Abstract
We investigated the genetic variation and relationships among 35 melon landraces collected from the Xinjiang Uygur Autonomous Region in northwestern China by using 19 polymorphic simple sequence repeat markers (SSRs). A total of 55 polymorphic alleles were amplified. The number of alleles per SSR locus ranged from 2 to 5 with an average of 2.89 alleles per locus. The average gene diversity (GD) was 0.42 with a range of 0.06–0.71, and the average observed heterozygosity was 0.22 with a range of 0.06–0.97, indicating that the genetic diversity among the Xinjiang melon landraces was abundant. Genetic variation was also detected between the landrace populations in different regions in Xinjiang. The most abundant genetic diversity was observed among the landraces in Eastern Xinjiang, with the highest GD of 0.45 and PIC of 0.39. Eleven alleles (20 %) were found exclusively in the landraces from Eastern Xinjiang, and two alleles (3.6 %) were unique to the landraces from Southern and Northern Xinjiang. The genetic similarity matrix was defined on the basis of Jaccard coefficient to determine the genetic relationships among Xinjiang landraces. Cluster analysis was performed using the unweighted pair group method with arithmetic means, showing that the ‘wild Hami’ (XJ-34) landrace was distinct from the 34 other landraces that were divided into three clusters. Therefore, the genetic background of XJ-34 differed from that of the other landraces. The landraces were not precisely separated on the basis of their geographic origins, although most of these landraces were likely grouped near one another, as visualized through principal coordinate analysis. Thus, western China is one of the primary or secondary centers of melon diversity because of the relatively higher genetic variation detected among Xinjiang landraces. Except the ‘wild Hami’ landrace, Xinjiang melon landraces could be classified into two botanical varieties, namely, var. inodorus and var. cantalupensis. However, the distinction between these two genotypes was not significantly different.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melon (Cucumis melo L., 2n = 2x = 24) is an outcrossing horticultural crop of economic importance that belongs to the family Cucurbitaceae. Depending on the type of fruit, people cultivate melon for different purposes, such as a dessert or a vegetable, which is pickled or cooked. The morphological variation of this species is high in terms of the leaf, plant, and fruit characteristics, especially the fruit characteristics, such as the size or color of the fruit and the color or taste of fruit flesh, thus, C. melo is considered as the most variable species in the genus Cucumis (Jeffrey 1980; Bates and Robinson 1995; Pitrat 2008, 2013). C. melo is subdivided into two sub-species namely, subsp. agrestis (Naud.) Pangalo and subsp. melo, on the basis of vegetative morphological characteristics and fruit variation, length and distribution of hairs on the ovary and young fruit (Kirkbride 1993). The latter is further subdivided into several groups or botanical varieties, including var. flexuosus (L.) Naud., var. inodorus Jacq., var. catalupensis Naud., var. reticulatus Ser., var. adana Pangalo, var. ameri Pangalo, var. chandalak Pangalo and var. tibish Moham. (Jeffrey 2001; Hammer and Gladis 2014).
Although the geographical origin of melon remains unclear, Africa (Robinson and Decker-Walters 1997; Pitrat 2008; Tanaka et al. 2013) and Asia (Renner et al. 2007; Schaefer et al. 2009) are considered as the origins of melon domestication. China is the primary or secondary center of cultivated melon diversity (Robinson and Decker-Walters 1997; Luan et al. 2008; Tzitzikas et al. 2009; Malik et al. 2014), and China is also a major world producer of var. cantalupensis, var. inodorus and var. conomon (Thunb.) Makino commercial cultivars (Luan et al. 2008; Aierken et al. 2011; Malik et al. 2014; Raghami et al. 2014), with 14.3 million tons of production and 423.1 thousands of hectares per area harvested in 2013 (FAOSTAT 2014). More than 1200 melon accessions are conserved in the Chinese National Genebank of Crop Genetic Resources, including approximately 380 local landraces originating and collected from China (Kong et al. 2011). In China, melon is traditionally defined by the thickness of fruit skin and is divided into two cultivated types, particularly thin and thick-skinned melons (Wang 2000; Luan et al. 2008; Aierken et al. 2011). Thin-skinned melon is classified into the conomon group exhibiting a higher tolerance to humidity and potential disease resistance (Akashi et al. 2002). This type of melon is mostly produced in the eastern and central provinces in China (Luan et al. 2008; Aierken et al. 2011). However, thick-skinned melon, with a longer shelf time and higher sugar content, is generally distributed in the Xinjiang Uygur Autonomous Region (Xinjiang), which is the largest province-level administrative region of China in terms of land area (Wang 2000; Aierken et al. 2011; Zhang et al. 2012). Although the geographical variation between thin- and thick-skinned melons is often significant (Akashi et al. 2002; Nakata et al. 2005; Luan et al. 2008), details of the genetic variation of these two types of melon with different origins, especially the genetic variation of thick-skinned melon, have yet to be elucidated.
