Abstract
Diversity among 36 snapmelon landraces, collected from 2 agro-ecological regions of India (9 agro-climatic sub-regions), was assayed using RAPD primers, morphological traits of plant habit and fruit, 2 yield-associated traits, pest and disease resistance and biochemical composition (TSS, ascorbic acid, titrable acidity). Typical differences among accessions were observed in plant and fruit characteristics and snapmelon germplasm with high titrable acidity and possessing resistance to downy mildew, Cucumber mosaic virus, Zucchini yellow mosaic virus, Papaya ringspot virus, Aphis gossypii and Meloidogyne incognita was noticed in the collection. RAPD based grouping analysis revealed that Indian snapmelon was rich in genetic variation and region and sub-region approach should be followed across India for acquisition of additional melon landraces. Accessions of var. agrestis and var. momordica clustered together and there was a separate cluster of the accessions of var. reticulatus. Comparative analysis of the genetic variability among Indian snapmelons and an array of previously characterized reference accessions of melon from Spain, Israel, Korea, Japan, Maldives, Iraq, Pakistan and India using SSRs showed that Indian snapmelon germplasm contained a high degree of unique genetic variability which was needed to be preserved to broaden the genetic base of melon germplasm available with the scientific community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snapmelon [Cucumis melo L. var. momordica (Roxb.) Duthie et Fuller] is native to India. It was intensively grown in the 19th century in northern India (Duthie 1905) where it is commonly known as ‘phut’ which means to split. Immature fruits are cooked or pickled, the low sugared mature fruits are eaten raw. Ripe fruits invariably crack.
Snapmelon germplasm has been found to be a very good source of disease and insect resistance. California melon breeders came to India in February 1929, and a powdery mildew [Podosphaera xanthii (Castagne) Braun et Shishkoff and Golovinomyces cichoracearum (DC.) V.P. Heluta] resistant snapmelon collection designated PI 79376, originating from the Kathiawar region of Gujarat, was presented to them by Mr. D. N. Mehta, Second Economic Botanist, Nagpur, Central Provinces, India (Swarup 2000, Kathleen R. Reitsma, personal communication) The present day varieties of muskmelon resistant to race 2 of P. xanthii and to G. cichoracearum owe their origin to this genetic stock. Another snapmelon accession PI 124111, collected from Calcutta (now Kolkatta), India, in 1937 is known for its resistance to powdery mildew (Harwood and Markarian 1968) and downy mildew [Pseudoperonospora cubensis (Berk et Curtis) Rostovzev] (Thomas et al. 1988). Subsequently, Indian snapmelon accessions PI 124112, PI 134192 and PI 414723 provided resistance to various diseases and pests like fusarium wilt (Fusarium oxysporum Schltdl. fsp. melonis Snyder et Hansen), mildews, Zucchini yellow mosaic virus (ZYMV), Papaya ringspot virus (PRSV), Cucurbit aphid borne yellow virus (CABYV) and Aphis gossypii Glover (Pitrat et al. 2000). This has been exploited by muskmelon breeders in developed countries. Recently, PI 124111F (F7 derivative of snapmelon line PI 124111) which was originally found to be resistant to the five pathotypes of P. cubensis, was reported to be resistant to the newly discovered pathotype 6 in Israel (Cohen et al. 2003). In India, during five independent screenings under natural epiphytotic conditions, More (2002) observed five Indian snapmelon genotypes viz. 55-1, 55-2, 77, 113, and 114, resistant to downy mildew. Isozyme analysis indicated this germplasm genetically distinct from PI 124111F and PI 124112. Cucumber green mottle mosaic virus (CGMMV) resistance from Indian snapmelon has been incorporated into Indian muskmelon cultivars (Pan and More 1996).
Furthermore, Indian snapmelon accessions have also been used for creating mapping populations (Baudracco-Arnas and Pitrat 1996; Wang et al. 1997) and establishing taxonomic relationships with other melons (Silberstein et al. 1999; Stepansky et al. 1999a, b; Akashi et al. 2002; Monforte et al. 2003).
Despite this extensive utilization of Indian snapmelons, a comprehensive analysis of genetic variation available in this taxon, has not been performed. To increase the usefulness of this type of melon germplasm for melon conservationists, breeders and growers, the morphological, biochemical and molecular characterization of Indian snapmelons is required.
Different types of genetic markers have been employed to assess the genetic diversity in melon viz. isozymes (Akashi et al. 2002; McCreight et al. 2004), restriction fragment length polymorphisms (RFLPs; Neuhausen 1992), amplified fragment length polymorphism (AFLPs; Garcia-Mas et al. 2000), random amplified polymorphic DNA (RAPDs; Staub et al. 2004) and simple sequence repeats (SSRs; Monforte et al. 2003). All of these have been equally effective in establishing genetic relationships between melon genotypes.
A National Agricultural Technology Project (NATP), funded by the World Bank, has enabled us to collect snapmelon landraces from two agro-ecological regions of India representing nine agro-climatic sub-regions, dispersed over three states in the north-western plains of India. We used morphological and disease/pest resistance data, biochemical traits and RAPDs to (1) assess snapmelon genetic diversity and; (2) determine the relationships of snapmelon to other Indian melons. The variation detected among Indian snapmelons was compared to the reference accessions of melon from diverse origin (S.E. Asia, S. Asia, W. Asia, Europe) (Monforte et al. 2003, 2005) using a set of SSR markers (Danin-Poleg et al. 2001; Gonzalo et al. 2005). These analyses provided the insight into the horticultural worth of Indian snapmelons which is imperative for the organization and conservation of snapmelon genetic resources and its further utilization.
