Abstract
Snake melon is an important cucurbit crop especially in the Southeastern and the Mediterranean region of Turkey. It is consumed as fresh or pickled. The production is mainly done with the local landraces in the country. Turkey is one of the secondary diversification centers of melon and possesses valuable genetic resources which have different morphological characteristics in case of snake melon. Genetic diversity of snake melon genotypes collected from different regions of Turkey and reference genotypes obtained from World Melon Gene Bank in Avignon-France was examined using 13 simple sequence repeat (SSR) markers. A total of 69 alleles were detected, with an average of 5.31 alleles per locus. The polymorphism information content of SSR markers ranged from 0.19 to 0.57 (average 0.38). Based on cluster analysis, two major groups were defined. The first major group included only one accession (61), while the rest of all accessions grouped in the second major group and separated into different sub-clusters. Based on SSR markers, cluster analysis indicated that considerably high genetic variability exists among the examined accessions; however, Turkish snake melon accessions were grouped together with the reference snake melon accessions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snake melon (Cucumis melo L. subsp. melo flexuosus Group; 2n = 2x = 24) is a member of Cucurbitaceae family. The fruits of this non-sweet melon are long to very long having a ratio of 4:1 or higher fruit length to fruit width (Pitrat et al. 2000; Pitrat 2008). The exterior color of the immature fruits can be yellow-green, light green, and dark green. The surface of the slender fruits are ribbed or wrinkled. The mealy fruit flesh is not sweet and has no aroma. Young fruits are eaten fresh in salad as an alternative to cucumber (Robinson and Decker Walters 1997; Pitrat 2008; Pandey et al. 2010).
Snake melon is a well-known cucurbit crop in Northern Africa, Turkey, Iraq, and India (Pitrat 2008). In Turkey, it is commonly called as “acur,” “hitta,” or “hiti” and the production amount is 27886 tons on 17258 ha area (Anonymous 2014). Southeastern Anatolia region ranks first in production and followed by Mediterranean and Aegean regions (Anonymous 2014). It is widely grown in open field using landraces or local populations and consumed as fresh or as pickles in local markets.
Eventhough Turkey is accepted to be one of the diversification center of snake melon (Vural et al. 2000), few studies were performed on the genetic resources of snake melon. Besirli and Yanmaz (1997), collected and morphologically characterized the local genotypes from Southeastern Anatolia to identify the types of snake melon. Seedling, plant, leaf, flower, fruit, and seed characteristics of snake melon genotypes collected from different regions of Turkey were examined by Solmaz et al. (2004). Morphological and genetic diversity were assessed among snake melon accessions and their relationships with other melons and Cucumis species by random amplified polymorphic DNA markers (RAPD) by Kose (2008).
Genetic diversity analysis facilitates utilization of genetic resources for conservation germplasms, association genetics, and development of breeding programs (Hu et al. 2015). To increase the usefulness of snake melon germplasm for melon conservationists, breeders, and growers, the morphological and molecular characterizations of snake melon are required (Ali-Shtayeh et al. 2015).
Molecular markers have significantly contributed to our knowledge and understanding of genetic differentiation and relatedness in many crops (Nantoume et al. 2013). They are not affected by environmental factors and developmental stages of plant (Esposito et al. 2007; Wang et al. 2015).
Several molecular markers have been effectively used to assess the genetic diversity of melons; however, simple sequence repeats (SSRs) have been proved to be the most useful marker system in recent diversity studies (Tzitzikas et al. 2009; Aierken et al. 2011; Escribano et al. 2012; Kacar et al. 2012; Ning et al. 2014; Raghami et al. 2014; Hu et al. 2015).
The objective of this study was to analyze the genetic diversity of 40 snake melon accessions collected from Turkey and reference accessions obtained from World Melon Gene Bank using SSR markers.
Materials and Methods
This study was carried out in the Department of Horticulture, Faculty of Agriculture, University of Cukurova, Turkey.
Plant Material and DNA Isolation
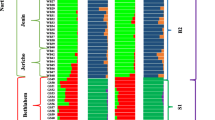
Forty different accessions from the snake melon genetic resources collection of Cukurova University, Faculty of Agriculture, Department of Horticulture were used in this study. Among them, 26 accessions were collected from geographically diverse areas of Turkey and 14 reference accessions supplied from INRA (Institut Nationale de la Recherche Agronomique Avignon, France). Details about names, origins, and seed suppliers of these accessions are listed in Table 1.