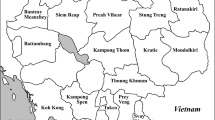
Xinjiang, located in northwest China in the hinterland of the Eurasian continent (Fig. 1), is adjacent to eight countries, namely, Russia, Kazakhstan, Kyrgyzstan, Tajikistan, Pakistan, Afghanistan, India, and Mongolia. Among these countries, India, Tajikistan, and Afghanistan are considered as primary diversity centers for melon (Tzitzikas et al. 2009). In history, Xinjiang served as a key controlling region of the well-known Silk Road from China (Shanxi Province) to Mediterranean countries, which passed through most of the central and western Asian countries. Xinjiang occupies approximately one-sixth of the Chinese territory and covers an area of approximately 1,660,000 km2 with some unique climatic characteristics, such as scant rainfall, large daily fluctuations of air temperature, strong sunshine, and long sunshine duration. These climatic conditions are advantageous for the cultivation of high-quality sweet and thick-skinned melon, which has been called Hami melon since two hundred years ago. With a total production of 1.1 million tons and a cultivation area of 41.0 thousand hectares annually, Hami melon is a local featured crop of economic important in Xinjiang where is the major producer of Hami melon cultivars to the other provinces in China (Aierken et al. 2011).
The three main agro-ecological regions of Xinjiang are naturally separated by the Tian Mountains into Eastern, Southern, and Northern Xinjiang, and these regions differ in terms of rainfall pattern, air temperature, and growing period (Fig. 1). More than 100 landraces were distributed in Xinjiang and collected several decades ago (Wu 1982). These local landraces were primarily classified into four groups, namely, var. cassaba, var. chandalak Pangalo (extra early Guadan melon), var. ameri (summer melon), and inodorus (winter melon), on the basis of the fruit maturation period (Lin and Su 1985; Lin 1991; Wang 2000). The local melon landraces in Xinjiang are very rich in phenotypic diversity, and the extent of morphological diversity between agro-ecological regions of Xinjiang is different (Zhang et al. 2012). In the past decade, the genetic diversity of a few or some melon landraces in Xinjiang has been investigated using molecular markers (Stepensky et al. 1999; Luan et al. 2008; Aierken et al. 2011). Nevertheless, a rigorous molecular analysis of the genetic variation among landraces, especially between landraces from different agro-ecological regions in Xinjiang, has yet to be comprehensively performed.
Local landraces have adapted to the natural environment in which they originate, and these landraces are important resources of the germplasm management and genetic improvement of melon. Most commercial cultivars released in Xinjiang have been selected directly or indirectly from local landraces collected decades ago, which is significant to the genetic improvement and the production of Hami melon (Yi et al. 2013). However, the lack of detailed description on the genetic variation in local melon landraces can be a serious disadvantage to the preservation of genetic variability and can interfere with the development of a genetic enhancement plan that focuses on the important traits of economic interests in Xinjiang melon landraces. The genetic diversity of local melon landraces or germplasm of regional or national collections has been effectively characterized by molecular analyses (Dhillon et al. 2007; Sensoy et al. 2007; Yi et al. 2009; Fergany et al. 2011; Kong et al. 2011; Yildiz et al. 2011; Malik et al. 2014). Therefore, this study used a set of SSR markers valuable for melon genetic characterization (Fernandez-Silva et al. 2008) to describe and analyze the genetic diversity of Xinjiang melon landraces and to compare the genetic relationship among and between these landraces from three agro-ecological regions in Xinjiang. Our study helps enhance our understanding of the distribution of genetic variability among the local melon germplasm in China.
Materials and methods
Plant materials
Thirty-five landraces, including 33 landraces and 2 wild melons (‘wild Hami’ and ‘wild Turpan’), distributed in the province were analyzed in the present study to evaluate the genetic diversity of local melon germplasm in Xinjiang. These melon landraces were collected from three agro-ecological regions and eight prefectures/autonomous prefectures in Xinjiang. These prefectures, two (Turpan and Hami) in Eastern Xiniang, four (Kashgar, Hotan, Akesu, and Bayingol Mongolian autonomous prefecture) in Southern Xiniang, and two (Tacheng and Urumqi) in Northern Xinjiang were considered. Table 1 and Fig. 1 provide the details of the origin and distribution of these landraces. Figure 2 shows the images of the landrace fruits.