Materials and methods
Plant material
Thirty six landraces of snapmelon (Table 1) were collected from the three states in north west India, namely Punjab, Haryana and Rajasthan, representing two agro-ecological regions (Sehgal et al. 1992) and nine agro-climatic sub-regions (Ghosh 1991) (Fig. 1). In addition, sixteen accessions corresponding to a broad range of melon horticultural types (Monforte et al. 2003, 2005) and Indian accessions belonging to agrestis, reticulatus and one unknown type (Wanga) were also included in the study as reference genotypes (Table 2). Accessions shown in Table 1 were used for RAPD analysis. Twenty seven accessions of snapmelon (Table 1; No 1–3, 13–36) were assessed for morphological, biochemical and disease/pest resistance analyses and these twenty seven accessions together with accessions depicted in Table 2 (reference genotypes) were used for SSR analysis. (Nine snapmelon accessions i.e. SM-1 to SM-9 were not available during morphological, biochemical, disease/pest evaluation and SSR analysis). Original germplasm maintained through sibbing was used for molecular study and ZYMV, PRSV and A. gossypii resistance evaluations, while the inbreds derived from this germplasm were used for rest of the evaluations. The nomenclature of C. melo followed by us and its equivalents in Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops has been provided in Table 3.
Morphological evaluation
An evaluation of the morphology and productivity of snapmelon accessions was carried out in 2004 at the Punjab Agricultural University, Ludhiana, India. Accessions were initially sown in compost and seedlings at the three-leaf stage were transplanted to the field. Each of the three replications containing ten plants were arranged in a randomized complete block design such that plant spacing was equivalent to 1.3 plants/m2. Plants were furrow irrigated and fertilized and treated against pathogens and pests according to standard agronomic practice. Five central plants of each entry in each replication were used for taking observations. The following traits were recorded: (1) vine length at fruit maturity, (2) number of primary branches, (3) extent of leaf lobing, (4) peduncle attachment, (5) fruit shape, (6) mature fruit colour, (7) fruit cracking pattern, (8) mature fruit flesh colour, (9) fruit flesh texture, (10) fruit number/vine, (11) fruit weight, (12) mature fruit length/diameter ratio.
Biochemical analysis
Five fruits of five plants of each accession in each replication were harvested at fruit splitting stage for biochemical assays. Total soluble solids (TSS) (°B) were examined from fruit juice using a hand refractometer. Ascorbic acid was bioassayed as described by Bajaj and Kaur (1981). Titrable acidity was measured by titration of a fruit juice sample with 0.05 N NaOH, using phenolphthalein as indicator.
Screening for downy mildew and Cucumber mosaic virus (CMV) resistance
Screening for downy mildew resistance was done under natural epiphytotic conditions in the field, during the rainy season (August–September) in 2003 and 2004 for downy mildew resistance and separately for CMV resistance in 2004 and 2005. Crop husbandry practices were the same as those adopted for the morphological evaluation experiment. The test plots for both the resistance studies were surrounded by Luffa aegyptriaca Mill. which became heavily infested with P. cubensis as well as CMV during the September month. When the mildew symptoms were conspicuous, 5 plants with 15 infected leaves per plant were randomly selected from each genotype for disease scoring. A 0–5 scale (Pan and More 1996) was used for individual leaf scoring (0 = no symptoms, 1 = less than 10 isolated spots, 2 = 11–20 isolated spots, 3 = more than 20 spots + patches with more than 30 percent leaf area affected, 4 = necrotic patches and 50% leaf area affected, 5 = necrotic patches and more than 50 percent leaf area affected. Percent Disease Index (PDI) was calculated for each accession by assessing 75 leaves (5 plants × 15 leaves per plant), using the formula: PDI = (Summation of numerical ratings/No of leaves × highest numerical rating) × 100. The genotypes were grouped into five categories on the basis of PDI: (1) 0 = immune (I), (2) 1–12 = resistant (R), (3) 13–25 = moderately susceptible (MS), (4) 26–50 = susceptible (S), (5) > 50 = highly susceptible (HS). Mean PDI ratings for the 2 years was used to define the disease reaction categories in Table 4. For CMV resistance ratings, a 0–4 scale was adopted for individual leaf grading (0 = no symptoms, 1 = 1–25% leaf area with symptoms, 2 = 26–50% leaf area with symptoms, 3 = 51–75% leaf area with symptoms, 4 = 76–100% leaf area with symptoms). PDI was calculated for each accession as described for the downy mildew screening. The accessions were grouped into five categories on the basis of PDI: (1) 0–10 = resistant (R), (2) 11–20 = moderately resistant (MR), (3) 21–30 = moderately susceptible (MS), (4) 31–40 = susceptible (S), (5) > 40 = Highly susceptible (HS) (Table 5)
Screening for root knot nematode (Meloidogyne incognita Chitwood) resistance
The assessment was carried out in infested potted soil, using three replications and five plants per replication. For analysis, 6 week old seedlings were uprooted, washed and scored. An 1–5 root galling index scale was used for scoring the plants: (1) 1–10% galling = resistant (R), (2) 11–25% galling = moderately resistant (MR), (3) 26–50% galling = moderately susceptible (MS), (4) 51–75% galling = susceptible (S), (5) 76–100% galling = highly susceptible (HS)
Screening for ZYMV, PRSV and A. gossypii resistance
Artificial inoculations were performed on plantlets at the first leaf stage by mechanical inoculation of the strain R1A belonging to the wilting pathotype of ZYMV or the strain E2 of PRSV. Plants were incubated in a growth chamber at 25°C/18 °C and 12 h day/12 h night. Visual symptoms were recorded 2–3 weeks later and plants were classified in three groups: resistant (no symptoms), wilting or mosaic symptoms. Plants were tested for A. gossypii (strain NM1) resistance by placing adult wingless adults on plantlets at the first leaf stage. Plants with aphid colonies and leaf curling 8–10 days after inoculation were considered as susceptible, whereas plants with only a few adults, very few larvae and no leaf curling were classified as resistant. Accessions were also tested for resistance to virus transmission by A. gossypii using I17F strain of CMV by placing 3–5 viruliferous aphids per plant. Plants with no virus symptoms 2 weeks later were considered as resistant.