Seedlings of snake melon accessions were grown in greenhouse. Young leaf samples were collected from each genotype when seedlings were at 2–3 true leaf stage and directly frozen in liquid nitrogen. Genomic DNA was extracted from the leaf samples following the CTAB miniprep protocol (Edwards et al. 1991). The concentration and quality of extracted DNA was measured with a NanoDrop, ND-100 spectrophotometer (NanoDrop Technologies, Inc.). DNA was diluted in water to a final concentration of 50 ng/μl and stored at −20 °C.
SSR Analysis
Thirteen SSR primers (CMACC146, CMCTT144, CMGA172, CMCCA145, CMAGN59, CSWCT10, CMAGN68, CMMS2-3, CMTCN9, TJ 24, CMAGN73, CMGT108, and CMCT160a) described previously (Danin-Poleg et al. 2001; Fazio et al. 2002; Chiba et al. 2003; Gonzalo et al. 2005) were used. PCR amplification was performed in a 10-μL volume containing 2× PCR Mastermix (Fermentas K0171), 1 unit of Taq DNA polymerase (Fermentas EP0402), 25 mM MgCl2, 1 μM of each primer, and 25 ng of sample DNA. The amplification was performed in a Master Gradient thermal cycler (Eppendorf) using a program consisting of an initial denaturation step of 2 min at 94 °C followed by 35 cycles of 2 min at 94 °C, 1 min at 55 °C, and 2 min at 72 °C; the program ended with a 10-min elongation step at 72 °C. PCR products were stored at 4 °C prior to analysis. After amplification, 1–25 µl of loading buffer containing 95 % formamide, 10 mM EDTA (ph 8.0), 0.025 % xylene cyanol, and 0.025 % bromophenol blue was added to each reaction tube. The samples were heat denatured for 5 min at 95 °C and quickly cooled on ice. After loading 1.0 µl of each sample, PCR products were separated in a 25-cm, 6 % denaturing polyacrylamide gel (Long Ranger, FMC Biozym, Hessisch Oldendorf, Germany) that had been preheated for 25 min. Electrophoresis was conducted at 1500 V, 50 W, 35 mA, and 48 °C using a Li-Cor DNA Analyzer 4300 (Licor Biosciences, Bad Homburg, Germany). A 50–350 bp DNA ladder (MWG Biotech AG, Ebersberg, Germany) was used to determine DNA sizes.
Statistical Analysis
Bands were scored as present (1) or absent (0), and then data were used to generate a pair-wise similarity matrix using Jaccard’s coefficient (Jaccard 1908). The un-weighted pair-group method using UPGMA was employed to create the clustering dendrograms using the NTSYS-PC software (Rohlf 1998). Polymorphism information content (PIC) values were calculated according to Smith et al. (1997), using the algorithm for all primer combinations as follows:
where i = 1 is the frequency of the ith allele.
Results
Characterization of SSR Polymorphism
With the 13 SSRs loci used, a total of 69 alleles were detected among 40 snake melon accessions (Table 2). The number of alleles detected by each primer set ranged from 2 (CMCCA145) to 11 (CMAGN68) with an average of 5.31. Variation of allele sizes ranged from 58 to 226 bp. All SSRs were found to be polymorphic (polymorphism rate: 100 %) among snake melon accessions. PIC values for SSRs ranged from 0.19 to 0.57 (Table 2) with a mean PIC of 0.38. The highest PIC value (0.57) was recorded for CMCCA145 and followed by CMMS2-3 (0.51) and CMGA172 (0.47).
Genetic Similarity
Genetic similarities among the accessions ranged from 0.08 to 0.94 (data not shown). The most related accessions were AC 4 (Siyah acur from Turkey) and AC 75 (PI 220170 from Afghanistan) with 0.94 genetic similarity followed by 0.89 between AC 4 and AC 33. The lowest similarity rate was 0.07 and obtained between AC 51 (Hitta from Turkey) and AC 62 (Ames 20836 from India). Low level of similarity (0.08) was also found between AC 61 (Ames 20443 from India) and AC 14 (Midyat 1 from Turkey), AC 21 (Sanliurfa, from Turkey), AC 27 (TR 40517 from Turkey), and AC 39 (TR 40328 from Turkey).
