Abstract
Mucins are highly O-glycosylated glycoproteins that carry a heterogenous variety of O-glycan structures. Tumor cells tend to overexpress specific mucins, such as the cell surface mucins MUC1 and MUC4 that are engaged in signaling and cell growth, and exhibit abnormal glycosylation. In particular, the Tn and T antigens and their sialylated forms are common in cancer mucins. We review herein methods chosen to use cancer-associated glycans and mucins as targets for the design of anti-cancer immunotherapies. Mucin peptides from the glycosylated and transmembrane domains have been combined with immune-stimulating adjuvants in a wide variety of approaches to produce anti-tumor antibodies and vaccines. These mucin conjugates have been tested on cancer cells in vitro and in mice with significant successes in stimulating anti-tumor responses. The clinical trials in humans, however, have shown limited success in extending survival. It seems critical that the individual-specific epitope expression of cancer mucins is considered in future therapies to result in lasting anti-tumor responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a leading cause of death worldwide, and the majority of cancer deaths is due to the formation of metastases. Cancer treatments include anti-hormone receptor analogs, cytostatic drugs, immunotherapy and vaccines. Although many different treatment options exist, the side effects are often daunting and the hope is for safe, individual-specific and effective methods of immune stimulation to mobilize the rejection of tumor cells. Multiple cancer-associated tumor antigens have been identified and proposed as diagnostic markers and therapeutic targets. This includes mucins, mucin-like glycoproteins and glycoproteins that are overexpressed in tumors. Tumor-associated glycans include the Tn and T (Thomsen-Friedenreich) antigens, Lewis antigens and a number of gangliosides.
Cancer-associated glycans in cancer vaccines are in context with proteins that are overexpressed in cancer. Thus, vaccines should not target O-glycans or N-glycans that are found on normal glycoproteins. Stem cells are critical for tumor development and mucin glycopeptides found specifically on stem cells would be ideal targets to stop tumor growth. Although additional cancer therapy may aid in the effectiveness of vaccines, mucin-based vaccines are likely to be more successful in patients that do not undergo immunosuppressive treatments.
Glycoproteins and large mucins can carry O-glycans O-linked to Ser/Thr or N-glycans that are N-linked to Asn residues. In tumor cells, the O-glycans are often truncated and highly sialylated, while N-glycans can be altered in their branching patterns and exposure epitopes such as the Lewis antigens. The mechanisms of these alterations include the up- and downregulation of the expression of glycosyltransferases (GTs) involved in the synthesis of glycans [1]. However, very little is known about the control of GT gene expression in cancer cells. Some of the specific factors modifying GT activities are cytokines and growth factors.
Glycoproteins that are overexpressed in tumor cells and can serve as markers or targets include human epidermal growth factor receptor2 (HER2), mucins MUC1, MUC4 (Fig. 1) and MUC16, epithelial-cell adhesion molecule (EpCAM) and prostate-specific antigen (PSA). A proportion of cell surface-bound glycoproteins is cleaved and can interact with antibodies in the serum. Carbohydrate-binding proteins (lectins) on immune cells and tumors facilitate interactions within the tumor microenvironment (TME) and cancer cell adhesion, thereby promoting cancer progression and metastasis. Sialic acid-binding lectins (Siglecs) expressed on specific hematopoietic cells such as natural killer (NK) cells and macrophages regulate immune responses while soluble Gal-binding galectins are involved in cell proliferation and apoptosis. Selectins expressed on endothelial and immune cells adhere to Lewis-type glycans expressed on tumor cells and promote their invasion and dissemination. Cancer cells that detach from the tumor enter the blood or lymph and can form metastases. The composition of the cancer environment, the extracellular matrix (ECM) and TME control the diffusion of immune cells to the tumor and act in an immunosuppressive fashion. The antigenicity of tumor epitopes is in constant flux and it is important to search for key targets involved in tumor growth without eliciting cross-reactivity. Vaccine preparations containing conjugates of non-human mucins or cancer-associated carbohydrates have been tested since 1992, but with minimal success in cancer treatment and prevention. In order to confirm the expression of mucins on tumors, anti-mucin antibodies could be used to label biopsies or tumors in vivo. Efforts have been made to produce safe anti-cancer vaccines with high specificity and efficacy that can reduce tumor growth [2].
Cell surface mucins MUC1 and MUC4. a MUC1 contains a C-terminal cytoplasmic domain (Cyt) with several phosphorylation sites (P). The transmembrane domain (tm) spans the membrane and is continuous with extracellular sea urchin sperm protein, enterokinase and agrin (SEA) domain, the site for proteolytic cleavage of the mucin. Potential N-glycosylation sites are indicated by red “Y” glycan structures. The majority of MUC1 is composed of variable number tandem repeats (VNTR) which are heavily O-glycosylated. O-glycans are shown in purple. MUC1 terminates in an N-terminal signal peptide (SP). b MUC4 is composed of two subunits: N-terminal α domain and C-terminal β domain. MUC4 has a similar C terminus to MUC1 but having only one phosphorylation site. The tm is linked to epidermal growth factor (EGF) domains and von Willebrand factor (vWF) domain containing several potential N-glycosylation sites. The α subunit is composed of an adhesion-associated domain (AMOP), nidogen (NIDO)-like domain, O-glycosylated VNTR and N-terminal SP.
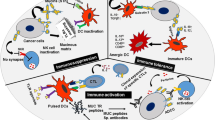
Microbiota function in detoxification and maintaining a balance in host cell growth and proliferation. While microbes including viruses can trigger amplification of genes involved in cancer progression and immunosuppression, they are also involved in the stimulation of the immune system and host responses [3]. Epithelial cell mucins bind to microbes but also form a barrier against pathogens. Studies in mice show that the microbiota collaborate with the immune system in anti-tumor responses. For example, killed Streptococci and Sarratia have been used in immunotherapy. Live bacteria in the bladder (Bacillus Calmette-Guérin, BCG) trigger immune responses against the tumor cells and are being used to successfully treat invasive bladder cancer [4]. Immune cells with roles in supporting BCG therapy include CD4+ and CD8+ lymphocytes, NK cells, macrophages and dendritic cells (DC). Bladder cancer cells are killed through direct cytotoxicity by these immune cells and by soluble apoptosis-inducing factors such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). BCG is able to stimulate anti-tumor immune responses in patients with bladder cancers that highly express sialyl-Tn and sialylα2–6(Galβ1–3)GalNAc- antigens (Fig. 2) [5].
Synthesis of cancer-associated O-glycans. Ser/Thr residues of mucin peptides are O-glycosylated by one of several ppGalNAc-Ts that transfer GalNAc in an α linkage to form the Tn antigen. The Tn antigen can be converted to many different O-glycan structures, and GTs compete for the available acceptor substrates. Tn antigen can be sialylated by ST6GalNAc-I but can also be converted to core 1 (T antigen) by β3 Gal-transferase C1GALT (with coexpressed chaperone Cosmc) or to core 3 by β3 GlcNAc-transferase B3GnT6. T antigen is converted to core 2 via the addition of a β6-GlcNAc residue by C2GnT1,2,3. However, it can also be converted by several other GTs including ST3Gal-I and ST6GalNAc-III,IV, Fuc-transferase FUT2 and sulfotransferase Gal3ST-2. Core 3 is converted to core 4 by β6 GlcNAc-T C2GnT2, or is galactosylated (by B3Gal-T5) or sialylated. Core 2, 3, 4 structures can be extended by Gal- and GlcNAc-transferases in various pathways in the synthesis of antigens such as fucosylated blood group and Lewis antigens. The structures in cancer are mostly truncated and sialylated Tn and T antigens
This review describes the approaches taken for the design of vaccines based on epitopes found on cell surface mucins of cancer cells with a focus on the major mucin targets MUC1 and MUC4. The goal is to stimulate safe, natural defenses against tumor cells.
Mucins
Mucins are expressed on epithelial and goblet cells and are targets for immunotherapy because of their overexpression and abnormal glycosylation patterns in a variety of different tumors [6]. The secreted mucins include small mucins (MUC7 and 8), large polymeric mucins (MUC2, 5 AC, 5B, 6, 19) and the cell surface-bound signaling mucins (MUC1, 3, 4, 12, 13, 15, 16, 17, 20, 21, 22) as well as their circulating cleavage products that are of particular importance for cancer. Mucins have variable number of tandem repeat regions (VNTR) that are densely O-glycosylated with GalNAc O-glycans attached while N-glycosylation sites are found mostly outside of this region. Mucins are extremely large glycoproteins but are functionally diverse with distinct domains. While soluble mucins are thought to function in the protection of the epithelium, the roles of cell surface mucins include cell adhesion, receptors for glycan binding proteins, interaction with other cell surface receptors, regulation of the immune system as well as cell signaling, growth and proliferation, and are thus of particular importance for the biology of tumor cells. Mucins bind to mammalian lectins such as siglecs expressed on DC and interact with platelets, neutrophils and macrophages via specific glycans [7]. These interactions regulate the immune responses against tumors. Infections and chronic inflammation also play a role in tumorigenesis [8]. For example, Toll-like receptor 4 (TLR4) is activated by bacteria leading to the production of inflammatory cytokines. Mucins also bind to TLR4 thus controlling inflammation, while inflammatory cytokines can regulate both mucin expression and glycosylation [9, 10].