DNA extraction
DNA from ten plants of each accession was extracted from young leaf tissue using the method described by Doyle and Doyle (1990), with modifications suggested by Garcia-Mas et al. (2000) and bulked for subsequent analysis.
RAPD analysis
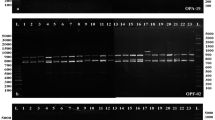
RAPD reactions were performed using random decamers (Biogene, USA, the primers were S 103, S 104, S 106, S 107, S 109, S 112, S 113, S 119, S 120), according to Williams et al. (1990). The reaction contained 15 ng of DNA, 1.5 μM primer, 0.2 mM dNTPs, 2.5 mM MgCl2, commercial Taq DNA polymerase buffer (10 ×) and 1.5 unit of Taq DNA polymerase (Biogene, USA) in a 20 μl final volume. PCR reaction was performed using a Eppendorf Mastercycler with the following cycling profile: An initial denaturation for 5 min at 94 °C was followed by 45 cycles. Each cycle consisted of denaturation at 94 °C for 1 min, primer annealing at 35 °C for 1 min, and elongation at 72 °C for 2 min. This was followed by a final extension step at 72 °C for 10 min. PCR products (20 μl) were electrophoresed on 1.5% agarose gels, stained in ethidium bromide (0.5 μg/μl) and documented using PC based gel documentation system. Each amplicon generated from a primer was treated as a discrete variable and those of the same size were assumed to represent the same genetic locus. Amplicons were scored as either present (1) or absent (0). A pair-wise similarity matrix was determined using Jaccards’ coefficient. UPGMA cluster analysis was performed to develop a dendrogram. All the computations were done using the NTSYSpc-2.02e programme.
SSR analysis
The 18 SSR markers used in the current study (CMGA128, CMTAA166, CMCCA145, CMCTN86, CMAGN75, CMAT35, CMTA134, CMTC160A+B, CMTCN41, CSCCT571, TJ27, TJ2, CMCT38, CMTC168, TJ10, TJ31, CMAT141, CMATN89) were developed previously by Danin-Poleg et al. (2001) and Gonzalo et al. (2005). All SSRs were amplified in a total volume of 15 μl of 1 × SSR buffer (20 mM (NH4)SO4, 75 mM Tris–HCl pH 8.8, 0.01% (v/v) Tween 20), 2 mM MgCl2, 166 μM dNTPs, 2 pmol of each primer and 2 units of Taq DNA polymerase (Applied Biosystems, Foster City, CA). SSR amplifications were performed in PTC 200 thermocyclers with one of the primers labeled with IRD-800. Cycling conditions were as follows; an initial cycle at 94 °C for 1 min followed by 35 cycles at 94 °C for 30 s, 51 °C for 30 s and 72 °C for 1 min and a final cycle at 72 °C for 5 min. Five microlitre of loading buffer (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol) were added to the PCR mix, and the samples were denatured at 100 °C for 10 min. About 0.8 μl were loaded on to a LICOR IR2 sequencer (Li-Cor Inc, Lincoln, Nebraska, USA) using 25 cm plates with 6% acrylamide, 1 × TBE (90 mM Tris-borate, 2 mM EDTA pH 8.0 and 7.5 M urea) and electrophoresis was performed at 1500 V, 35 mA and 31 W at 50 °C until the PCR products were visible. The molecular weight of each microsatellite band was estimated from the acrylamide gel by comparing its migration with the IRD-labelled STR molecular size marker (Li-Cor Inc. Lincoln, Nebraska, USA).
Due to the bulk sampling DNA extraction method, the observation of two or more SSR alleles in a single genotype could have been due to the presence of several heterozygous plants, or homozygous plants for the alternative alleles, or a mixture of both. Under the experimental conditions used for the PCR amplification, it was not possible to quantify the frequency of an SSR allele in the sample based on the band intensity in the gel. Thus, all the detected alleles were assumed to have a frequency of 1/n (n = number of alleles). Factor Correspondence Analysis (FCA) was performed with NTSYSpc 2.11W.