Cluster Analysis Based on SSR Markers
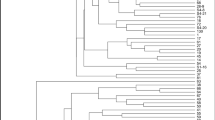
Cluster analysis (UPGMA) employing SSR data resulted in a dendrogram with two main groups (Fig. 1). In the first group, AC 61 (Ames 20443 from India) was clearly separated from all remaining accessions (Node 1). The second main group was also partitioned into several sub-clusters. AC 62 (Ames 20836 from India) and AC 5 (Hitta from Turkey) clustered together and differentiated from the other accessions with 0.40 similarity rate (Node 2). In Node 3, AC 51 (Hitta from Turkey), AC 55 (Unknown from Turkey), and AC 14 (Midyat from Turkey) were separated from the other accessions and grouped together. The clustering dendrogram was also divided into two sub-groups from Node 4. The first sub-group contained AC 78 (PI 525133 from Egypt), AC 26 (TR 40308 from Turkey), and AC 20 (Minehoyuk from Turkey) while the second contained a large clustering group which was further sub-divided from Node 5. Two main sub-groups containing most of the accessions were defined and partitioned into several sub-clusters (Node 5). The first sub-group contained 18 accessions (AC 44, AC 33, AC 80, AC 74, AC 8, AC 77, AC 27, AC 21, AC 63, AC 60, AC 18, AC 6, AC 82, AC 39, AC 66, AC 79, AC 9, AC 75, and AC 4) from Turkey and reference accessions originated from different countries. Accessions AC 4 (Siyah acur from Turkey) and AC 75 (PI 220170 from Afghanistan) were clustered very closely (genetic similarity: 0.94) even they have diverse countries of origin and variation in morphological traits. The second sub-group consisted of 12 accessions (AC 12, AC 81, AC 67, AC 16, AC 47, AC 54, AC 25, AC 28, AC 57, AC 32, AC 40, and AC 64) which was further sub-divided into different groups.
Discussion
Genetic diversity among Turkish snake melon genotypes was evaluated by SSR markers. Microsatellites or SSRs have been considered one of the most powerful Mendelian markers (Jarne and Lagoda 1996) because of their high reproducibility, co-dominance inheritance, multi-allelic character, and extensive genome coverage (Powell et al. 1996). They have been proven useful molecular markers in recent diversity studies of Turkish melons (Kacar et al. 2012; Yildiz et al. 2014) which focused on assessing genetic diversity of different groups of Cucumis melo L. and reveal a high genetic variation. In this present study, we studied genetic diversity of only Flexuosus Group genotypes collected from different regions of Turkey and reference genotypes using SSR markers.
In our study, the genetic variation among 40 snake melon accessions was assessed with the 13 SSRs loci and a total of 69 alleles were detected. The number of alleles by each primer set ranged from 2 to 11 which was similar to that reported by Ning et al. (2014) who analyzed the genetic diversity among Chinese Hami melon and its relationship with melon germplasm of diverse origins by microsatellite markers. The average number of alleles per SSR marker was 5.31 and found to be higher than those obtained by Ning et al. (2014) in 36 SSR markers (4.03) and Yildiz et al. (2014) in 16 SSR markers (4.9) who characterized twenty-four accessions covering different groups of Cucumis melo L. from Eastern and Southeastern Anatolian regions of Turkey using morphological traits, ISSR and SSR markers. However, higher number of alleles were detected in some previous studies. Kacar et al. (2012) reported 6.55 alles on 96 melon genoytpes consisted of var. inodorus, var. dudaim, var. cantalupensis, var. agrestis, and var. flexuosus genotypes. Similarly, Monforte et al. (2003) detected 6.3 alleles on a collection of 27 melon (Cucumis melo L.) accessions, representing a broad range of wild and cultivated melons. High values in these reports may be due to different germplasms and various subspecies of melons used in these studies.
The polymorphism percentage among snake melon accessions using 13 SSR markers was 100 % in this present study which was in agreement with Tzitzikas et al. (2009) who used 17 SSR markers to investigate the genetic diversity and population structure of traditional Greek and Cypriot melon cultigens. Monforte et al. (2003) found 100 % polymorphism using 18 SSR markers which is in accordance with our results. These findings proved the usefulness of SSR markers in melon diversity analysis.