DNA extraction
Three weeks after the landraces were sown at the experimental base-station of the Center for Hami Melon, Xinjiang Academy of Agricultural Sciences (XAAS), Sanya City, China, mixed young leaves were collected from five seedlings of each landrace and stored at approximately −80 °C until DNA was extracted. Genomic DNA was isolated in accordance with the modified CTAB procedure described by Staub et al. (2004). DNA was quantified with a spectrophotometer and diluted to approximately 10 ng/μL for PCR amplification.
SSR amplification
The 36 SSR primer pairs used in this study were previously developed in melon by Fernandez-Silva et al. (2008). The SSR loci were amplified with a total volume of 20 μL, containing 50 ng of template DNA, 0.5 μM of each primer, 0.1 mM dNTPs, 2.5 mM MgCl2, 1× buffer (20 mM (NH)4SO4; 75 mM Tris–HCl, pH 8.8; 0.01 %, v/v, Tween-20), and 1 U of Taq DNA polymerase (Sangon Biotech Co. Ltd., Shanghai, China). PCR amplifications were performed in a TC-512 thermocycler (Bibby Scientific Ltd., OSA, UK). The following program was used: an initial step of 5 min at 94 °C, followed by 34 cycles of 30 s at 94 °C, 30 s at 55 °C, and 80 s at 72 °C, with a final step of 5 min at 72 °C. PCR products were separated on 6 % native polyacrylamide gels in 1× TBE buffer with a constant voltage of 10 V/cm for approximately 2.5 h and visualized by silver-staining.
Data analysis
The amplified fragments of each SSR locus were recorded as present (1) or absent (0) codominant markers. Two or more SSR alleles in a single genotype were observed because of the presence of several heterozygous plants, homozygous plants for the alternative alleles, or a combination of both as a consequence of the bulk samples during DNA extraction. The binary matrix was converted to the required data input format on the basis of the manuals for the respective computer program. The number of alleles, gene diversity (GD), observed heterozygosity, and polymorphism information content (PIC) were calculated with PowerMarker version 3.25 (Liu and Muse 2005). The genetic similarity between landraces was determined on the basis of pairwise Jaccard similarity coefficient (JC) by using the SIMQUAL module of NTSYS-PC version 2.10e (Exeter Software, Setauket, NY, USA). Cluster analysis was performed on the genetic similarity matrix by using the unweighted pair group method with arithmetic means (UPGMA) via the SAHN module of NTSYS-PC version 2.10e to visualize the relationships among landraces based on the JCs. The bootstrapping of UPGMA tree was performed by the FreeTree program (Hampl et al. 2001) with 1000 iterations. Principal coordinate analysis (PCA) based on the genetic similarity matrix was performed using DCENTER, EIGEN, and MOD3D algorithms in the NTSYS-PC software package to further elucidate the relationship among these landraces.
Results
Polymorphism characterization based on SSR markers
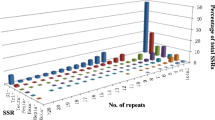
A total of 36 SSR markers were used to evaluate the genetic diversity of the 35 melon local landraces in Xinjiang in western China. These markers were evenly distributed on the genetic linkage map. Twelve SSR markers generated undistinguished bands, and five SSR markers amplified monomorphic bands. These markers were excluded from the subsequent analysis. The 19 polymorphic SSR markers were chosen for the final analysis and amplified 55 high-quality alleles among 35 local landraces. These polymorphic markers were distributed on nine linkage groups and were used for statistical analysis. The results of the analysis are presented in Table 2.
The number of alleles per locus ranged from 2 to 5, with an average of 2.89 alleles for the 19 polymorphic loci. Only 8 loci had 2 alleles each among the 35 landraces, whereas the CMCT505, CMAGN33, CMAGN61, and CMAGN73 loci exhibited more than 4 alleles each. GD ranged from 0.06 (at CMCTN65) to 0.71 (at CMAGN33), with an average of 0.42. The PIC value ranged from 0.05 (at CMCTN65) to 0.65 (at CMAGN33), with an average of 0.36. Five markers with PIC values of >0.50 were considered informative markers for landrace identification. Except for CMTC168, CMCTN4, CMCTN35, CMTCN41, and CMCTN65, all the other loci could identify heterozygous individuals across 35 landraces. The proportion of heterozygous landraces ranged from 0.06 at CMTCN1, CMCTN2, and CMGAN80 to 0.97 at CMCT505, with an average heterozygosity of 0.22.