Results
Morphological comparisons and field observations
A detailed description of the snapmelon accessions used in the present study is given in Tables 4 and 5. All the snapmelon accessions were monoecious. Long (>250 cm), medium (150–250 cm) and short (<150 cm) vines were observed in 44%, 37% and 19% of the accessions respectively (Table 3). Leaf lobing was absent in two accessions and an equal number of accessions had high leaf lobing whereas the majority (95.5%) of the accessions had intermediate lobing. A single line IC 274023 possessed frilled leaves. During extraction of inbred lines, the accession IC 274029 segregated for a leaf mutant (75 plants normal and 22 with shoe-string leaves). The range for primary branches/plant was from 2.9 to 11.8. The highest number of primary branches was observed in accessions with medium vine length (Gill Patti Phut and KP 7). There was no association between the number of fruits/vine and the number of primary branches/vine. Sixty six percent accessions exhibited no slip peduncle abscission whereas almost equal number showed half slip (18%) and full slip (15%) mode of abscission. Accession IC 274029 had a conspicuous eight spoke peduncle disc pattern (Fig. 2) which was absent from the rest of the germplasm. Seven types of fruit shape were present in the germplasm viz. round, acorn, oblate, ovate, elongated, elliptical and pyriform (Table 4). The majority of accessions belonged either to ovate (10) or oblate (8) category; the categories elliptical, acorn and pyriform fruit shape were represented by one accession each. Light yellow to yellow mature fruit colour was seen in the majority of accessions (81%) whereas only one accession (IC 274014) had whitish fruit. Likewise, the majority (74%) of the accessions had light yellow to orange fruit flesh. There was no association of mature fruit colour with fruit flesh colour. Snapmelons are better known for mealy flesh texture. Three kind of flesh textures namely soft, crispy and intermediate were tasted in these accessions. Fruit cracks on maturity are a characteristic of snapmelons. We observed distinct genetic polymorphism for fruit cracking pattern (Fig. 2). Fruit cracking was either longitudinal or random starting in the middle of the fruit, whereas round fruits always displayed blossom end cracking. Fruit cracking was absent in two accessions (IC 267360, IC 267353). In some cases, instead of fruit cracking, only skin peeling (again either longitudinal or random) occurred (Fig. 2). Fruits of two accessions IC 274029 and IC 274030, burst on the second day after cracking (Fig. 2). This bursting led to the forced ejection of seed which was seen scattered at some distance (15–20 cm) from the fruit. Farmers at the site of collection are aware of this trait and sell fruits before the cracking commences. The higher fruit weight of these two lines was liked by the farmers. The average number of fruit/plant ranged between 1.0 and 3.5. Average accession fruit weight ranged between 0.239 kg and 1.4 kg. The two accessions with highest fruit weight had produced the lowest number of fruits/vine. Furthermore, in the farmers’ field, under rained conditions, variability in snapmelon landraces was also observed for maturity, rind thickness, seed cavity size and flesh thickness (data not presented). Skin luster was prominent in five accessions (IC 297360, IC 267384, IC 274013, IC 274017, IC 274023).
Biochemical comparison
The TSS, ascorbic acid and titrable acidity values of the 27 snapmelon accessions are shown in Table 6. Their total sugars ranged between 2.0 and 5.3°B. Ascorbic acid and titrable acidity of mature fruits ranged between 1.6 and 34.1 mg/100 g of fresh weight and 0.08–0.61% respectively. Accessions SP 3 and IC 267378 contained significantly (P < 0.05) more ascorbic acid than other accessions (34.1 and 33.8 mg/100 g of fruit weight, respectively). Accessions IC 274021 (0.61%) and IC 267360 (0.57%) were significantly (P < 0.05) more acidic than the other landraces.
Evaluation for pest and disease resistance
Three accessions (IC 267353, IC 274029, KP 7) were resistant to downy mildew (Table 6). One accession (IC 274014) was resistant to CMV and it was confirmed through artificial inoculation also but symptoms were not severe overall, with the average PDI for the non-resistant accessions being c. 39 (range of 24–56). The least root galling index (1.3, resistant category) was observed on landrace IC 274023. IC 267377 was the only accession which provided some plants with wilting symptoms after inoculation with ZYMV. Accessions IC 274007 and IC 274014 were heterogeneous for their susceptibility to ZYMV as some plants exhibited no symptoms. All other accessions (except PI 414723 as a control) were susceptible with mosaic symptoms. For PRSV, several accessions (IC 267360, IC 267363, IC 267374, IC 267384, IC 274006, IC 274007, IC 274010, IC 274011 and IC 274013) were heterogeneous with susceptible plants showing typical mosaic symptoms and there were plants without symptoms or necrotic local lesions. When tested with A. gossypii, three accessions (IC 267353, IC 267384 and IC 274010) were heterogeneous and comprised resistant plants. The same accessions were also segregating for CMV transmission resistance. All the plants of other accessions were fully susceptible to A. gossypii and to the CMV transmission.
Molecular characterization
RAPD analysis
Among 104 bands observed, only three were monomorphic and the remaining were polymorphic in at least two pair wise comparisons between accessions. The highest number of bands (20) was generated by the primer S 113 while the lowest number (8) was generated with S 120 and the mean number of bands amplified was 11.5. The highest number of polymorphic bands per primer was 20, generated by S 113. The percentage of polymorphic bands detected ranged from 80 to 100, with a mean of 96.6. This high polymorphism is in agreement with previous work on melon (Silberstein et al. 1999; Lopez-Sese et al. 2003). The resultant phenogram (Fig. 3) grouped 36 snapmelon accessions into 6 clusters. One accession remained unclustered. Cluster 1 is represented by the six accessions collected from the same sub-region, the Tonk district of Rajasthan. In cluster 2, out of seven accessions, five were from the Karnal district of Haryana while the other two were from Punjab or a different sub-region of Haryana. Cluster 3 contained snapmelon accessions from the Punjab. Cluster 4 and 5 contained two accessions each of different sub-regions of Rajasthan. The unclustered accession of snapmelon (IC 267360), originated from the Jalor district of Rajasthan. This accession was very rich in ascorbic acid and had the second highest number of fruits of moderate weight and was devoid of fruit cracking. The two snapmelon accessions in cluster 7 originated from the two different ecological regions as well as the sub-regions of Punjab. Two accessions of var. agrestis (Wild Chibber and Ra Chibber) and one unknown variety of melon (Wanga) were grouped together in cluster 3 with var. momordica. Two cultivars of var. reticulatus (Hara Madhu, Punjab Sunehri) were clustered separately (cluster 6).
