M, Baldantoni D, Proto A, Cicatelli A, Castiglione S (2014) Polyaspartate, a biodegradable chelant that improves the phytoremediation potential of poplar in a highly metal-contaminated agricultural soil. J Environ Manage 132:9–15. https://doi.org/10.1016/j.jenvman.2013.10.015
Lin Y, Aarts MGM (2012) The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci 69(19):3187–3206. https://doi.org/10.1007/s00018-012-1089-z
Liu J, Xin X, Zhou Q (2018) Phytoremediation of contaminated soils using ornamental plants. Environ Rev 26(1):43–54. https://doi.org/10.1139/er-2017-0022
Liu Y, Yu XF, Feng YM, Zhang C, Wang C, Zeng J, Huang Z, Kang HY, Fan X, Sha L, Zhang HQ, Zhou YH, Gao SP, Chen QB (2017) Physiological and transcriptome response to cadmium in cosmos (Cosmos bipinnatus Cav.) seedlings. Sci Rep 7(1):14691. https://doi.org/10.1038/s41598-017-14407-8
Liu Z, Bai Y, Yang TM (2019) Effect of Cd on the uptake and translocation of Pb, Cu, Zn, and Ni in potato and wheat grown in Sierozem. Soil Sediment Contam 28(7):650–669. https://doi.org/10.1080/15320383.2019.1643289
Li X, Wang YJ, Pan YS, Yu H, Zhang XL, Shen YP, Jiao S, Wu K, La GX, Yuan Y (2017) Mechanisms of Cd and Cr removal and tolerance by macrofungus Pleurotusostreatus. J Hazard Mater 330:1–8. https://doi.org/10.1016/j.jhazmat.2017.01.047
Marques MC, Nascimento CW, Silva AJ (2017) Tolerance of an energy crop (Jatropha curcas L.) to zinc and lead assessed by chlorophyll fluorescence and enzyme activity. S Afr J Bot 112:275–282. https://doi.org/10.1016/j.sajb.2017.06.009
Meers E, Ruttens A, Hopgood MJ, Samson D, Tack MG (2005) Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58(8):1011–1022. https://doi.org/10.1016/j.chemosphere.2004.09.047
Meng Q, Zou J, Zou JH, Jiang WS, Liu DH (2007) Effect of Cu2+ concentration on growth, antioxidant enzyme activity and malondialdehyde content in garlic (Allium sativum L). Acta Biol Crac 49:95–101
Muro DA, Mussali P, Valencia L, Flores K, Tovar E (2020) Morphological, physiological, and genotoxic effects of heavy metal bioaccumulation in Prosopislaevigata reveal its potential for phytoremediation. Environ Sci Pollut Res 27:40187–40204. https://doi.org/10.1007/s11356-020-10026-5
Peco JD, Higueras P, Campos JA, Olmedilla A, Romero MC, Sandalio LM (2020) Deciphering lead tolerance mechanisms in a population of the plant species Biscutella auriculata L. from a mining area: Accumulation strategies and antioxidant defenses. Chemosphere 261:127721. https://doi.org/10.1016/j.chemosphere.2020.127721
Qureshi FF, Ashraf MA, Rasheed R, Ali S, Hussain I, Ahmed A, Iqbal M (2020) Organic chelates decrease phytotoxic effects and enhance chromium uptake by regulating chromium-speciation in castor bean (Ricinus communis L.). Sci Total Environ 716:137061. https://doi.org/10.1016/j.scitotenv.2020.137061
Ramana S, Tripathi AK, Bharati K, Singh AB, Kumar A, Sahu A, Rajput PS, Dey P, Saha JK, Patra AK (2021) Tolerance of cotton to elevated levels of Pb and its potential for phytoremediation. Environ Sci Pollut Res 28(25):32299–32309. https://doi.org/10.1007/s11356-021-13067-6
Rizvi A, Khan MS (2017) Biotoxic impact of heavy metals on growth, oxidative stress and morphological changes in root structure of wheat (Triticumaestivum L.) and stress alleviation by Pseudomonas aeruginosa strain CPSB1. Chemosphere 185:942–952. https://doi.org/10.1016/j.chemosphere.2017.07.088
Shahid M, Austruy A, Echevarria G, Arshad M, Sanaullah M, Aslam M, Nadeem M, Nasim W, Dumat C (2014) EDTA-enhanced phytoremediation of heavy metals: A Review. Soil Sediment Contam 23(4):389–416. https://doi.org/10.1080/15320383.2014.831029
Shen X, Li RL, Chai MW, Cheng SS, Niu ZY, Qiu GY (2019) Interactive effects of single, binary and trinary trace metals (lead, zinc and copper) on the physiological responses of Kandeliaobovata seedlings. Environ Geochem Health 41(1):135–148. https://doi.org/10.1007/s10653-018-0142-8
Sheoran V, Sheoran A, Poonia P (2011) Role of hyperaccumulators in phytoextraction of metals from contaminated mining sites: a review. Crit Rev Environ Sci Technol 41:168–214. https://doi.org/10.1080/10643380902718418