Various parameters of landraces collected from three agro-ecological regions in Xinjiang were assessed to identify the degree of genetic variation of landraces within the same region and the degree of genetic differentiation between different regions, and the statistical results are shown in Table 3. Genetic variation within and among landraces from different regions differed when examined in terms of their origin. The number of alleles amplified by 19 SSR markers for landraces from Eastern Xinjiang was the highest (2.75). The genetic variation of the landraces from Eastern Xinjiang was larger than that of the landraces from the other two regions and had the highest GD value (0.45), PIC value (0.39), and percent of polymorphic loci (89.47). However, the observed heterozygosity of landraces from Southern Xinjiang was slightly higher than that of landraces from the other two regions.
Eleven alleles (20.0 %) were exclusively present in the landraces from Eastern Xinjiang, whereas two alleles each (3.6 %) were unique to the landraces of Southern and Northern Xinjiang (Table 3).
Genetic relationships among and between melon landraces
The binary data matrix containing 55 polymorphic alleles was used to assess the genetic similarity of the 35 melon landraces, and the JC were calculated. JC values ranged from 0.20 (between XJ-1 and XJ-34) to 0.96 (between XJ-24 and XJ-25), with a mean of 0.51 (raw data not presented). XJ-1 is the ‘Huangdanzi’ landrace from Eastern Xinjiang, whereas XJ-34 is a wild melon from the same region. The low JC value between these landraces indicated that considerable genetic differences existed between XJ-1 and XJ-34. XJ-24 and XJ-25 are two landraces from the same agro-ecological region in Southern Xinjiang. The high JC value suggested a similar genetic background between these two landraces.
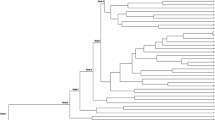
The phylogenetic tree was constructed by the UPGMA method based on the JC matrix, as shown in Fig. 3. The 34 landraces were divided into 3 clusters, except for ‘wild Hami’ (XJ-34). The ‘wild Hami’ remained unclustered, thereby indicating its distinct genetic relationship with the other landraces in Xinjiang. Cluster I was relatively distantly related to the other 2 clusters and consisted of two landraces (XJ-1 and -2) that were collected from the same agro-ecological region (Eastern Xinjiang). Cluster II included most landraces from 3 regions and was divided into 5 sub-clusters, among which 2 sub-clusters (IIa and IIe) were comprised of 2 landraces from the same region (Southern Xinjiang). The other wild melon, ‘wild Turpan’ (XJ-35), was grouped in sub-cluster IIa with two landraces from Southern Xinjiang (XJ-3 and -17). Out of the 17 closely related landraces in sub-cluster IIb, 11 were from Southern Xinjiang (XJ-12, -13, -18, -19, -20, -21, -24, -25, -26, -28, and -32). Sub-cluster IIc was represented by four landraces, in which three were from Eastern Xinjiang (XJ-10, -23, and -33) and one from Southern Xinjiang (XJ-16). The two landraces in sub-cluster IId were collected from Southern (XJ-8) and Northern (XJ-7) Xinjiang. Cluster III contained three landraces from each of the three regions, namely, Southern (XJ-14), Eastern (XJ-4), and Northern (XJ-11) Xinjiang.
Genetic relationships among the 35 landraces were visualized by PCA as shown in Fig. 4. The first, second, and third PCA components accounted for 12.4, 9.4, and 8.1 % of the total variation, respectively, and were abstracted to examine the genetic variation. PCA clearly displayed the genetic differences among the landraces, which was consistent in terms of various aspects with the results of the JC phylogenetic tree. Most landraces from the same region in Southern Xinjiang (e.g., XJ-3, -5, -12, -13, -17, -18, -19, -20, -21, -24, -25, -26, -28, and -32) tended to be grouped together, thereby exhibiting their moderate genetic diversity. However, landraces collected from Southern Xinjiang were not distinctly separated from landraces of the other regions. Landraces collected from Eastern Xinjiang were scattered in a large region along the third dimension, demonstrating their higher genetic variation than the landraces from Southern and Northern Xinjiang.