The cell surface mucin MUC1 has been found to be dramatically increased in most tumor cells studied [11]. The VNTR domain of MUC1 has multiple Pro/Ser/Thr-rich sequences (PDTRPAPGSTAPPAHGVTSA), each carrying up to five O-glycans. The potential of MUC1 as a vaccine candidate is demonstrated by the fact that natural immunity against MUC1 has been found in cancer patients. These naturally occurring polyclonal antibodies bind to a variety of mucin epitopes [12]. The anti-Tn antibodies isolated by GalNAc-affinity chromatography were shown to bind to a great variety of GalNAc-containing oligosaccharides and GalNAc-peptide epitopes.
Both anti-MUC1 IgG and IgM antibodies were found in breast and gastric cancer, and the levels of antibodies correlated with increased survival of patients [13,14,15].
The extracellular O-glycosylated domain of cell surface mucins can be released and found in the circulation which may interfere with mucin immunotherapy. Autocleavage sites are near the membrane and in several mucins include the SEA (sea urchin sperm protein, enterokinase, and agrin) domain. After cleavage, the N-terminal (β) membrane-bound and the C-terminal (α) O-glycosylated domains (Fig. 1) form a non-covalently linked heterodimer [16]. The cytoplasmic tail of MUC1 (MUC1-C) has been well characterized and facilitates their dimerization through disulfide bonds involving the CQC sequence. There are five N-glycosylation sites of MUC1 close to the transmembrane domain [17].
The C-terminal cytoplasmic domain of MUC1 is involved in signal transduction and regulating cell growth, differentiation and survival signals. It can be phosphorylated, associate with galectin-3, block apoptosis and has been named ‘oncogenic’ MUC1-C or ‘oncoprotein’. Multiple Tyr, Thr and Ser residues in the cytoplasmic domain are phosphorylation sites and can activate signaling pathways involving Ras and catenins. The epidermal growth factor (EGF) domains of cell surface-bound mucins form complexes with other proteins, e.g. HER2 Tyrosine kinase. MUC1 also associates with the oncogene product p53. MUC1-C forms complexes with transcription factors, and translocates to the nucleus by an unknown mechanism, where it is believed to influence gene transcription. MUC1-C has also been proposed to attenuate apoptotic pathways and increase resistance to apoptosis-inducing drugs [6, 17, 18]. A number of different cancer cells have been examined for the effect of blocking dimerization of MUC1-C. The cell-penetrating peptide GO-203 blocks MUC1-C homodimerization leading to a reduction in the oncogenic potential of MUC1-C in cultured cancer cells and in mice [19,20,21,22].
MUC4 is another cell surface mucin that is increased in pancreatic, breast, lung and ovarian tumors. Especially in pancreatic cancer, MUC4 is expressed at high levels while it is almost undetectable in normal pancreatic tissue [23]. MUC4 has highly O-glycosylated VNTR and also multiple N-glycosylation sites near the EGF domains and within the von Willebrand factor (vWF) domain (Fig. 1). The nidogen (NIDO)-like domain, adhesion associated domain (AMOP) present in some splice variants and vWD type D domains synergistically participate in MUC4 function in cancer [24, 25]. MUC4 interacts with other proteins and is involved in cell signaling. Thus, MUC4 is a tumor marker as well as a target for immunotherapy [26]. MUC4 is autocatalytically cleaved releasing the O-glycosylated α domain from the β domain (Fig. 1). MUC4 is thought to promote tumorigenicity and aggressive behavior of tumors, and its high expression correlates with poor outcomes in various types of epithelial carcinomas. Both MUC1 and MUC4 play a role in the progression and metastasis formation of pancreatic cancer.
The cell surface mucin MUC16 is known as the ovarian cancer antigen CA 125. MUC16 is also overexpressed in pancreatic cancer and accompanied by MUC16 antigen-specific T cell clones, and could serve as a potential target for developing cancer immunotherapy [7, 23, 27]. In addition, secreted MUC5AC is found in the serum of pancreatic cancer patients and can be used for diagnosis. Thus, MUC1, MUC4, MUC5AC and MUC16 play a role in disease progression in pancreatic cancer [28] and are valuable diagnostic biomarkers, especially in combination with the expression of sialyl-Lewisa glycans [29]. The expression of large polymeric, secreted mucins has been studied in a number of tissues, cells and cancer types and these mucins have been related to tumor biology [30]. There is no clear correlation between the expression of specific mucins with malignant potential and tumor progression [31]. It appears though that the ratio of mucins expressed varies between tissues and cancer types and in different patients, but may nevertheless be useful in determining the primary site of metastatic tumors. The expression of soluble MUC2, MUC6 and MUC5A mucins has been found to be down regulated in gastric cancer, while MUC2 and MUC5AC expression was up regulated in colorectal cancer [32]. It was revealed that the expression of MUC2 in colorectal cancer was associated with a poor prognosis while MUC5Acand MUC6 expression could predict a more favorable prognosis [33].
Mechanisms of aberrant mucin glycosylation in cancer cells
Mucins can carry hundreds of O-glycan chains with different structures. The glycan structures are variable between glycosylation sites - and between mucins within a tumor - and are a result of complex regulation of GT activities [1]. A common tumor-associated glycan is the unmodified GalNAcα-Ser/Thr (Tn antigen), sialylα2–6GalNAc (sialyl-Tn antigen) and the unmodified T antigen, O-glycan core 1 (Galβ1–3GalNAc) and its sialylated versions (Fig. 2). The sialyl-T antigens are not cancer-specific but can regulate overall O-glycosylation [34], cellular interactions and cell death [35]. In addition, more complex neutral, sialylated and fucosylated glycans and those carrying Lewis epitopes are often found in increased amounts in O-glycans, N-glycans or glycolipids and have been proposed as biomarkers [36]. The mechanisms of these changes include the up- or downregulation of GTs, possible relocalization of GTs within the Golgi stacks and availability of donor and mucin acceptor substrates. Enzymes often compete for the same acceptor substrate and the relative GT activities determine the final outcome.
The first sugar of all O-glycans, GalNAc (Tn antigen), is normally substituted by Galβ1–3 (in core 1) or by GlcNAcβ1–3 (in core 3). Twenty different polypeptide GalNAc-transferases (ppGalNAc-Ts) synthesize the Tn antigen and contribute to differentiation, cancer development and metastasis. These enzymes differ in function, depending on the cell type and isoform [37, 38]. Several of these enzymes have been shown to be aberrantly expressed in cancer and involved in cancer progression. In hepatocellular carcinoma, mainly ppGalNAc-T1 and 2 are expressed. Overexpression of ppGalNAc-T2 blocks Tyr phosphorylation of the EGF receptor and reduces tumor growth in mice [39]. Pancreatic tumors highly express ppGalNAc-T3, while downregulation of the gene induces apoptosis and decreases tumorigenicity of pancreatic cancer cells [40]. ppGalNAc-T4 is highly expressed in breast cancer and is involved in the regulation of estrogen levels in breast cancer cell lines [41]. Another enzyme, ppGalNAc-T6, is particularly well expressed in breast tumors and is used as a biomarker [42]. The gene encoding ppGalNAc-T6 is frequently methylated at later stages of cancer but infrequently methylated in primary breast tumors. The ppGalNAc-T9 is a brain-specific enzyme that appears to be involved in brain metastases of breast tumors [43]. Overexpression of ppGalNAc-T14 in breast cancer cells MCF-7 indicates the critical role of ppGalNAc-T14 in cell invasion and migration [44]. Knockdown of ppGalNAc-T14 in MCF-7 cells affected the gene expression patterns of a number of proteins and decreased the expression of MUC1. Overexpression of ppGalNAc-Ts, or possibly their abnormal localization within the Golgi membrane assembly line, might increase the amount of unmodified GalNAc residues.
Cell surface glycosylation has been shown to control tumorigenesis and metastasis [45]. The Tn antigen has been correlated with metastasis and poor outcomes in cancer patients [46]. The lack of further processing of GalNAc to more complex chains could be due to a downregulation of core 1 β1,3-Gal-transferase (C1GalT) or core 3 β3-GlcNAc-transferase (B3GnT6) (Fig. 2). C1GalT activity may also be absent due to the lack of co-expression of its specific chaperone Cosmc [47]. The T antigen is normally converted to branched and elongated O-glycans. However, low activities of specific GlcNAc-, sialyl- or Fuc-transferases that modify core 1 result in the overexpression of the T antigen in cancer cells.
The sialylation of GalNAc to form the cancer-associated Sialyl-Tn antigen will also prevent the synthesis of core 1. Overexpression of α2,6-sialyl-transferases ST6GalNAc-I [48] and ST6GalNAc-II [49] has been observed in tumor cells that display the sialyl-Tn epitope. ST6GalNAc-I is the main enzyme that synthesizes and correlates with sialyl-Tn [50]. However, the sialic acid residue can be O-acetylated which would prevent binding of antibodies to the cryptic sialyl-Tn [51]. ST6GalNAc-I was found to contribute to cell proliferation and invasion of mouse hepatocarcinoma cancer cells [52] and to tumor growth of breast cancer cells in mice [48].