SSR analysis
A total of 232 SSR alleles (12.2 alleles per SSR locus) were observed among the studied genotypes. An average of 10.3 alleles per SSR was found in the Indian snapmelon collection) and 7.5 among the reference genotypes. Eighty nine alleles (38.4%) were present only within the Indian snapmelon accessions and 36 alleles present in the reference genotypes (15.5%) were not found among the Indian snapmelon accessions. Important differences were also observed in within accession variability, the average heterozygosity was 0.42 for the Indian snapmelon accessions, whereas it was only 0.09 for the reference genotypes.
The plot of the two first axis of the FCA performed with the SSR data is shown in Fig. 4. All the snapmelon accessions (including snapmelons used as reference genotypes) are grouped in the center of the plot. Cultivars belonging to the var. reticulatus, ameri, flexuosus and inodorus genotypes are clustered on the right side of the plot, whereas reference accessions of Indian, Korean, Maldivian and Japanese origin were plotted on the left.
Discussion
Analysis of plant diversity using precise morphological and molecular evaluation of regional collections is useful for germplasm curators and plant geneticists as it helps to define accessions by geographical regions and gives a solid historical reference data for future genetic studies aimed at assessing genetic erosion, exploration potential and the site conservation priorities. We assembled a set of 36 snapmelon landraces from two agro-ecological regions of India (comprising three agro-climatic sub-regions of Punjab, two sub-regions of Haryana and four sub-regions of Rajasthan) representing the three states of the northwestern plains of India. These regions and sub-regions have been classified according to National Bureau of Soil Survey and Land Use Planning (NBSS & LUP) and National Agricultural Research Project (NARP) classification, respectively (Ghosh 1991; Sehgal et al. 1992). Each agro-ecological region has fairly uniform growing period and climatic land form and soil type. The sub-regions have been delineated in each ecological zone on the basis of the characteristics of soil, topography, climate and water resources.
The common perception of a snapmelon is that it is an Indian fruit which is low in sugar, has a mealy texture and which cracks at maturity. Our study of morphological traits of Indian snapmelon landraces sampled from the northwestern Indian plains, revealed a plethora of diversity in plant habit, fruit traits, biochemical value, resistance to pests and diseases. Various types of fruit shapes (7), fruit colours (4), fruit flesh colour (5), flesh texture (3) exist in snapmelons. Genotypic specific patterns of fruit cracking and skin peeling are apparent. Even absence of fruit disintegration at maturity was noticed in two landraces. We were informed that the farmers had consciously practiced selection for this trait because the non-cracked mature snapmelon fruits are preferred by the modern consumer. This is also important from the transport, storability and hygienic point of view. All the three kinds of peduncle abscission, typical of the present day cultivars of sweet melon, are available in snapmelons. Different kinds of leaf lobing, frilled leaves in one accession, and fruit bursting on the second day after cracking are specific traits of few snapmelon accessions. It seems India abounds in snapmelon variability. Snapmelon accessions SP 3 and IC 267378 contain appreciable amount of ascorbic acid (34.1 and 33.8 mg/100 g of fruit weight, respectively). The range of ascorbic acid is wide in snapmelons. More germplasm of snapmelon should be surveyed for higher ascorbic acid content.
In general, muskmelon sweetness alone determines muskmelon quality (Yamaguchi et al. 1977) while in other horticultural fruits, the sugar/acid ratio is the indicator of fruit quality (flavour) A combination of high sugar and high acid was unavailable in a previous survey of C. melo (Stepansky et al. 1999a, b; Burger et al. 2003). The range of titrable acidity (%) in commercial Indian sweet melons is 0.12–0.2. Interestingly, Burger et al. (2000) have demonstrated that sugar and acid accumulation in melon are under independent genetic control and it is possible to combine high sugar and acid content in one genotype. Our survey of Indian snapmelon landraces indicates a high genetic variability for acidity in this species. Accessions IC 274021 (0.61%) and 267360 (0.57%) appear to be very good sources of organic acid, needed for the genetic improvement of this trait in sweet melons.
Variability in snapmelon accessions was found for pest and disease resistance. Cohen et al. (2003) reported that the Indian population of P. cubensis (able to infest Luffa spp.) differ from the populations in Japan, USA and Israel, which are incompatible with Luffa spp., and therefore can be classified as a different pathotype. Accessions IC 267353, IC 274029 and KP 7, resistant to this Indian pathotype of P. cubensis, should be tested against the six pathotypes existing in the other parts of the world. Accession PI 124111F resistant to all the six classified pathotypes of P. cubensis, is susceptible to the Indian pathotype of P. cubensis (More 2002). The accession KP 7 has also been reported resistant to downy mildew in previous studies (Lal et al. 1994; Singh et al. 1996). Only one accession (IC 274014) was highly resistant to CMV. None of the accession was killed by the virus. It is known that snapmelons are generally tolerant to CMV in conditions under which muskmelon genotypes are killed at the four leaf stage, and will produce reasonable yields. CMV resistance has been described as quantitative, recessive and oligogenically controlled in a number of Oriental melon lines (Dogimont et al. 2000) and it is not easy to exploit this for developing melon F1 hybrids. It would be of interest to study the genetic control of CMV resistance in IC 274014. No commercial sweet melon is resistant to root knot nematode. Snapmelon accession IC 274023, resistant to M. incognita (root galling index of 1.3) is a potential source of nematode resistance in sweet melon breeding programmes. The resistance of this line has also been verified in the sick field. Sources of potential resistance to ZYMV have been observed in IC 274007 and IC 274014. Earlier, only PI 414723, another snapmelon accession from India, has been described as resistant to this virus with a monogenic (Pitrat and Lecoq 1984) or an oligogenic genetic control (Danin-Poleg et al. 1997). After achieving homozygosity for resistance through selfing, it would be interesting to compare the genetic control of resistance in these two IC accessions with PI 414723.