Discussion
The number of melon landraces from Xinjiang was relatively small in previous studies, wherein only one or two landraces of thick-skinned melon from the region were studied (Stepensky et al. 1999; Luan et al. 2008). Aierken et al. (2011) used neutral nuclear and chloroplastic genome molecular markers to characterize the genetic variation and relationship of 24 Hami melon landraces, of which the geographical origin was not exhaustively analyzed. In the present study, the genetic variation and relationships were assessed for 35 landraces collected from various regions in Xinjiang, and the geographical variation of these landraces in 3 main agro-ecological regions of Xinjiang was analyzed. Most of these landraces were assessed for the first time to the best of our knowledge. The selected SSRs in this study were sufficient to test melon landraces in Xinjiang, even though the number of SSRs was relatively small.
The average number of alleles per SSR marker was 2.89 among the 35 melon landraces in our study, which was similar to the results of López-Sesé et al. (2002), with 2.4 alleles among 15 Spanish melons, and Tzitzikas et al. (2009), with 2.47 alleles among 14 Greek and Cypriot melons. Monforte et al. (2003) found 6.3 alleles among 27 wild and cultivated melons, whereas Raghami et al. (2014) detected 4.38 alleles among 24 Iran melon accessions, most of which were collected from local farmers or stores. This high value was possibly caused by the wider range of examined melon accessions, such as the use of various subspecies or different groups of C. melo. The genetic variability measured by SSR markers revealed the high genetic diversity among melon landraces in Xinjiang. The genetic variation estimates for Xinjiang melon landraces in the present study, with GD of 0.42 and PIC of 0.36, were higher than those reported by Tzitzikas et al. (2009), with GD of 0.30 and PIC of 0.26, thereby indicating the different genetic backgrounds of the materials tested. The average PIC value of our study (0.36) was lower than those of Fergany et al. (2011) and Raghami et al. (2014) at 0.54 and 0.49, respectively, thereby reflecting the lower allele diversity and frequency among landraces in Xinjiang than among landraces in India and Iran. Aierken et al. (2011) detected a lower GD (0.32) among 24 Hami melon landraces based on 16 SSR markers compared with our study (0.42), which could have resulted from the small number of samples or the narrow distribution range of the selected landraces.
Heterozygosity could be considered a measure of genetic variability among samples. This value refers to how much variation exists in the population and how that variation is distributed across the alleles of an analyzed molecular marker (Raghami et al. 2014). The average observed heterozygosity per SSR marker in the 35 landraces examined in the present study was higher than those reported by López-Sesé et al. (2002), Monforte et al. (2003) and Tzitzikas et al. (2009), even though zero heterozygosity was observed for six markers in our study. All the tested landraces were collected from local farmers and maintained by the Center for Hami Melon, XAAS in Xinjiang. Therefore, any outcrossing with other landraces would have happened before collection, thereby resulting in higher levels of heterozygosity. Fergany et al. (2011) detected a mean observed heterozygosity of 0.23 in 50 landraces from southern India, which was similar to our results (0.22), thereby indicating a higher proportion of heterozygous individuals in the tested population samples. The observed heterozygosity exhibited lower values than the corresponding expected heterozygosity values in all but 4 markers, thereby indicating excessive homozygosity exited among some of the tested Xinjiang landraces. Six SSR markers showed zero heterozygosity, which meant 100 % observed homozygosity. These results indicated the lack of intercrossing between Xinjiang landraces or outcrossing with other groups, alternatively, a high rate of self-pollination occurred after collection from farmers. Raghami et al. (2014) found extremely low observed heterozygosity values in some Iranian landraces, which were attributed to the use of landraces originating from small populations or high levels of inbreeding selection.
Based on the tested genetic affinities and the historical record, Luan et al. (2008) confirmed that Chinese thick-skinned melon was introduced to western China from Middle East via the famous Silk Road. We detected the highest genetic variation among landraces collected from Eastern Xinjiang, which were similar to our previous study based on 32 morphological traits (Zhang et al. 2012). The Turpan and Hami prefectures in Eastern Xinjiang were regarded as important locations along the Silk Road, which could lead to the rich genetic diversity in the region and result in unique gene pools. In the present study, 20 % (11) of the tested SSR alleles were exclusively present in landraces from Eastern Xinjiang and have not been previously described, despite the relatively small number of SSR markers that were used. These results indicated that the current melon landraces collection in Eastern Xinjiang could contain a degree of unique genetic variability, which needs to be preserved for future breeding plans (Fig. 4).