In normal lung and intestinal tissues, the Tn antigen is converted to core 3 by B3GnT6 which competes with C1GalT for GalNAc-acceptor substrates [53]. A downregulation of the B3GnT6 gene expression and activity has been observed in cancer cells and tumors [54,55,56] and allows the increased synthesis of core 1 (T antigen) by C1GalT. Colon cancer cells that overexpress B3GnT6 exhibit a significantly reduced metastatic potential in nude mice. This suggests that the expression of B3GnT6 or the presence of core 3 in glycoproteins is a marker of healthy tissue. Deletion of the B3GnT6 gene in mice led to increased permeability of the intestinal barrier and increased susceptibility to colitis and colorectal adenocarcinoma [57]. B3GnT6 is not expressed in normal human pancreatic epithelial cells but is expressed in many of the pancreatic ductal adenocarcinoma and cancer cells, correlating with the expression of MUC5A [58]. The expression of B3GnT6 increased with differentiation towards gastric mucus-producing cells and may be a favorable prognostic factor. Re-expression of B3GnT6 in pancreatic cancer cells suppressed tumor growth, migration and invasion through matrigel in vitro. Mice injected with B3GnT6-expressing pancreatic cancer cells showed significantly lower tumor growth and metastasis. The mechanisms by which core 3 exerts its anti-tumor function remain to be established.
The branched core 2 structure, GlcNAcβ1–6(Galβ1–3)GalNAc-, can be synthesized from core 1 by three variants of C2GnT. C2GnT1 is variably active in cancer cells. In certain metastatic prostate cancer cells, C2GnT1 activity is present at high, low or undetectable levels [59]. The α2,3-sialyltransferase ST3Gal I also acts on core 1 and competes with C2GnT1. Many cancer cells have a high activity of ST3Gal I, and the enzyme controls cancer cell proliferation. The overexpression of ST3Gal I in mice is related to increased breast tumor growth [60] and downregulation of the enzyme reduced prostate tumor growth [35]. This may be due to the protective function of sialic acid that prevents removal of cells through anti-T antigen immunity. The synthesis of Sialylα2-3Galβ1–3GalNAc- by ST3Gal I in breast cancer cells and tumors [61] prevents the conversion of core 1 to core 2 and to complex O-glycans, although ST6GalNAc-III and IV can add a second sialic acid residue to core 1. As a consequence, breast cancer cells exhibit mainly short, sialylated O-glycans [34]. These smaller O-glycans attached to MUC1 allow the binding of anti-MUC1 peptide antibodies directed against normally cryptic peptide epitopes. Thus, the relative activities of competing GTs shape the glycan structures and control epitope exposure of mucins in cancer cells. This phenomenon is important for the design of a mucin-based cancer vaccine.
O-glycan core 3 can be converted to core 4 by C2GnT2 [62, 63]. Although both core 3 and 4 are restricted in their tissue expression, C2GnT2 is expressed in a number of colon and lung cancer cells with relatively low activity. Cytokines interleukin-4 and interleukin-13, and retinoic acid upregulate C2GnT2 activity in lung cancer cells [64]. The overexpression of C2GnT2 in HCT116 colon cancer cells resulted in decreased growth, cell adhesion and invasive properties [65]. In addition, overexpression of C2GnT2 in nude mice reduced tumor growth. This indicates that both core 3 and core 4 structures of mucins are associated with low tumorigenicity of cancer cells.
Gastric gland mucins express O-glycans with terminal GlcNAcα1–4Gal- epitopes that are primarily attached to MUC6. This epitope is also common in premalignant conditions such as pyloric gland adenoma of the stomach, chronic atrophic gastritis, Barrett’s esophagus and pancreatic intraductal papillary-mucinous neoplasms. The gene encoding α1,4-GlcNAc-transferase that synthesizes the GlcNAcα1–4 epitope is highly expressed and the enzyme is another marker for pancreatic cancer and other cancers [66].
Selection and immunogenicity of mucin epitopes
Knowledge of the molecular basis that governs epitope presentation by immune cells, as well as interactions between antigens and elicited antibodies is limited. Nuclear magnetic resonance spectroscopy of mucin peptide and glycopeptide antigens in solution as well as crystallization studies are excellent methods to determine antigen folding and conformations [67]. Molecular dynamics simulations are also helpful to determine the spatial orientation of glycan epitopes in mucins and to anticipate their binding abilities and antigenicity [68].
The glycosylation and conformations of MUC1 peptides strongly affect antibody binding [61, 67, 69, 70]. Thus, for the generation of high affinity therapeutic antibodies, both the peptide and the glycans need to be considered in context. Antibodies that bind to mucin peptides on cell surfaces can cause clustering of mucins, thus enhancing immune recognition or cell adhesion. This mucin clustering affects the exposure of tumor-associated carbohydrate antigens. Short glycans such as the Tn antigens generally exhibit low immunogenicity and have to be presented as clusters in order to produce effective mucin epitopes. However, Tn antigen attached to the Thr residue in the PDTRP sequence of MUC1 was used as a vaccine which led to an effective immune response without adjuvant and induced antibodies recognizing human breast cancer cells [71]. Antibodies recognize a spatial epitope which is difficult to predict due to the great variety of glycans, their complexity, large size and heterogeneous sugar composition and linkages but in silico modeling of epitopes has been attempted [72].
The synthesis of tumor epitopes has been highly successful for vaccine design [67, 73,74,75]. MUC1 peptides glycosylated with Tn and T antigens and their sialylated versions were synthesized and linked to tetanus toxoid peptide (T cell epitope). Dendrimers containing more than one MUC1 (glyco)peptide chain were shown to produce high antibody titers in mice. Multivalent constructs of mucin vaccines included glycosphingolipid GM2, glycopeptide from MUC1, and glycans Globo H, LewisY, Tn and T antigens conjugated to KLH (Keyhole limpet hemocyanin), as well as a purified tree bark extract adjuvant (QS-21) [76]. The vaccine was administered to prostate cancer patients and was found to be safe while raising IgM antibodies against Globo-H expressed on prostate tumors. The increase in PSA levels seen prior to vaccination was in part inhibited by the vaccine.
In order to create novel epitopes, MUC1 peptides carrying Sialyl-Tn antigen were linked to a mutagenized tetanus toxoid adjuvant to immunize mice as a preclinical breast cancer model. The vaccine showed immune responses, decreased tumor growth and increased survival [77]. KLH is another common adjuvant used in mucin vaccines [78]. While adjuvants are critical for an effective immune response, there are also self-adjuvanting vaccines where an immune stimulant is present in the same molecule as the antigen. This includes MUC1 linked to lipopeptides that provide amphipathic properties to the antigen to improve the in vivo efficacy.
Chemical synthesis permits the introduction of antigenic epitopes into the antigen. A novel Tn antigen mimic composed of GalNAc attached to α-methylserine in glycopeptides appeared to retain the bioactive conformation recognized by anti-MUC1 antibodies [67, 79]. This vaccine elicited high antibody titers in mice [80, 81]. A modified T antigen (3-F-Galβ1–3GalNAc-) linked to Thr in glycopeptides showed highly specific binding of anti-MUC antibody [82].
A monoclonal IgG1 antibody (PankoMab-GEX) recognizes a conformational epitope of MUC1 carrying Tn or T antigens. The antibody is safe, well tolerated, and showed promising anti-tumor recognition at higher dosages in patients with advanced disease. Adverse events in a small number of patients included diarrhea, intestinal perforation, peritonitis and tinnitus. However, further studies are required to evaluate the efficacy of PankoMab-GEX as a maintenance therapy in advanced cancers [83].
Immunotherapy
Cancer development is generally accompanied by naturally acquired IgG antibodies against tumor-associated epitopes, suggesting that natural immunity against tumors exists and could possibly be enhanced. Immunotherapy approaches are effective and safe strategies to increase natural immune defenses against cancer cells by therapeutic antibodies, immune checkpoint inhibitors, immune cell modulators and anti-cancer vaccines.
The scarcity and often poor antigenicity of cancer antigens and an immunosuppressive tumor microenvironment (TME) limits the success of immunotherapy in cancer [23]. The TME can favor the proliferation and metastasis of cancer cells, partly due to the presence of fibroblasts, regulatory T cells and macrophages and production of ECM (glyco) proteins like collagens and fibronectin, as well as cytokines secreted by immune or cancer cells. The ECM forms a physical barrier for T cell infiltration. Immunotherapy for cancer therefore should address the immunosuppressive properties of the ECM, stimulate the immune responses, facilitate immune cell infiltration of ECM and tumors, and recognize specific tumor epitopes.