Nine snapmelon accessions namely IC 267360, IC 267363, IC 267374, IC 267384, IC 274006, IC 274007, IC 274010, IC 274011 and IC 274013 were observed to be segregating for PRSV resistance. Two alleles (Prv 1 and Prv 2) at one locus have been described for PRSV resistance and both of these originated in Indian accessions PI 180280 and PI 180283, respectively. After stabilizing resistance through selfing of these nine snapmelon accessions, allelism tests would be performed.
Resistance to A. gossypii colonization and to CMV transmission has been observed in IC 267353, IC 267384 and IC 274010. This type of resistance has already been described in snapmelon accessions from India (PI 414723 – Kishaba et al. 1971), Far-East (PI 161375 – Lecoq et al. 1979; Yoshida and Kohyma 1986) or Zimbabwe (TGR 1551 – Soria et al. 2000) with a monogenic inheritance.
RAPD based cluster analysis has clearly indicated that there is no agro-ecological separation of snapmelon germplasm but most of the accessions can be grouped into clusters corresponding to agro-climatic sub-regions. Accessions KP 7, Gill Patti Phut and SP 3 are the exceptions. Accessions KP 7, Gill Patti Phut and IC 267360 are highly divergent from the other accessions. Evaluation of variation at 101 RAPD loci indicated that 36 snapmelon landraces exhibited greater genetic diversity and our assessment demonstrates that sub-region specific sampling of snapmelon germplasm is of benefit in accessing genetic variability. Our field tests have indicated that these snapmelons from the arid zones, survive and are productive under severe field drought conditions, whereas muskmelon varieties failed to survive under similar situations (data not presented). Isozyme studies by Akashi et al. (2002) have shown that Western Indian melons are rich in genetic variation and those originating in high rainfall areas of India (Assam) and of Myanmar are tolerant of wet conditions. Similarly, isozyme studies by McCreight et al. (2004) confirmed that Indian melon germplasm of Rajasthan origin is rich in genetic diversity.
Clustering together of the accessions of var. agrestis and var. momordica and separate clustering of the accessions of var. reticulatus is comparable to the infraspecific division proposed by Jeffrey (1980). Also, our results support the previous reports on molecular variation in C. melo based on RAPDs and ISSRs (Stepansky et al. 1999a, b) and SSRs (Monforte et al. 2003). Hara Madhu and Punab Sunehri, the two lines of var. reticulatus (cluster 6) are also related by descent. Punjab Sunehri is the F8 derivative of a cross between Hara Madhu and Edisto. Landraces of var. agrestis are found growing wild in waste places, along water channels, in the area also occupied by var. momordica (snapmelon). It might therefore be expected that there is an exchange of genetic material between these two taxa, hence their clustering together (Fig. 3). The two morphotypes of var. agrestis (Wild Chibber and Ra Chibber) differ in size and fruit weight. The fruits of Wild Chibber are <5 cm and weigh 15–20 g whereas those of Ra Chibber are 8–10 cm and weigh 60–80 g. Through repeated backcrossing of the F1 (Wild chibber × snapmelon) to the Wild Chibber, we were able to construct genotypes similar to the Ra Chibber. This suggests a genetic exchange between var. agrestis and var. momordica. Moreover, in the area (e.g. district of Amritsar) where snapmelon is not cultivated, the Ra Chibber is not found but Wild Chibber is abundant. The fact that var. agrestis from tropical Asia and var. momordica share the same isozyme alleles, led Morri et al. (1980) to postulate that there is genetic interchange between these two varieties. The var. agrestis germplasm was observed to be tolerant of moisture stress in the field. No systematic effort has been made to collect var. agrestis germplasm and study its diversity. The melon landrace ‘Wanga’ is genetically more closer to var. momordica and var. agrestis accessions (Fig. 3). The fruit of this accession is ovate. The exterior of the fruit is green and lightly sutured. It is of bland taste like var. agrestis and is co-cultivated with snapmelons only in rain-fed areas. Immature fruit (200–300 g) is used as salad. Var. momordica and var. agrestis landraces might have contributed to its origin.
Using 18 SSR markers, we performed FCA (Fig. 4) on Indian melons (Snapmelons: 1–3, 13–36, Table 1 and agrestis and reticulatus accessions), and melons originating from Spain, Korea, Japan, Israel, Iraq, Maldives, Pakistan (Table 2). The importance of the genetic variability in the snapmelon collection can be better appreciated on comparing it with the genetic variability observed in the reference genotypes. Most of the alleles (85%) observed in all the tested genotypes are present in the snapmelon collection and a high proportion (38%) are unique to the snapmelon germplasm. Though a larger sample of reference population should have been used, these results indicate that the current snapmelon collection contains a degree of unique genetic variability which needs to be preserved for future use. Also it supports the previous observations of Akashi et al. (2002) and Monforte et al. (2003) suggesting that the process of introduction and domestication of melons into these two regions could have led to the erosion of the variability. The snapmelon accessions were plotted in the center of the FCA plot. This result confirms that India is the primary centre of melon diversity and the Mediterranean and the countries around the China Sea correspond to two extremes of the geographical distribution of C. melo.