In our study, the PIC values for SSRs ranged from 0.19 to 0.57 with a mean of 0.38 which was lower than those reported by Raghami et al. (2014) who studied the genetic variation of 24 Iranian melon accessions using 18 SSR markers and obtained 0.49 mean PIC value. Similarly, Ning et al. (2014) also reported higher value of PIC (0.45) as well. This difference might be the result of different germplasm they used and various subspecies of melons they examined. Another reason might be more informative SSRs that they were tested when compared to our study.
The similarity coefficient ranged from 0.08 to 1.00 employing SSR analysis and all snake melon accessions could be distinguished. Cluster analysis also supported this finding; however, neither the geographical origin nor the morphological traits were correlated with the clustering groups of snake melon accessions. This result was in concordance with the report of Kacar et al. (2012) indicating no correlation between grouping and geographical origin of melon genotypes.
Our results revealed that high degree of genetic variability exists among Turkish and reference snake melon accessions in agreement with Kose (2008) who studied the genetic diversity of Turkish snake melon and its relation with other Cucumis species by RAPD markers. Similarly, Soltani et al. (2010) who studied Iranian melon accessions using RAPD markers and observed a high genetic diversity in var. flexuosus.
References
Aierken Y, Akashi Y, Phan TPN, Halidan Y, Tanaka K, Long B, Nishida H, Long CL, Wu MZ, Kato K (2011) Molecular analysis of the genetic diversity of Chinese Hami melon and its relationship to the melon germplasm from Central and South Asia. J Jpn Soc Hortic Sci 80:52–65
Ali-Shtayeh, Jamous RM, Shtaya MJ, Mallah OB, Eid IS, Abu Zaitoun SY (2015) Morphological characterization of snake melon (Cucumis melo var. flexuosus) populations from Palestine. Genet Resour Crop Evol. doi:10.1007/s10722-015-0329-0
Anonymous (2014) TUİK Turkish Statistical Institute, www.tuik.gov.tr
Besirli G, Yanmaz R (1997) Types of the snake cucumber (Cucumis melo var. flexuosus Naud.) Grown in the south east region of Turkey. In: Proceedings of the First International Symposium on Cucurbits, 20–23 May 1997, Adana Acta Hortic 492:37–40
Chiba N, Suwabe K, Nunome T, Hirai M (2003) Development of microsatellite markers in melon (Cucumis melo L.) and their application to major cucurbit crops. Breed Sci 53:21–27
Danin-Poleg Y, Reis N, Tzuri G, Katzir N (2001) Development and characterization of microsatellite markers in Cucumis. Theor Appl Genet 102:61–72
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349
Escribano S, Lazaro A, Cuevas HE, Loopez-Sese AI, Staub JE (2012) Spanish melons (Cucumis melo L.) of the Madrid provenance: a unique germplasm reservoir. Genet Resour Crop Evol 59:359–373
Esposito MA, Martin EA, Cravero VP, Cointry E (2007) Characterization of pea accessions by SRAP’s markers. Sci Hort 113:329–335
Fazio G, Staub JE, Chung SM (2002) Development and characterization of PCR markers in cucumber (Cucumis sativus L.). J Am Soc Hortic Sci 127:545–557
Gonzalo MJ, Oliver M, Garcia-Mas J, Monforte A, Dolcet-Sanjuan R, Katzir N, Arús P, Monforte AJ (2005) Development of a consensus map of melon (Cucumis melo L.) based on high-quality markers (RFLPs and SSRs) using F2 and double-haploid line populations. Theor Appl Genet 110:802–811
Hu J, Wang P, Su Y, Wang R, Li Q, Sun K (2015) Microsatellite diversity, population structure, and core collection formation in melon germplasm. Plant Mol Biol Rep 33:439–447
Jaccard P (1908) Nouvelles sur la distribution florale. Bulletin de la Société Vaudoise des Sciences Naturelles 44:223–270
Jarne P, Lagoda PJL (1996) Microsatellites, from molecules to populations and back. Trends Ecol Evol 11:424–429
Kacar YA, Simsek O, Solmaz I, Sari N, Mendi YY (2012) Genetic diversity among melon accessions (Cucumis melo) from Turkey based on SSR markers. Genet Mol Res 11:622–4631