Monoclonal antibodies used in passive immunotherapy are designed to bind to specific tumor epitopes. Mucin antigens should be selected according to their tumor-specific expression and the ability to elicit strong immune responses. These antibodies are raised by immunization of mice either with tumor extracts or with purified or synthetic tumor mucin epitopes. Monoclonal antibodies raised against MUC1 carrying Tn and T antigens successfully elicited immune responses in mice with a number of different cancers. Humanized antibodies that contain a human antibody scaffold with the complementarity-determining region specific for tumor antigen binding have potential for treatment in humans. A number of these anti-tumor antibodies are being tested in preclinical and clinical trials [84]. Unfortunately, mucin fragments of MUC1 are present in the serum in high amounts at late stages of cancer. These soluble mucins can compete for the binding sites of anti-MUC1 antibodies, thus decreasing their binding to tumors. Other problems are the poor penetration of antibodies into the tumor and the heterogeneity of epitopes within the tumor (Table 1).
A novel approach of immunotherapy is using chimeric antigen receptor (CAR) T cells that are engineered to potently target tumor-associated glycosylation. A monoclonal antibody specific for MUC1 carrying Tn antigens was used to construct CAR T cells [95]. These T cells successfully decreased tumor growth in T cell leukemia and pancreatic cancer mouse models and specifically targeted tumor cells.
Immune checkpoints play significant roles in downregulating the immune responses and are targeted in cancer immunotherapy. More than 60 different immune checkpoints targets, including proteins and reference antibodies could be targeted. Many of these constitute cell surface glycoproteins including galectins and siglecs involved in the control of the immune system. Immune checkpoint inhibitors allow enhanced immune responses to cancer. The cytotoxic T lymphocyte-associated protein 4 (CD152, CTLA-4) is one of the checkpoint receptors on activated T cells. Anti-CTLA-4 antibodies have been successful in stimulating anti-cancer responses and tumor regression in patients [85]. Another checkpoint is the activity of the programmed death receptor PD1 that inhibits T cell responses and promotes apoptosis in T cells. Inhibitors of PD-1 and its ligand PD-1 L can reduce apoptotic signals in T cells and have been used in the treatment of melanoma, lymphoma and a number of other cancer types. Anti-PD1 antibodies given to cancer patients prolonged overall survival [86]. The function of T cell immunoglobulin mucins (TIM-3 and TIM-4) is similar to that of PD-1 resulting in apoptosis. TIM-3 has a mucin domain and is an immune checkpoint that regulates T cell responses [96]. TIM-3 is activated by galectin-9 and mediates CD8+ T cell exhaustion and controls macrophage activation that reduces the inflammatory responses [97]. Mice with B16-induced melanomas were vaccinated with irradiated B16 melanoma cells that express FLt3L, a cytokine ligand of Tyrosine kinase FLt3 that induces signaling and increased immune cell numbers. When mice were injected with monoclonal antibodies against checkpoints TIM-3 or TIM-4, the therapeutic effects of the B16 vaccine were enhanced and the numbers of NK cells infiltrating the tumors were increased.
Several tumors including neuroblastoma, small-cell lung cancer, melanoma, glioma and sarcomas display abundant glycosphingolipids on their cell surfaces, including ganglioside GD2. The combination of mucin antigens and ganglioside epitopes could afford enhanced responses although anti-GD2 treatment could potentially lead to side effects related to the function of GD2 in normal cells. Monoclonal antibodies and bispecific antibodies against GD2 have been used in cancer treatment with limited success [98]. Other combinations of bispecific antibodies against mucins and cell surface receptors such as CD3 or CD28 have also been successful in slowing down tumor growth in mice [99].
Tumor suppression in mice has also been achieved by injection of cDNA encoding human MUC1 [100]. Tumors were induced in mice 5 days after the cDNA injection with mouse tumor cells MC38, and most mice with prior cDNA treatment were free of tumors. The anti-tumor protection lasted for several months.
Anti-cancer vaccine preparations require the design of antigens, adjuvants and carriers to deliver the antigens and cytotoxic T cell epitopes [98]. These anti-cancer vaccines are based on a specific protein, glycan or glycopeptide antigen of cancer cells to stimulate the immune system to attack the tumor. The epitope that is recognized by antibodies and T cells should be exposed and accessible on the cancer cell surface. Preliminary structural analyses of individual tumor antigens should identify the glycan chains and mucins present. The various types of vaccines developed include mucin glycopeptides, peptides of VNTR domains, signal peptides, C-terminal membrane-bound peptides (oncoprotein), or MUC1 DNA in a eukaryotic expression vector, and recombinant adenovirus or vaccinia virus encoding mucin peptides. The extracellular O-glycosylated domains of mucins are good candidates for cancer-specific vaccines.
DC play important roles in tumor development and are instrumental in engulfing vaccine epitopes. They interact between the innate and adaptive immune systems. DC have been primed with tumor associated epitopes or transfected with mucin cDNA in order to be more effective antigen presenters. DCs transfected with mRNA encoding MUC4 elicited strong CTL response in vitro in pancreatic ductal adenocarcinoma. Using MUC4 specific epitopes transduced into dendritic cells with universal DR-restricted T-helper epitope, a potent MUC4-specific cytotoxic T cell response was observed [101].
Several problems are inherent in immunotherapy and vaccines directed against glycosylated mucins: a) The glycan structures of mucins are heterogeneous and variable; b) both the peptides and the glycans of mucins are also found in normal cells and are not absolutely specific for cancer; c) mucins are extremely large and have many specific epitopes that can induce immunity; d) it is difficult to analyze the epitopes exposed in a tumor in individual cancer patients; e) side effects can be considerable and immune reactions against monoclonal antibodies prevent high dosage of antibodies; f) the glycosylation mucins within a tumor changes due to the aberrant expression and activities of glycosyltransferases, altering mucin antigenicity. To overcome these problems, a number of different mucin constructs have been developed as antigens, containing peptide and carbohydrate tumor antigens, with several different adjuvants and co-stimulating compounds, and various methods of immune stimulation.
Mucin vaccines based on MUC1
MUC1 is consistently overexpressed in cancer and has been a primary candidate for cancer vaccine design [67]. Vaccines are promising since spontaneous anti-MUC1 immune responses in cancer patients are associated with better prognosis or with a reduced lifetime risk of developing MUC1 positive cancers. A number of vaccine formulations based on MUC1 have been tested during the past 30 years in vitro using cancer cell lines, in mice and in patients with carcinomas. Most domains of MUC1 were used to prepare peptide or glycopeptide antigens for a vaccine. Important components of effective vaccines are adjuvants that induce strong immune responses of the innate and adaptive immune system. Thus, natural or modified mucin epitopes have been linked to KLH, bovine serum albumin (BSA) or Tetanus toxoid derived from Clostridium tetani. Tetanus toxoid is a strong immune stimulant and elicits immune responses and high titers of IgG antibodies in mice [73]. The levels of cell surface-bound and circulating mucins, as well as their glycosylation patterns are variable and may be tumor- and individual-specific, making the choice of appropriate vaccine very difficult. Glycosylated MUC1 carrying Tn or sialyl-Tn antigens showed a significant immune stimulation and, when linked to a cell-penetrating peptide, delayed tumor growth in mice [102].
MUC1 glycopeptides linked to BSA were produced containing unnatural GalNAcβ-Ser/Thr linkages as analogs of the Tn antigens and produced high antibody titers in mice [103]. In order to create new mucin glycopeptide epitopes and stable conformations of the O-glycans, the O-linkage of GalNAc to Ser/Thr was replaced by an unnatural S- or Se-linkage. The synthetic S- and Se-glycosides were conjugated to gold nanoparticles and bound to anti-MUC1 antibodies. Vaccination with this immunogen in mice resulted in a significant humoral immune response against cancer-MUC1 without adjuvants [69].
Conjugation of a MUC1 peptide antigen to a β-glucan polysaccharide, which serves both as a carrier and an immune activator in nanoparticles, facilitated the delivery of antigens leading to the activation of both innate and adaptive immunity [104]. Peptide-β-glucan conjugates can form uniform nanoparticles that facilitate the delivery of antigens. Beta-glucans also binds to multiple cell surface receptors and lectins stimulating immune processes such as T cell and B cell activation.
A successful anti-cancer vaccine was composed of MUC1 and a TLR7 agonist as adjuvant covalently conjugated to BSA. This adjuvant-protein-antigen [105] enhanced humoral and cellular immune responses in mice. The raised antibodies showed a potent anti-MUC1 response to human breast cancer cells MCF-7.
However, mucin-based vaccines have generally shown low responses in human patients. Therefore, further improvement has focused on the modifications of the mucin glycopeptide epitopes and choice of adjuvants [106]. Vaccine constructs range from synthetic mucin antigens conjugated to carrier proteins, encapsulations in liposomes or nanoparticles, or addition of T-helper epitopes to stimulate the induction of memory B-cells to permit long-term immunity. The combination of vaccine and additional drugs such as indomethacin can result in a significant reduction in tumor burden [107].
The bacteriophage Qβ is a powerful virus-like particle for antigen delivery due to its highly organized three-dimensional structure and ability to display cancer antigens. A conjugate of Qβ and MUC1 carrying the T antigen has shown success in vitro in killing of B16 melanoma cells expressing MUC1 after addition of complement. Furthermore, mice vaccinated with Qβ-MUC1 glycopeptide conjugates were protected from forming pulmonary metastases induced by B16-MUC1 melanoma cells [108].