In the present report, cluster analysis reflected no precise association between landrace separation and geographical origin except for two landraces in cluster I, although most landraces from the same agro-ecological region (Southern or Eastern Xinjiang) were grouped near each other (Figs. 3, 4). However, some landraces from the same agro-ecological region were scattered in different clusters or sub-clusters, thereby indicating the occurrence of out-crossing (Fig. 3). All but one of the tested landraces belonged to the three clusters, and most landraces were grouped into cluster II. Cluster I had distinctive position from the other clusters, and the JC between each pair of landraces in clusters II and III averaged 0.52. However, the JC was 0.42 between 2 landraces in cluster I and 32 landraces in the other 2 clusters (data not shown). These results indicated that 2 landraces in cluster I were distantly related to most of the other landraces, which were relatively more related each other. The biggest cluster was divided into 5 sub-clusters further, among which one sub-cluster (IIb) was comprised of 17 closely related landraces. Two landraces in cluster I were collected from Eastern Xinjiang, with distinctive fruit traits of very poor shelf life, climacteric growth, and soft flesh. Nevertheless, most of the other landraces in sub-cluster IIb were collected from Southern Xinjiang and possessed the typical fruit traits of a very long shelf life, non-climacteric growth, and crisp flesh. Aierken et al. (2011) found that most Hami melon landraces closely clustered together with landraces from Central Asia and should be classified in the inodorus group, even though these tested landraces were primarily classified into the cassaba, chankdalak, ameri, and inodorus groups based on the fruit maturation period (Lin 1991). However, the maturation-related differences could be controlled by a small number of key-genes and did not represent extensive genetic divergence (Staub et al. 1997). Therefore, Xinjiang melon landraces, except the wild melons (‘wild Hami’), which represents C. melo subsp. agrestis (Naud.) Pangalo, are representatives of C. melo subsp. melo (groups inodorus and cantalupensis). In this study, SSR resolution between the inodorus and cantalupensis groups was ambiguous despite the significant differences in their morphology. This trend was similar to the observations on melon accessions from Spain (López-Sesé et al. 2003), Greece, Cyprus (Tzitzikas et al. 2009), and Iran (Raghami et al. 2014).
The ‘wild Hami’ (XJ-34) was collected as feral melon among weeds near the cultivated area and named ‘Gou Gua’ by local residents (Wu 1982; Lin 1991). This group is distinctly separated from the other landraces (Fig. 3). Stepensky et al. (1999) noted that ‘Gou Gua’ was more distantly related to two dessert melon groups (cantalupensis and inodorus) but was closely related to subsp. agrestis based on the morphological traits or molecular data (RAPD and ISSR markers), as confirmed by SSR analysis in the present study. By contrast, the ‘wild Turpan’ (XJ-35) was grouped in cluster II (Fig. 3) and had relatively similar genetic properties with the other examined landraces. The fruit traits of ‘wild Turpan’ were not consistent with the previous descriptions (data not present), thereby indicating that inter-crossing with other landraces had possibly occurred during its preservation after collection by local residents. Investigations on the resistance or tolerance to biological and non-biological stress in wild melons from Xinjiang could be important for the genetic improvement of local germplasm.
Melon has a long history of cultivation from approximately 1000 B.C.E. in Egypt, Mesopotamia, eastern Iran, China, and India (Pitrat et al. 2000). According to ancient historical and archaeological records, melon has been cultivated in Xinjiang for at least 2000 years (Wu 1982; Lin and Su 1985). The thick- and thin-skinned melons were independently introduced into China along different routes, thereby resulting in two unique gene pools, one from the Middle East to western China via the Silk Road and another from India to China via Laos and eastern China (Luan et al. 2008; Aierken et al. 2011). The melon landraces tested in the present study exhibited relatively high genetic variation (i.e., GD of 0.42 and observed heterozygosity of 0.22), which would support the idea of China as a primary or secondary center of melon diversity (Robinson and Decker-Walters 1997; Luan et al. 2008; Tzitzikas et al. 2009; Malik et al. 2014). Furthermore, a certain number of unique SSR alleles were detected among landraces between different agro-ecological regions (Table 3). These results indicated the importance of Xinjiang melon landraces for long-term conservation and future use in breeding programs.