Sidhu S, Batish K (2016) Effect of lead on oxidative status, antioxidative response and metal accumulation in Coronopusdidymus. Plant Physiol Biochem 105:290–296. https://doi.org/10.1016/j.plaphy.2016.05.019
Srivastava RK, Pandey P, Rajpoot R, Rani A, Dubey RS (2014) Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251(5):1047–1065. https://doi.org/10.1007/s00709-014-0614-3
Tabelin CB, Toshifumi I, Tabelin V, Mylah IP, Einstine M, Naoki H (2018) Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci Total Environ 645:1522–1553. https://doi.org/10.1016/j.scitotenv.2018.07.103
Usharani B, Vasudevan N (2017) Root exudates of Cyperusalternifolius in partial hydroponic condition under heavy metal stress. Pharmacogn Res 9(3):294–300. https://doi.org/10.4103/pr.pr_107_16
Wang S, Zhao Y, Guo JH, Liu Y (2018) Antioxidative response in leaves and allelochemical changes in root exudates of Ricinuscommunis under Cu, Zn, and Cd stress. Environ Sci Pollut Res 25(32):32747–32755. https://doi.org/10.1007/s11356-018-3283-5
Wang ZY, Wang H, Xu C, Lv GH, Luo ZC, Zhu HH, Wang S, Zhu QH, Huang DY, Li BZ (2020) Foliar application of Zn-EDTA at early filling stage to increase grain Zn and Fe, and reduce grain Cd, Pb and grain yield in rice (Oryza sativa L.). Bull Environ Contam Toxicol 105(3):428–432. https://doi.org/10.1007/s00128-020-02949-z
Weigel HJ, Jager HJ (1980) Subcellular distribution and chemical form of cadmium in bean plants. Plant Physiol 65(3):480–482. https://doi.org/10.1104/pp.65.3.480
Xin J, Zhang Y, Tian R (2018) Tolerance mechanism of Triarrhenasaccharifiora (Maxim.) Nakai. seedlings to lead and cadmium: Translocation, subcellular distribution, chemical forms and variations in leaf ultrastructure. Ecotox Environ Safe 165:611–621. https://doi.org/10.1016/j.ecoenv.2018.09.022
Xu P, Wang Z (2014) A comparison study in cadmium tolerance and accumulation in two cool-season turfgrasses and Solanum nigrum L. Water Air Soil Pollut 225(5):1–9. https://doi.org/10.1007/s11270-014-1938-5
Yang W, Wang Y, Liu D, Hussain B, Ding Z, Zhao F, Yang X (2020) Interactions between cadmium and zinc in uptake, accumulation and bioavailability for Salix integra with respect to phytoremediation. Int J Phytoremediat 22(6):628–637. https://doi.org/10.1080/15226514.2019.1701981
Yi XY (2018) Study on the responses of Ricinus communis L. to Lead and Zinc stress and their mechanisms. Hunan: Central South University of Forestry and Technology 162–163.
Zeng J, Li XY, Wang XX, Zhang KH, Wang Y, Kang HY, Chen GD, Lan T, Zhang ZW, Yuan S, Wang CQ, Zhou YH (2020) Cadmium and lead mixtures are less toxic to the Chinese medicinal plant Ligusticum chuanxiong Hort. than either metal alone. Ecotox Environ Safe 193:110342. https://doi.org/10.1016/j.ecoenv.2020.110342
Zhang Q, Chen YH, Du L, Zhang MY, Han LZ (2019) Accumulation and subcellular distribution of heavy metal in Paulownia fortunei cultivated in lead-zinc slag amended with peat. Int J Phytoremediat 21(11):1153–1160. https://doi.org/10.1080/15226514.2019.1612844
Zhang W, Hu YJ, Cao YR, Huang FG, Xu H (2012) Tolerance of lead by the fruiting body of Oudemansiellaradicata. Chemosphere 88:467–475. https://doi.org/10.1016/j.chemosphere.2012.02.079
Funding
This work was supported by the General Project of the Key Research and Development Plan of Shaanxi Province (2021NY-070); Project of Science and Technology Plan of Xi’an (20NYYF0064).
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration with all authors. BZ put forward to the concept, ensured the feasibility and rationality of the experiment, and also proofread the manuscript. All authors designed the experiment and prepared the manuscript. YPD and YZ carried out the experimental work and data analyses. All of authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Duan, Y., Zhang, Y. & Zhao, B. Lead, zinc tolerance mechanism and phytoremediation potential of Alcea rosea (Linn.) Cavan. and Hydrangea macrophylla (Thunb.) Ser. and ethylenediaminetetraacetic acid effect. Environ Sci Pollut Res 29, 41329–41343 (2022). https://doi.org/10.1007/s11356-021-18243-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18243-2