In order to achieve strong tumor-specific immune responses, an antigenic epitope linear Gal (Galα1–3Gal) on MUC1 was produced. MUC1 was expressed in human pancreatic cancer cells transfected with α1,3-Gal-transferase that synthesize the linear Gal epitope. Mice lacking the α1,3-Gal-transferase were treated with pig tissue (expressing the epitope) to pre-immunize them, followed by B16F10 melanoma cells to induce tumors. The linear Gal-MUC1 vaccine successfully reduced tumor burden in these mice and significantly extended survival time [109]. Antibodies to the linear Gal epitope enhanced recognition and uptake of linear Gal-MUC1 by antigen presenting cells and thus improved the effectiveness of vaccination. Cancer stem cells are instrumental in proliferation of tumors and are often resistant to therapies. When tumors were induced with human pancreatic cancer cells PANC1 expressing the linear Gal epitope in mice, the immune responses were also directed against pancreatic cancer stem cells. Most of these stem cells express CD44, CD24 and epithelial-specific antigen. Stem cells were isolated with anti-CD44 antibodies and were shown to express MUC1 with the linear Gal epitope [109]. This suggests that antibodies against MUC1 with linear Gal epitope could eradicate stem cells. It remains to be shown if this strategy is applicable to human tumors.
MUC1 participates in the tumorigenicity and metastasis of colorectal cancer stem cells and has preferred epitopes for stem cell vaccination [110]. Stem cells from human colorectal adenocarcinoma cells SW620 express MUC1 and other markers such as the cell surface glycoprotein CD133 and aldehyde dehydrogenase. The injection of stem cell lysates into mice significantly reduced the growth of tumors induced by SW620 cells and increased NK killing activity. A comparison to lysates from SW620 cells after knockdown of MUC1 showed that the anti-tumor effect of the vaccine was dependent on the presence of MUC1. The vaccination did not show any toxicity.
A new peptide ligation technology is the diselenide-selenoester ligation (DSL) used for the preparation of self-adjuvanting glycolipopeptide vaccines. Rapid ligation of MUC1 glyco- and lipopeptides is achieved via DSL together with a lipopeptide adjuvant. This vaccine construct elicited MUC1-specific antibody and cytotoxic T lymphocyte responses in mice. An additional helper T cell peptide epitope was able to boost the antibody response as well as cytokine production [111].
An interesting approach was taken in vaccine design by Glaffig et al. [112]. They utilized the Man receptor CD206, a C-type lectin, present on macrophages and dendritic cells to facilitate the uptake of vaccine consisting of MUC1 peptide carrying the Tn antigen and modified by Man residues. After vaccination of BALB/c mice, the Man-linked vaccine bound to CD206 was taken up by cells and induced a high titer of IgG1 and IgG2 against MUC1.
A recombinant vaccine was produced with MUC1 linked to maltose-binding protein (MBP) and a human TLR-9 agonist as a DNA-based adjuvant [113]. Melanomas were induced in mice with MUC1-expressing B16 cells and T cell responses were examined after repeated injections of the vaccine. MUC1-specific responses included Th1 responses and cytotoxic lymphocyte killing activity with an increase in tumor infiltration of lymphocytes. Thus, a significant anti-tumor response was observed although additional injections of the vaccine decreased the numbers of immune stimulatory cells within tumors. This indicated that the timing of vaccine administration was crucial for the efficacy of vaccination.
Another covalent construct of seven VNTR peptides of MUC1 and MBP expressed in E. coli was used together with BCG as adjuvant to examine the effect on cell growth of MUC1-expressing BL16–induced melanomas in mice [114]. The combined vaccine induced Th1 helper cells and cytotoxic T-lymphocytes that resulted in decreased tumor growth. BCG was critical for enhancing the effect of MUC1-MBP.
Plant cells can naturally encapsulate antigens and a vaccination is possible by ingestion [115] of plant cells that prevent proteins from being degraded in the upper digestive tract. A fusion protein was constructed with the C-terminal peptide of HER2 and seven tandem repeat peptides of MUC1 with minimal N- and O-glycosylation. The gene encoding the antigen construct was transfected into hairy root culture. Mice orally immunized with the vaccine based on these cells showed increased humoral and cellular immunity with a considerable rise in serum IgG and IgA directed against MUC1 and HER2.
In a self-adjuvanting, highly effective anti-MUC1 vaccine, the N-termini of MUC1 peptides were conjugated to one or two palmitoyl chains (to form amphiphilic Pam-MUC1 or Pam2-MUC1) [116]. These lipid-tailed MUC1 antigens were assembled into liposomes containing Galα-ceramide as an adjuvant and resulted in a significant immune response in mice. Vaccines containing other hydrophobic components as adjuvants were similarly effective.
L-Rhamnose (Rha) is an antigenic sugar that is foreign to humans, and anti-Rha antibodies have been found in humans. The priming of both CD4+ and CD8+ T cells toward MUC1 carrying Tn antigen was enhanced in mice that received human anti-L-Rha antibodies prior to vaccination with an L-Rha-modified MUC1-Tn cancer vaccine [117]. In order to exploit Rha as an adjuvant, a liposomal preparation of the TLR2 ligand Pam3Cys (having 3 palmitoyl chains) linked to a Rha-containing glycopeptide from MUC1 carrying GalNAc (Tn) was evaluated as a vaccine in groups of C57BL/6 mice. Mice showed a strong humoral response. Some groups of mice were previously immunized to generate anti-Rha antibodies and exhibited particularly high cellular immunogenicity [118], indicating that Rha is a suitable adjuvant.
A tricomponent antitumor vaccine Pam3CysSer was used as an immune stimulator together with MUC1 glycopeptides, a tetanus toxin peptide as a T-helper epitope and fibroblast stimulating lipopeptide 1 (FSL-1) as adjuvant in covalent linkages [119]. B16 cell-induced tumors were grown in mice. The vaccine elicited both humoral and cellular immune responses, high IgG antibody levels and achieved a reduction in tumor size. Similarly, a vaccine was prepared with MUC1 glycopeptides (carrying GalNAc, the Tn antigen) linked to a TLR2 agonist (Pam3CysSK4, a peptide T-helper epitope derived from polio virus) or to a TLR9 agonist. Using a mouse model of mammary cancer, it was shown that vaccination resulted in potent antibody and cellular immune responses against tumor-associated glycopeptides [120, 121].
The SEA domain present in the extracellular moiety of MUC1 was chosen as an immunogen to raise monoclonal antibodies (mAbs) with high affinity for SEA [16]. The antibody reacted with MUC1 from tumors and cancer cells but only weakly with MUC1 from normal cells. A chimeric protein complex containing an IgG binding protein fused to exotoxin from Pseudomonas aeruginosa linked to an anti-SEA domain humanized antibody was tested in cancer cells in vitro and in immunodeficient nude and SCID mice in vivo. Tumors were induced in these mice with MUC1 positive pancreatic cancer cells Colo357. The chimeric protein complex showed high cytotoxicity and suppression of tumor volume.
Algae can be used to produce proteins and as delivery vehicles of vaccines. A multiepitope vaccine was produced with tumor-associated epitopes from HER2, MUC1, Wilm’s tumor antigen and mammaglobin. The vaccine also contained the recombinant B subunit of enterotoxin from Escherichia coli as an immunogen that binds the glycosphingolipid GM1 and was expressed in algae. After injection of the vaccine, mice developed serum IgG antibodies that were shown to be directed against mouse breast cancer cells 4 T1 [122].
As DC are the major antigen-presenting cells, they can be primed with an antigen and then injected into the patient to enhance responses. MUC1 peptides can be used as antigen with various adjuvants. These primed cells were successful in reducing tumor load or growth [87]. Pre-activation of patient’s T cells is another method to stimulate the immune response [123].
Clinical studies using MUC1 based vaccines
The initial studies of immune responses to mucin epitopes were conducted in cell lines, and then in mice before initiating clinical trials. Tecemotide vaccine contains a synthetic 25 amino acid, non-glycosylated MUC1 lipopeptide (BLP25), and the immunoadjuvant monophosphoryl lipid A in a liposomal preparation. Tecemotide injected into in mice with lung cancer showed significant T cell responses against MUC1. Therefore, tecemotide was examined in lung cancer patients. Tecemotide with or without prior chemotherapy did not significantly increase survival time in patients with unresectable stage III non-small-cell lung cancer, but the vaccine appeared to be successful in patients receiving chemo- and radiotherapy concurrent with the vaccine [88] (Table 1). The vaccine did not produce toxicity but did not show the expected effectiveness in reducing tumor burden. Further study in this population is warranted. Tecemotide vaccine was also tested in a clinical phase II study of patients with HER2 positive breast cancer [89], and the vaccine was found to be safe but did not improve tumor burden.
A vaccine based on vaccinia virus and MUC1 (TG4010) was examined in a phase II study of patients with metastatic renal clear-cell cancer with and without additional cytokines [90]. The side effects were minimal, and patients developing CD8+ T cell responses against MUC1 showed a longer survival. However, the study is still considered preliminary as many factors could influence the outcome.