In conclusion, this work shows that Indian snapmelon landraces display a considerable diversity coupled with good horticultural characteristics and there appears to be sub-regional differentiation at the molecular level. India is divided into 21 agro-ecological regions comprising 131 agro-climatic sub-regions. This concept of regions and sub-regions can be adopted for future snapmelon explorations in India for ensuring the retention of existing variability. Threats to biodiversity are increasing in India due to population pressure on cultivatable land. Sweet melon evolution under domestication has resulted in better productivity and fruit quality. However, this process narrowed the genetic basis. Natural variation among the relatives of sweet melon (var. momordica for increased high acidity, high tolerance to biotic and abiotic stresses; var. agrestis for large number of fruit per vine, drought tolerance and disease resistance) provides an opportunity to enrich the gene pool of sweet melon with novel alleles that eventually could improve productivity, quality and adaptation and lessen the risk of genetic vulnerability. In the recent past, such agronomically inferior germplasm has helped to break yield, quality and adaptability barriers in various crops (Tanksley and McCouch 1997; Schaffer et al. 1999; Gur and Zamir 2004).
References
Akashi Y, Fukuda N, Wako T, Masuda M, Kato K (2002) Genetic variation and phylogenetic relationships in East and South Asian melons, Cucumis melo L., based on the analysis of five isozymes. Euphytica 125:385–396
Bajaj LK, Kaur G (1981) Spectrophotometric determination of l-ascorbic acid in vegetables and fruits. Analyst 106:117–120
Baudracco-Arnas S, Pitrat M (1996) A genetic map of melon (Cucumis melo L.) with RFLP, RAPD, isozyme, disease resistance and morphological markers. Theor Appl Genet 93:57–64
Burger Y, Sa’ar U, Distelfeld A, Katzir N (2003) Development of sweet melon (Cucumis melo) genotypes combining high sucrose and organic acid content. J Amer Soc Hort Sci 128:537–540
Cohen Y, Meron I, Mor N, Zurial S (2003) A new pathotype of Pseudoperonospora cubensis causing downy mildew in cucurbits in Israel. Phytoparasitica 31:452–466
Danin-Poleg Y, Paris HS, Cohen S, Rabinowitch HD, Karchi Z (1997) Oligogenic inheritance of resistance to zucchini yellow mosaic virus in melons. Euphytica 93:331–337
Danin-Poleg Y, Reis N, Tzuri G, Katzir N (2001) Development and characterization of microsatellite markers in Cucumis. Theor Appl Genet 102:61–72
Dogimont C, Lecomte L, Perin C, Thabuis A, Lecoq H, Pitrat M (2000) Identification of QTLs contributing to resistance to different strains of cucumber mosaic cucumovirus in melon. Acta Hort 510:391–398
Doyle JJ, Doyle JL (1990) Isolation of DNA from fresh tissue. Focus 12:13–15
Duthie JF (1905) Flora of the Upper Gangetic Plain and the adjacent Sivalik and sub-Himalyan tracts. Vol 1. Ranunculaceae to Companulaceae. Office of the Supereintendent of Government Printing, Calcutta
Garcia-Mas AJ, Oliver M, Gomez-Paniagua H, DeVicente MC (2000) Comparing AFLP, RAPD and RFLP markers for measuring genetic diversity in melon. Theor Appl Genet 101:860–864
Ghosh SP (1991) Agroclimatic zones specific research–Indian Perespective under NARP. ICAR, Pusa, New Delhi, India
Gonzalo MJ, Oliver M, Garcia-Mas J, Monfort A, Dolcet-Sanjuan R, Katzir N, Arus P, Monforte AJ (2005) Development of a consensus map of melon (Cucumis melo L.) based on high-quality markers (AFLPs and SSRs) using F2 and double-haploid line populations. Theor Appl Genet 110:802–811
Gur A, Zamir D (2004) Unused natural variation can lift yield barriers in plant breeding. PloS Biol 2:1610–1615
Harwood RR, Markarian D (1968) A genetic survey of resistance to powdery mildew in muskmelon. Heredity 59:213–217
Jeffrey C (1980) A review of the Cucurbitaceae. Bot J Linn Soc 81:233–247
Jeffrey C (2001) Cucurbitaceae. In: Hanelt P and Institute of Plant Genetics and Crop Plant Research (eds), Mansfeld’s encyclopedia of agricultural and horticultural crops. Berlin: Springer, pp 1510–1557
Kishaba AN, Bohn GW, Toba HH (1971) Resistance to Aphis gossypii in muskmelon. J Econ Entom 64:935–937
Lal T, Dhiman JS, Dhaliwal MS (1994) Evaluation of snapmelon genotypes for downy mildew resistance. Adv Hort Sci 8:153–155
Lecoq H, Cohen S, Pitrat M, Labonne G (1979). Resistance to cucumber mosaic virus transmission by aphids in Cucumis melo. Phytopathology 69:1223–1225
Lopez-Sese A, Staub JE, Gomez-Guillamon ML (2003) Genetic analysis of Spanish melon (Cucumis melo L.) germplasm using a standardized molecular marker array and reference accessions. Theor Appl Genet 108:41–52
McCreight JD, Staub JE, Lopez-Sese AI, Chung SM (2004) Isozyme variation in Indian and Chinese melon (Cucumis melo L.) germplasm collections. J Amer Soc Hort Sci 129:811–818