References
Aierken Y, Akashi Y, Phuong NPT, Halidan Y, Tanaka K, Long B, Nishida H, Long C, Wu MZ, Kato K (2011) Molecular analysis of the genetic diversity of Chinese Hami melon and its relationship to the melon germplasm from central and south Asia. J Jpn Soc Hortic Sci 80:52–65
Akashi Y, Fukuda N, Wako T, Masuda M, Kato K (2002) Genetic variation and phylogenetic relationships in East and South Asian melons, Cucumis melo L., based on the analysis of five isozymes. Euphytica 125:385–396
Bates DM, Robinson RW (1995) Cucumber, melons and watermelons, Cucumis and Citrullus (Cucurbitaceae). In: Smartt J, Simmonds NW (eds) Evolution of crop plants. Wiley, New York, pp 89–111
Dhillon NPS, Ranjana R, Singh K, Eduardo I, Monforte AJ, Pitrat M, Dhillon NK, Singh PP (2007) Diversity among landraces of Indian snapmelon (Cucumis melo var. momordica). Genet Resour Crop Evol 54:1267–1283
FAOSTAT (2014) Statistic database. http://faostat3.fao.org
Fergany M, Kaur B, Monforte AJ, Pitrat M, Rys C, Lecoq H, Dhillon NPS, Dhaliwal SS (2011) Variation in melon (Cucumis melo) landraces adapted to the humid tropics of southern India. Genet Resour Crop Evol 58:225–243
Fernandez-Silva I, Eduardo I, Blanca J, Esteras B, Picó F, Nuez P, Arús P, Garcia-Mas J, Monforte AJ (2008) Bin mapping of genomic and EST-derived SSRs in melon (Cucumis melo L.). Theor Appl Genet 118:139–150
Hammer K, Gladis T (2014) Notes on infraspecific nomenclature and classifications of cultivated plants in Compositae, Cruciferae, Cucurbitaceae, Gramineae (with a remark on Triticum dicoccon Schrank) and Leguminosae. Genet Resour Crop Evol 61:1455–1467
Hampl V, Pavlicek A, Flegr J (2001) Construction and bootstrap analysis of DNA fingerprinting-based phylogenetic trees with the freeware program FreeTree: application to trichomonad parasites. Int J Syst Evol Microbiol 51:731–735
Jeffrey C (1980) A review of the Cucurbitaceae. Bot J Linn Soc 81:233–247
Jeffrey C (2001) Cucurbitaceae. In: Hanelt P, IPK (eds) Mansfeld’s encyclopedia of agricultural and horticultural crops. Springer, Berlin, pp 1512–1515
Kirkbride JH (1993) Biosystematic monograph of the genus Cucumis (Cucurbitaceae). Parkway Publishers, Boone
Kong QS, Xiang CP, Yang J, Yu ZW (2011) Genetic variations of Chinese melon landraces investigated with EST-SSR markers. Hortic Environ Biotechnol 52:163–169
Lin DP (1991) Classification of melon. In: Qi SK, Wu DK, Lin DP (eds) Chinese Melon. Popular Science Press, Beijing, pp 9–21
Lin DP, Su XP (1985) Records of Melon and Watermelon in Xinjiang. In: Investigating Group on Resources of Xinjiang Melon and Watermelon (ed) The origin and taxonomy of melon and watermelon in Xinjiang. Xinjiang People’s Publishing House, Urumqi, pp 36–84
Liu K, Muse SV (2005) PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
López-Sesé AI, Staub JE, Katzir N, Gómez-Guillamón ML (2002) Estimation of between and within accession variation in selected Spanish melon germplasm using RAPD and SSR markers to assess strategies for large collection evaluation. Euphytica 127:41–51
López-Sesé AI, Staub JE, Gómez-Guillamón ML (2003) Genetic analysis of Spanish melon (Cucumis melo L.) germplasm using a standardized molecular-marker array and geographically diverse reference accessions. Theor Appl Genet 108:41–52
Luan FS, Delannay I, Staub JE (2008) Chinese melon (Cucumis melo L.) diversity analyses provide strategies for germplasm curation, genetic improvement, and evidentiary support of domestication patterns. Euphytica 164:445–461
Malik AA, Vashisht VK, Singh K, Sharma A, Singh DK, Singh H, Monforte AJ, McCreight JD, Dhillon NPS (2014) Diversity among melon (Cucumis melo L.) landraces from the Indo-Gangetic plains of India and their genetic relationship with USA melon cultivars. Genet Resour Crop Evol 61:1189–1208
Monforte AJ, Garcia-Mas J, Arus P (2003) Genetic variability in melon based on microsatellite variation. Plant Breed 122:153–157
Nakata E, Staub JE, López-Sesé AI, Katzir N (2005) Genetic diversity in Japanese melon (Cucumis melo L.) as assessed by random amplified polymorphic DNA and simple sequence repeat markers. Genet Resour Crop Evol 52:405–419