MUC1 glycopeptides carrying Tn antigens were examined in the Rhesus macaque monkeys. The MUC1 (glyco) peptides were derived from Rhesus monkey mucin and were mixed with an adjuvant or with autologous DC. The vaccine showed high immune response when the Tn antigen was present [91]. Similarly, in a Phase I/II clinical trial with a limited number of patients with nonmetastatic castrate-resistant prostate cancer, the vaccine was found to be safe and able to induce significant T cell responses with a lesser increase in PSA levels (Table 1).
Theratope (from Biomira) is a vaccine comprising the Sialyl-Tn antigen linked to KLH protein that has shown success in patients with metastatic breast cancer to prevent relapse of the disease [92]. The initial injections contained oil emulsions with lipid A and cell wall fragments from Mycobacterium phlei as immune stimulants. An increase in survival of breast cancer patients was observed in a Phase II study with minimal toxicity of the vaccine. However, a follow up Phase III study could not confirm the survival results.
Another approach was a vaccine construct against multiple myeloma, consisting of the signal peptide of MUC1 which specifically addressed MUC1 but not its glycosylated or C terminal domains. This ImMucin vaccine was successful in Phase I/II human trials in eliciting antibodies as well as cell-mediated immunity against the construct, stabilizing the disease [93].
For adoptive immunotherapy, autologous DC were treated with full-length MUC1 mRNA, and autologous cytotoxic T-lymphocytes were generated in vitro. These cells together with the chemotherapeutic gemcitabine were injected into late-stage pancreatic cancer patients and led to a significantly higher survival rate [94]. Several other clinical trials have been conducted using MUC1 based vaccines with results that require further modification of vaccines and study designs [124] (Table 1).
Mucin vaccines based on MUC4
MUC4 is not expressed in normal pancreatic tissues at detectable levels but is present at high levels in pancreatic cancer [23, 125, 126] and contributes to tumor aggressiveness. It has been shown that MUC4 expression modulates cell signaling, promotes tumorigenesis and metastasis and contributes to the resistance of pancreatic tumor cells to chemotherapy [126]. MUC4 expression progressively increases with the progression to pancreatic ductal adenocarcinoma, thus MUC4 can be used for diagnosis and is a target for cancer therapy. When the expression of MUC4 has been knocked down, proliferation, invasion, and metastasis of pancreatic cancer cells in vitro and in vivo are decreased [126, 127]. The appearance of anti-MUC4 antibodies and increased Th1 and Th2 cytokines in pancreatic cancer suggest strong immunogenicity of the mucin in cancer.
MUC4 is autocatalytically cleaved N-terminal to its vWF domain, forming the O-glycosylated domain MUC4α and the membrane-anchored domain MUC4β (Fig. 2). MUC4 has 3 EGF domains and interacts with a variety of proteins involved in tumor progression, including HER2, proteins of the Wnt pathway, galectin and integrins [128]. MUC4 modulates the interaction of tumor cells with their TME and contributes to the resistance of tumor cells to chemotherapy [129]. The proliferative action of MUC4 includes the inhibition of apoptotic pathways.
Several therapeutic avenues have been taken using MUC4 epitopes. Polyanhydride nanoparticles were synthesized containing encapsulated purified MUC4β chain for their ability to activate dendritic cells and induce adaptive immunity. The MUC4β protein can be released from the nanoparticles with minor loss of epitope. Immunization in mice with this nanovaccine led to MUC4β-specific antibody responses [130, 131]. Immature dendritic cells when pulsed with MUC4β-nanovaccine exhibited significant increase in the surface expression of MHC I and MHC II and costimulatory molecules (CD80 and CD86) and in the secretion of pro-inflammatory cytokines (interferon-γ, IL-6, and IL-12). The MUC4β-nanovaccine elicited IgG2b and IgG1 anti-MUC4β-antibodies in a ratio that suggested a predominantly Th1-like class switching.
Glycopeptides from the VNTR of MUC4 carrying T and sialyl-Tn antigens, conjugated to tetanus toxoid were used to vaccinate mice. The strong immune responses were specifically directed against MUC4. Analyses of antibody specificity by glycopeptide microarrays demonstrated that antibodies could distinguish between epitopes at specific glycosylation sites [132]. These studies suggest that MUC4 is an excellent candidate for an anti-tumor vaccine for pancreatic, lung, gastric, endometrial and other tumors expressing MUC4.
Conclusions
About 80% of patients with a variety of different tumors experience an increased expression of cell surface signaling mucins MUC1 or MUC4 with abnormal glycosylation, making these mucins suitable targets for immunotherapy in cancer. Significant immune responses have been noted in in vitro and mouse models upon immunization with many different mucin constructs, often carrying cancer-associated O-glycans and a variety of adjuvants. However, there is a need for vaccines that induce effective anti-tumor responses and decrease tumor growth. The results of immunotherapy that suggest a positive outcome remain to be validated in clinical trials. The development of vaccines, antibody and T cell therapies that combine the tumor glycans (sialyl-)Tn or (sialyl-)T antigens and adjuvants for immunostimulation should be continued. Before choosing the appropriate vaccine, patient tumor antigens and serum mucins should be established to confirm aberrant expression, shedding, and glycosylation in order to direct the optimal immunotherapy with the highest probability of success. Mucin vaccination is a safe anti-tumor prevention method and can be considered adjuvant immunotherapy accompanied by additional anti-tumor strategies. Any alterations in cancer-associated antigens need to be monitored after treatment that may require therapy modification. Cell surface mucins MUC1 and MUC4 exhibit a more consistent expression phenotype in cancer compared to soluble mucins. With the exception of patients with high risk of cancer, mucin vaccines should probably not be a general preventative method due to the presence of a small number of cancer epitopes in normal tissues. Future studies on the glycosylation and antigenicity of cancer stem cells may lead to new approaches. However, efforts should continue to produce vaccine with targeted delivery to patient’s tumor cells based on the high expression of glycosylated cancer-associated epitopes, together with support and effective stimulation of the patient’s immune system.
References
Brockhausen, I., Gao, Y.: In: Yurevics, E. (ed.) Structural Glycobiology: Applications in Cancer Research. Chapter 8, pp. 177–213. CRC Press, Taylor & Francis Group, Abingdon (2012)
Tati, S., Fisk, J.C., Abdullah, J., et al.: Humanization of JAA-F11, a highly specific anti-Thomsen-Friedenreich Pancarcinoma antibody and InVitro efficacy analysis. Neoplasia. 19, 716–733 (2017)
Garrett, W.S.: Cancer and the microbiota. Science. 348, 80–86 (2015)
Redelman-Sidi, G., Glickman, M.S., Bochner, B.H.: The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat. Rev. Urol. 11, 153–162 (2014)
Lima, L., Severino, P.F., Silva, M., Miranda, A., Tavares, A., Pereira, S., Fernandes, E., Cruz, R., Amaro, T., Reis, C.A., Dall'Olio, F., Amado, F., Videira, P.A., Santos, L., Ferreira, J.A.: Response of high-risk of recurrence/progression bladder tumours expressing sialyl-Tn and sialyl-6-T to BCG immunotherapy. Br. J. Cancer. 109, 2106–2114 (2013)
Beatson, R.E., Taylor-Papadimitriou, J., Burchell, J.M.: MUC1 immunotherapy. Immunotherapy. 2, 305–327 (2010)