Monforte AJ, Garcia-Mas J, Arus P (2003) Genetic variability in melon based on microsatellite variation. Plant Breed 122:153–157
Monforte AJ, Eduardo I, Arus P (2005) Inheritance mode of fruit traits in melon-heterosis for fruit shape and its correlation with genetic distance. Euphytica 144:31–38
More TA (2002) Enhancement of muskmelon resistance to disease via breeding and transformation. Acta Hort 588:205–211
Morii S, Fujishita N, Momodani Y (1980) Phylogenetic analysis in C. melo based on isozyme polymorphism, with special reference to the relationship between weed melon and cultivated melon. J Japan Soc Hort Sci 49:188–189 (Japanese)
Neuhausen SL (1992) Evaluation of restriction fragment length polymorphism in Cucumis melo. Theor Appl Genet 83:379–384
Pan RS, More TA (1996) Screening of melon (Cucumis melo L.) germplasm for multiple disease resistance. Euphytica 88:125–128
Pitrat M, Lecoq H (1984) Inheritance of Zucchini Yellow Mosaic Virus resistance in Cucumis melo L. Euphytica 33:57–61
Pitrat M, Hanelt P, Hammer K (2000) Some comments on infraspecific classification of cultivars of melon. Acta Hort 510:29–36
Schaffer A, Petreikov M, Miron D, Fogelman M, Spiegelman M, Bnei-Moshe Z, Shan S, Granot D, Hadas R, Dai N, Levine I, Bar M, Friedman M, Pilowsky M, Gilboa N, Chen L (1999) Modification of carbohydrate content in developing tomato fruit. HortScience 34:12–15
Sehgal JL, Mandal DK, Mandal C, Vadivelu S (1992) Agro ecological regions of India. National Bureau of Soil Survey and Land Use Planning (ICAR), Nagpur, India
Silberstein L, Kovalski I, Huang R, Anagnostou K, Jahn M, Perl-Treves R (1999) Molecular variation in melon (Cucumis melo L.) as revealed by RFLP and RAPD markers. Sci Hort 79:101–111
Singh PP, Thind TS, Lal T (1996) Reaction of some muskmelon genotypes against Pseudoperonospora cubensis under field and artificial epiphytotic conditions. Indian Phytopathol 49:188–190
Sorria C, Diaz JA, Moriones E, Gomez-Guillamon ML (2000) Resistance to Aphis gossypii and to virus transmission by this aphid in melon. In: Katzir N, Paris HS (eds) Proceedings Cucurbitaceae 2000. Acta Hortic. (ISHS 2000), 510, 305–312
Staub JE, Lopez-Sese AI, Fanourakis N (2004) Diversity among melon landraces (Cucumis melo L.) from Greece and their genetic relationships with other melon germplasm of diverse origins. Euphytica 136:151–166
Stepansky A, Kovalski I, Perl-Treves R (1999a) Intraspecific classification of melons (Cucumis melo L.) in view of their phenotypic and molecular variation. Plant Syst Evol 217:313–332
Stepansky A, Kovalski I, Schaffer AA, Perl-Treves R (1999b) Variation in sugar levels and invertase activity in mature fruit representing a broad spectrum of Cucumis melo genotypes. Genet Resour Crop Evol 46:53–62
Swarup V (2000) Genetic resources in vegetable crops in India. In: Kallo G, Singh K (eds) Emerging scenario in vegetable research and development. Research Periodicals and Book Publishing Home, India, pp 346–355
Tanksley SD, McCouch SR (1997). Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Thomas CF, Cohen Y, McCreight JD, Jourdain EL, Cohen S (1988) Inheritance of resistance to downy mildew in Cucumis melo. Plant Dis 72:33–35
Wang YH, Thomas CE, Dean RA (1997) A genetic map of melon (Cucumis melo L.) based on amplified fragment length polymorphism (AFLP) markers. Theor Appl Genet 95:791–798
Williams JGK, Kubelik AR, Livak KJ, Rafalski J, Tingey SV (1990) DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucl Acid Res 18:6531–6535
Yamaguchi M, Hughes DL, Yabumoto K, Jennings WC (1977) Quality of cantaloupes: variability and attributes. Sci Hort 6:59–70
Yoshida Y, Kohyma T (1986) Mechanisms, genetics and selection methods of aphid resistance in melons, Cucumis melo. Bull Veg Ornam Crops Res Station 9:1–12
Acknowledgements
Ranjana was supported by a Junior Research Fellowship of Indian Council of Agricultural Research. The authors thank Professor G. J. Jellis for helpful comments and F. García for technical assistance. We are grateful to Prof. Karl Hammer and Dr. Klaus Pistrick for providing valuable comments. This work was funded in part by grants AGL2003–09175-C02–01 (to AJM) from the Spanish Ministry of Education and Science. AJM was partly supported by a contract from Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), Spain. IE was supported by a fellowship from the Spanish Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
N. P. S. Dhillon and Ranjana contributed equally to this work and are considered the first authors.
Rights and permissions
About this article
Cite this article
Dhillon, N.P.S., Ranjana, ., Singh, K. et al. Diversity among landraces of Indian snapmelon (Cucumis melo var. momordica). Genet Resour Crop Evol 54, 1267–1283 (2007). https://doi.org/10.1007/s10722-006-9108-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-006-9108-2