Pitrat M (2008) Melon. In: Prohens J, Nuez F (eds) Handbook of plant breeding, vol vegetables I: Asteraceae, Brassicaceae, Chenopodiaceae, and Cucurbitaceae. Springer, Heidelberg, pp 283–315
Pitrat M (2013) Phenotypic diversity in wild and cultivated melons (Cucumis melo). Plant Biotechnol 30:273–278
Pitrat M, Hanelt P, Hammer K (2000) Some comments on infraspecific classification of cultivars of melon. Acta Hortic 510:29–36
Raghami M, Lopez-Sese AI, Hasandokht MR, Zamani Z, Moghadam MRF, Kashi A (2014) Genetic diversity among melon accessions from Iran and their relationships with melon germplasm of diversity origins using microsatellite markers. Plant Syst Evol 300:139–151
Renner SS, Schaefer H, Kocyan A (2007) Phylogenetics of Cucumis (Cucurbitaceae): Cucumber (C. sativus) belongs in an Asian/Australian clade far from melon (C. melo). BMC Evol Biol 7:58–69
Robinson RW, Decker-Walters DS (1997) Cucurbits. CAB Int. University Press, Cambridge
Schaefer H, Heibl C, Renner SS (2009) Gourds afloat: a dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Proc R Soc B 276:843–851
Sensoy S, Büyükalaca S, Abak K (2007) Evaluation of genetic diversity in Turkish melon (Cucumis melo L.) based on phenotypic characters and RAPD markers. Genet Resour Crop Evol 54:1351–1365
Staub J, Box J, Melic V, Horejsi TF, McCreight JD (1997) Comparison of isozyme and random amplified polymorphic DNA data for determining intraspecific variation in Cucumis. Genet Resour Crop Evol 44:257–269
Staub JE, López-Sesé AI, Fanourakis N (2004) Diversity among melon landraces (Cucumis melo L.) from Greece and their genetic relationships with other melon germplasm of diverse origins. Euphytica 136:151–166
Stepensky A, Kovalski I, Perl-Treves R (1999) Intraspecific classification of melons (Cucumis melo L.) in view of their phenotypic and molecular variation. Plant Syst Evol 217:313–332
Tanaka K, Akashi Y, Fukunaga K, Yamamoto T, Aierken Y, Nishida H, Long CL, Yoshino H, Sato YI, Kato K (2013) Diversification and genetic differentiation of cultivated melon inferred from sequence polymorphism in the chloroplast genome. Breed Sci 63:183–196
Tzitzikas EN, Monforte AJ, Fatihi A, Kypriotakis Z, Iacovides TA, Ioannides IM, Kalaitzis P (2009) Genetic diversity and population structure of traditional Greek and Cypriot melon cultigens (Cucumis melo L.) based on simple sequence repeat variability. HortScience 44:1820–1824
Wang J (2000) Chinese watermelon and melon. Chinese Agricultural Press, Beijing (in Chinese)
Wu MZ (1982) Genetic resources of thick-skinned melon collected from Turpan depression and their utilization. China Fruits 2:28–33
Yi SS, Akashi Y, Tanaka K, Cho TT, Khaing MT, Yoshino H, Nishida H, Yamamoto T, Win K, Kato K (2009) Molecular analysis of genetic diversity in melon landraces (Cucumis melo L.) from Myanmar and their relationship with melon germplasm from East and South Asia. Genet Resour Crop Evol 56:1149–1161
Yi HP, Wu MZ, Feng JX, Zhang YB (2013) Advances in genetic improvement of Hami melon in Xinjiang, China. Aca Hortic Sin 40:1779–1786
Yildiz M, Ekbic E, Keles D, Sensoy S, Abak K (2011) Use of ISSR, SRAP, and RAPD markers to assess genetic diversity in Turkish melons. Sci Hortic 130:349–353
Zhang YB, Li MH, Wu HB, Yi HP, Wu MZ (2012) Genetic diversity of melon landraces (Cucumis melo L.) in Xinjiang based on phenotypic characters. Acta Hortic Sin 39:305–314
Acknowledgments
We are grateful to Professor Q.S Kong for providing helpful and valuable comments. This work was supported by grants from the National Science Foundation of China (Project No. 31260480), in part by the Chinese Post Doctor Research Plan (Project No. 92193), the National Science & Technology Pillar Program during the 12th Five-year Plan Period (2012BAD02B03-4) and the Modern Agro-industry Technology Research System (CARS-26-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Fan, X., Aierken, Y. et al. Genetic diversity of melon landraces (Cucumis melo L.) in the Xinjiang Uygur Autonomous Region on the basis of simple sequence repeat markers. Genet Resour Crop Evol 64, 1023–1035 (2017). https://doi.org/10.1007/s10722-016-0422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-016-0422-z