Bhatia, R., Gautam, S.K., Cannon, A., Thompson, C., Hall, B.R., Aithal, A., Banerjee, K., Jain, M., Solheim, J.C., Kumar, S., Batra, S.K.: Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. 38, 223–236 (2019)
Peng, C., Ouyang, Y., Lu, N., Li, N.: The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: recent advances. Front. Immunol. 11, 1387 (2020)
Barrera, M.J., Aguilera, S., Veerman, E., Quest, A.F., Diaz-Jimenez, D., Urzua, U., et al.: Salivary mucins induce a toll-like receptor 4-mediated pro-inflammatory response in human submandibular salivary cells: are mucins involved in Sjogren’s syndrome? Rheumatology (Oxford). 54, 1518–1527 (2015)
Yang, X., Yip, J., Anastassiades, T., Harrison, M., Brockhausen, I.: The action of TNFalpha and TGFbeta include specific alterations of the glycosylation of bovine and human chondrocytes. Biochim. Biophys. Acta. 1773, 264–272 (2007)
Apostolopoulos, V., McKenzie, I.F.C.: Cellular Mucins: targets for immunotherapy. Crit. Rev. Immunol. 37, 421–437 (2017)
Dobrochaeva, K., Khasbiullina, N., Shilova, N., Antipova, N., Obukhova, P., Ovchinnikova, T., Galanina, O., Blixt, O., Kunz, H., Filatov, A., Knirel, Y., LePendu, J., Khaidukov, S., Bovin, N.: Specificity of human natural antibodies referred to as anti-Tn. Mol. Immunol. 120, 74–82 (2020)
Von Mensdorff-Pouilly, S., Gourevitch, M.M., Kenemans, P., et al.: Humoral immune response to polymorphic epithelial mucin (MUC-1) in patients with benign and malignant breast tumours. Eur. J. Cancer. 32A, 1325–1331 (1996)
Von Mensdorff-Pouilly, S., Verstraeten, A.A., Kenemans, P., et al.: Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J. Clin. Oncol. 18, 574–583 (2000)
Kurtenkov, O., Klaamas, K., Mensdorff-Pouilly, S., Miljukhina, L., Shljapnikova, L., Chuzmarov, V.: Humoral immune response to MUC1 and to the Thomsen–Friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 46, 316–323 (2007)
Pichinuk, E., Chalik, M., Benhar, I., Ginat-Koton, R., Ziv, R., Smorodinsky, N.I., Haran, G., Garbar, C., Bensussan, A., Meeker, A., Guillaume, T., Rubinstein, D.B., Wreschner, D.H.: In vivo anti-MUC1+ tumor activity and sequences of high-affinity anti-MUC1-SEA antibodies. Cancer Immunol. Immunother. 69, 1337–1352 (2020)
Taylor-Papadimitriou, J., Burchell, J.M., Graham, R., Beatson, R.: Latest developments in MUC1 immunotherapy. Biochem. Soc. Trans. 46, 659–668 (2018)
Singh, P.K., Hollingsworth, M.A.: Cell surface-associated mucins in signal transduction. Trends Cell Biol. 16, 467–476 (2006)
Kharbanda, A., Rajabi, H., Jin, C., Tchaicha, J., Kikuchi, E., Wong, K.K., Kufe, D.: Targeting the oncogenic MUC1-C protein inhibits mutant EGFR-mediated signaling and survival in non-small cell lung cancer cells. Clin. Cancer Res. 20, 5423–5434 (2014)
Raina, D., Agarwal, P., Lee, J., Bharti, A., McKnight, C.J., Sharma, P., Kharbanda, S., Kufe, D.: Characterization of the MUC1-C cytoplasmic domain as a cancer target. PLoS One. 10, e0135156 (2015)
Hasegawa, M., Sinha, R.K., Kumar, M., Alam, M., Yin, L., Raina, D., Kharbanda, A., Panchamoorthy, G., Gupta, D., Singh, H., Kharbanda, S., Kufe, D.: Intracellular targeting of the oncogenic MUC1-C protein with a novel GO-203 nanoparticle formulation. Clin. Cancer Res. 21, 2338–2347 (2015)
Hiraki, M., Suzuki, Y., Alam, M., Hinohara, K., Hasegawa, M., Jin, C., Kharbanda, S., Kufe, D.: MUC1-C stabilizes MCL-1 in the oxidative stress response of triple-negative breast cancer cells to BCL-2 inhibitors. Sci. Rep. 6, 6643 (2016)
Gautam, S.K., Kumar, S., Dam, V., Ghersi, D., Jain, M., Batra, S.K.: MUCIN-4 (MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy. Semin. Immunol. 47, 101391 (2020)
Zhu, Y., Zhang, J.J., Peng, Y.P., Liu, X., Xie, K.L., Tang, J., Jiang, K.R., Gao, W.T., Tian, L., Zhang, K., Xu, Z.K., Miao, Y.: NIDO, AMOP and vWD domains of MUC4 play synergic role in MUC4 mediated signaling. Oncotarget. 8(6), 10385–10399 (2017)
Tang, J., Zhu, Y., Xie, K., Zhang, X., Zhi, X., Wang, W., Li, Z., Zhang, Q., Wang, L., Wang, J., Xu, Z.: The role of the AMOP domain in MUC4/Y-promoted tumour angiogenesis and metastasis in pancreatic cancer. J. Exp. Clin. Cancer Res. 35, 91 (2016)
Xia, P., Choi, A.H., Deng, Z., Yang, Y., Zhao, J., Wang, Y., Hardwidge, P.R., Zhu, G.: Cell membrane-anchored MUC4 promotes tumorigenicity in epithelial carcinomas. Oncotarget. 8, 14147–14157 (2017)
Aithal, A., Rauth, S., Kshirsagar, P., Shah, A., Lakshmanan, I., Junker, W.M., Jain, M., Ponnusamy, M.P., Batra, S.K.: MUC16 as a novel target for cancer therapy. Expert Opin. Ther. Targets. 22, 675–686 (2018)
Wang, S., You, L., Dai, M., Zhao, Y.: Mucins in pancreatic cancer: a well-established but promising family for diagnosis, prognosis and therapy. J. Cell. Mol. Med. 24, 10279–10289 (2020)
Kaur, S., Smith, L.M., Patel, A., Menning, M., Watley, D.C., Malik, S.S., Krishn, S.R., Mallya, K., Aithal, A., Sasson, A.R., Johansson, S.L., Jain, M., Singh, S., Guha, S., Are, C., Raimondo, M., Hollingsworth, M.A., Brand, R.E., Batra, S.K.: A combination of MUC5AC and CA19-9 improves the diagnosis of pancreatic cancer: a multicenter study. Am. J. Gastroenterol. 112, 172–183 (2017)
Mereiter, S., Balmaña, M., Campos, D., Gomes, J., Reis, C.A.: Glycosylation in the era of cancer-targeted therapy: where Are we heading? Cancer Cell. 36, 6–16 (2019)
Niv, Y., Ho, S.B., Fass, R., Rokkas, T.: Mucin expression in the esophageal malignant and pre-malignant states: a systematic review and meta-analysis. J. Clin. Gastroenterol. 52, 91–96 (2018)
Terada, T.: An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int. J. Clin. Exp. Pathol. 6, 613–621 (2013)
Betge, J., Schneider, N.I., Harbaum, L., Pollheimer, M.J., Lindtner, R.A., Kornprat, P., Ebert, M.P., Langner, C.: MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: expression profiles and clinical significance. Virchows Arch. 469, 255–265 (2016)
Dalziel, M., Whitehouse, C., McFarlane, I., Brockhausen, I., Gschmeissner, S., Schwientek, T., Clausen, H., Burchell, J.M., Taylor-Papadimitriou, J.: The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem. 276, 11007–11015 (2001)
Bai, R., Luan, X., Zhang, Y., Robbe-Masselot, C., Brockhausen, I., Gao, Y.: The expression and functional analysis of the sialyl-T antigen in prostate cancer. Glycoconj. J. 37, 423–433 (2020)
Dabelsteen, E.: Cell surface carbohydrates as prognostic markers in human carcinomas. J. Pathol. 179, 358–369 (1996)
de Las Rivas, M., Lira-Navarrete, E., Gerken, T.A., Hurtado-Guerrero, R.: Polypeptide GalNAc-Ts: from redundancy to specificity. Curr. Opin. Struct. Biol. 56, 87–96 (2019)
Bagdonaite, I., Pallesen, E.M., Ye, Z., Vakhrushev, S.Y., Marinova, I.N., Nielsen, M.I., Kramer, S.H., Pedersen, S.F., Joshi, H.J., Bennett, E.P., Dabelsteen, S., Wandall, H.H.: O-glycan initiation directs distinct biological pathways and controls epithelial differentiation. EMBO Rep. 21, e48885 (2020)
Wu, Y.M., Liu, C.H., Hu, R.H., Huang, M.J., Lee, J.J., Chen, C.H., Huang, J., Lai, H.S., Lee, P.H., Hsu, W.M., Huang, H.C., Huang, M.C.: Mucin glycosylating enzyme GALNT2 regulates the malignant character of hepatocellular carcinoma by modifying the EGF receptor. Cancer Res. 71, 7270–7279 (2011)
Taniuchi, K., Cerny, R.L., Tanouchi, A., Kohno, K., Kotani, N., Honke, K., Saibara, T., Hollingsworth, M.A.: Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene. 30, 4843–4854 (2011)
Niang, B., Jin, L., Chen, X., Guo, X., Zhang, H., Wu, Q., Padhiar, A.A., Xiao, M., Fang, D., Zhang, J.: GalNAc-T4 putatively modulates the estrogen regulatory network through FOXA1 glycosylation in human breast cancer cells. Mol. Cell. Biochem. 411, 393–402 (2016)
Freire, T., Berois, N., Sóñora, C., Varangot, M., Barrios, E., Osinaga, E.: UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int. J. Cancer. 119, 1383–1388 (2006)