Kose MT (2008) Genetic and morphological characterization of Turkey’s snake melon (Cucumis melo var. flexuosus) M.Sc. Thesis University of Cukurova, Adana
Monforte AJ, Garcia-Mas J, Arus P (2003) Genetic variability in melon based on microsatellite variation. Plant Breed 122:153–157
Nantoume AD, Andersen SB, Jensen BD (2013) Genetic differentiation of watermelon landrace types in Mali revealed by microsatellite (SSR) markers. Gen Res Crop Evol 60:2129–2141
Ning X, Xiong L, Wang X, Gao X, Zhang Z, Zhong L, Li G (2014) Genetic diversity among Chinese Hami melon and its relationship with melon germplasm of diverse origins revealed by microsatellite markers. Bioch Syst and Ecol 57:432–438
Pandey S, Dhillon NPS, Sureja AK, Singh D, Malik AA (2010) Hybridization for increased yield and nutritional content of snake melon (Cucumis melo L. var. flexuosus). Plant Genet Resour 8:127–131
Pitrat M (2008) Melon. In: Prohens J, Nuez F (eds) Handbook of Plant Breeding, vol 1. Springer, New York, pp 283–315
Pitrat M, Hanelt P, Hammer K (2000) Some comments on infraspecific classification of cultivars of melon. Acta Hortic 510:29–36
Powell W, Machray GC, Provan J (1996) Polymorphism revealed by simple sequence repeats. Trends Plant Sci 1:215–222
Raghami ML, Lopez-Sese AI, Hasandokht MR, Zamani Z, Moghadam MRF, Kashi A (2014) Genetic diversity among melon accessions from Iran and their relationships with melon germplasm of diverse origins using microsatellite markers. Plant Syst Evol 300:139–151
Robinson RW, Decker-Walters D (1997) Cucurbits. Cab International, New York
Rohlf FJ (1998) NTSYS-PC Numerical Taxonomy and Multivariate Analysis System. Version 2.00. Exeter software, Setauket
Smith JSC, Chin ECL, Shu H, Smith OS, Wall SJ, Senior ML, Mitchel SE, Kresorich S, Tiegle J (1997) An evaluation of the utility of SSR loci as molecular markers in maize (Zea mays L.): comparisons with data from RFLPs and pedigree. Theor Appl Genet 95:163–173
Solmaz I, Aras V, Unlu H, Sarı N (2004). Türkiye’nin farklı bölgelerinden toplanan acur (Cucumis melo var. flexuosus) genotiplerinde karakterizasyon. Turkiye V. Sebze Tarimi Sempozyumu Bildirileri 21–24 Eylul 2004 Canakkale, 75-81 (In Turkish)
Soltani F, Akashi Y, Kashi A, Zamani Z, Mostofi Y, Kato K (2010) Characterization of Iranian melon landraces of Cucumis melo L. Groups Flexuosus and Dudaim by analysis of morphological characters and random amplified polymorphic DNA. Breed Sci 60:34–45
Tzitzikas NE, Monforte AJ, Fatihi A, Kypriotakis Z, Iacovides TA, Ioannides IM, Kalaitzis P (2009) Genetic diversity and population structure of traditional Greek and Cypriot melon cultigens (Cucumis melo L.) based on simple sequence repeat variability. HortScience 44:1820–1824
Vural H, Esiyok D, Duman I (2000) Kultur Sebzeleri (Sebze Yetistirme) Ege Universitesi Ziraat Fakultesi Bahce Bitkileri Bolumu, Ege Universitesi Basımevi, Izmir (In Turkish)
Wang P, Li Q, Hu J, Su Y (2015) Comparative analysis of genetic diversity among Chinese watermelon germplasms using SSR and SRAP markers, and implications for future genetic improvement. Turk J Agric For 39:322–331
Yildiz M, Akgul N, Sensoy S (2014) Morphological and molecular characterization of Turkish landraces of Cucumis melo L. Not Bot Horti Agrobo 42:51–58
Acknowledgments
This research was supported by the Scientific Research Projects Coordination Unit of Cukurova University (FBA-2014-2380). The authors are grateful to Dr. Aydin Uzun for his kindly help in the PIC analysis; AARI, TAEA, EMTZARI, INRA (Michel Pitrat), and Veysel Aras for seed supplying.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solmaz, I., Kacar, Y.A., Simsek, O. et al. Genetic Characterization of Turkish Snake Melon (Cucumis melo L. subsp. melo flexuosus Group) Accessions Revealed by SSR Markers. Biochem Genet 54, 534–543 (2016). https://doi.org/10.1007/s10528-016-9739-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-016-9739-8