Pangeni, R.P., Channathodiyil, P., Huen, D.S., Eagles, L.W., Johal, B.K., Pasha, D., Hadjistephanou, N., Nevell, O., Davies, C.L., Adewumi, A.I., Khanom, H., Samra, I.S., Buzatto, V.C., Chandrasekaran, P., Shinawi, T., Dawson, T.P., Ashton, K.M., Davis, C., Brodbelt, A.R., Jenkinson, M.D., Bièche, I., Latif, F., Darling, J.L., Warr, T.J., Morris, M.R.: The GALNT9, BNC1 and CCDC8 genes are frequently epigenetically dysregulated in breast tumours that metastasise to the brain. Clin. Epigenetics. 7, 57 (2015)
Huanna, T., Tao, Z., Xiangfei, W., Longfei, A., Yuanyuan, X., Jianhua, W., Cuifang, Z., Manjing, J., Wenjing, C., Shaochuan, Q., Feifei, X., Naikang, L., Jinchao, Z., Chen, W.: GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol. Carcinog. 54, 1159–1171 (2015)
Tarp, M.A., Clausen, H.: Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim. Biophys. Acta. 1780, 546–563 (2008)
Liu, Z., Liu, J., Dong, X., Hu, X., Jiang, Y., Li, L., Du, T., Yang, L., Wen, T., An, G., Feng, G.: Tn antigen promotes human colorectal cancer metastasis via H-Ras mediated epithelial-mesenchymal transition activation. J. Cell. Mol. Med. 23, 2083–2092 (2019)
Ju, T., Aryal, R.P., Kudelka, M.R., Wang, Y., Cummings, R.D.: The Cosmc connection to the Tn antigen in cancer. Cancer Biomark. 14, 63–81 (2014)
Julien, S., Adriaenssens, E., Ottenberg, K., Furlan, A., Courtand, G., Vercoutter-Edouart, A.S., Hanisch, F.G., Delannoy, P., Le Bourhis, X.: ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology. 16, 54–64 (2006)
Schneider, F., Kemmner, W., Haensch, W., Franke, G., Gretschel, S., Karsten, U., Schlag, P.M.: Overexpression of sialyltransferase CMP-sialic acid: Galbeta1,3GalNAc-R alpha6-Sialyltransferase is related to poor patient survival in human colorectal carcinomas. Cancer Res. 61, 4605–4611 (2001)
Marcos, N.T., Bennett, E.P., Gomes, J., Magalhaes, A., Gomes, C., David, L., Dar, I., Jeanneau, C., DeFrees, S., Krustrup, D., Vogel, L.K., Kure, E.H., Burchell, J., Taylor-Papadimitriou, J., Clausen, H., Mandel, U., Reis, C.A.: ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front. Biosci. (Elite Ed). 3, 1443–1455 (2011)
Ogata, S., Koganty, R., Reddish, M., Longenecker, B.M., Chen, A., Perez, C., Itzkowitz, S.H.: Different modes of sialyl-Tn expression during malignant transformation of human colonic mucosa. Glycoconj. J. 15, 29–35 (1998)
Yu, X., Wu, Q., Wang, L., Zhao, Y., Zhang, Q., Meng, Q., Pawan, Wang, S.: Silencing of ST6GalNAc I suppresses the proliferation, migration and invasion of hepatocarcinoma cells through PI3K/AKT/NF-κB pathway. Tumour Biol. 37, 12213–12221 (2016)
Iwai, T., Inaba, N., Naundorf, A., Zhang, Y., Gotoh, M., Iwasaki, H., Kudo, T., Togayachi, A., Ishizuka, Y., Nakanishi, H., Narimatsu, H.: Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J. Biol. Chem. 277, 12802–12809 (2002)
Vavasseur, F., Yang, J.M., Dole, K., Paulsen, H., Brockhausen, I.: Synthesis of O-glycan core 3: characterization of UDP-GlcNAc: GalNAc-R beta 3-N-acetyl-glucosaminyltransferase activity from colonic mucosal tissues and lack of the activity in human cancer cell lines. Glycobiology. 5, 351–357 (1995)
Iwai, T., Kudo, T., Kawamoto, R., Kubota, T., Togayachi, A., Hiruma, T., Okada, T., Kawamoto, T., Morozumi, K., Narimatsu, H.: Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc. Natl. Acad. Sci. U. S. A. 102, 4572–4577 (2005)
An, G., Wei, B., Xia, B., McDaniel, J.M., Ju, T., Cummings, R.D., Braun, J., Xia, L.: Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 204, 1417–1429 (2007)
Doi, N., Ino, Y., Angata, K., Shimada, K., Narimatsu, H., Hiraoka, N.: Clinicopathological significance of core 3 O-glycan synthetic enzyme, β1,3-N-acetylglucosaminyltransferase 6 in pancreatic ductal adenocarcinoma. PLoS One. 15, e0242851 (2020)
Radhakrishnan, P., Grandgenett, P.M., Mohr, A.M., Bunt, S.K., Yu, F., Chowdhury, S., Hollingsworth, M.A.: Expression of core 3 synthase in human pancreatic cancer cells suppresses tumor growth and metastasis. Int. J. Cancer. 133, 2824–2833 (2013)
Gao, Y., Chachadi, V.B., Cheng, P.W., Brockhausen, I.: Glycosylation potential of human prostate cancer cell lines. Glycoconj. J. 29, 525–537 (2012)
Picco, G., Julien, S., Brockhausen, I., Beatson, R., Antonopoulos, A., Haslam, S., Mandel, U., Dell, A., Pinder, S., Taylor-Papadimitriou, J., Burchell, J.: Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology. 20, 1241–1250 (2010)
Burchell, J., Poulsom, R., Hanby, A., Whitehouse, C., Cooper, L., Clausen, H., Miles, D., Taylor-Papadimitriou, J.: An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology. 9, 1307–1311 (1999)
Brockhausen, I., Matta, K.L., Orr, J., Schachter, H.: Mucin synthesis. UDP-GlcNAc:GalNAc-R beta 3-N-acetylglucosaminyltransferase and UDP-GlcNAc:GlcNAc beta 1–3GalNAc-R (GlcNAc to GalNAc) beta 6-N-acetylglucosaminyltransferase from pig and rat colon mucosa. Biochemistry. 24, 1866–1874 (1985)
Yang, J.M., Byrd, J.C., Siddiki, B.B., Chung, Y.S., Okuno, M., Sowa, M., Kim, Y.S., Matta, K.L., Brockhausen, I.: Alterations of O-glycan biosynthesis in human colon cancer tissues. Glycobiology. 4, 873–884 (1994)
Beum, P.V., Basma, H., Bastola, D.R., Cheng, P.W.: Mucin biosynthesis: upregulation of core 2 beta 1,6 N-acetylglucosaminyltransferase by retinoic acid and Th2 cytokines in a human airway epithelial cell line. Am. J. Phys. Lung Cell. Mol. Phys. 288, L116–L124 (2005)
Huang, M.C., Chen, H.Y., Huang, H.C., Huang, J., Liang, J.T., Shen, T.L., Lin, N.Y., Ho, C.C., Cho, I.M., Hsu, S.M.: C2GnT-M is downregulated in colorectal cancer and its re-expression causes growth inhibition of colon cancer cells. Oncogene. 25, 3267–3276 (2006)
Ishizone, S., Yamauchi, K., Kawas, S., Suzuki, T., Shimizu, F., Harada, O., Sugiyama, A., Miyagawa, S., Fukuda, M., Nakayama, J.: Clinical utility of quantitative RT-PCR targeted to alpha1,4-N-acetylglucosaminyltransferase mRNA for detection of pancreatic cancer. Cancer Sci. 97, 119–126 (2006)
Martínez-Sáez, N., Peregrina, J.M., Corzana, F.: Principles of mucin structure: implications for the rational design of cancer vaccines derived from MUC1-glycopeptides. Chem. Soc. Rev. 46, 7154–7175 (2017)
Rhinehardt, K.L., Srinivas, G., Mohan, R.V.: Molecular dynamics simulation analysis of anti-MUC1 Aptamer and Mucin 1 peptide binding. J. Phys. Chem. B. 119, 6571–6583 (2015)
Barnett, C.B., Senapathi, T., Naidoo, K.J.: Comparative ligand structural analytics illustrated on variably glycosylated MUC1 antigen-antibody binding. Beilstein J. Org. Chem. 16, 2540–2550 (2020)
Movahedin, M., Brooks, T.M., Supekar, N.T., Gokanapudi, N., Boons, G.J., Brooks, C.L.: Glycosylation of MUC1 influences the binding of a therapeutic antibody by altering the conformational equilibrium of the antigen. Glycobiology. 27, 677–687 (2017)
Huang, Z.H., Shi, L., Ma, J.W., Sun, Z.Y., Cai, H., Chen, Y.X., Zhao, Y.F., Li, Y.M.: A totally synthetic, self-assembling, adjuvant-free MUC1 glycopeptide vaccine for cancer therapy. J. Am. Chem. Soc. 134, 8730–8733 (2012)
Carmicheal, J., Atri, P., Sharma, S., Kumar, S., Chirravuri Venkata, R., Kulkarni, P., Salgia, R., Ghersi, D., Kaur, S., Batra, S.K.: Presence and structure-activity relationship of intrinsically disordered regions across mucins. FASEB J. 34, 1939–1957 (2020)
Gaidzik, N., Westerlind, U., Kunz, H.: The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem. Soc. Rev. 42, 4421–4442 (2013)
Wilson, R.M., Danishefsky, S.J.: A vision for vaccines built from fully synthetic tumor-associated antigens: from the laboratory to the clinic. J. Am. Chem. Soc. 135, 14462–14472 (2013)
Feng, D., Shaikh, A.S., Wang, F.: Recent advance in tumor-associated carbohydrate antigens (TACAs)-based antitumor vaccines. ACS Chem. Biol. 11, 850–863 (2016)
Slovin, S.F., Ragupathi, G., Fernandez, C., Diani, M., Jefferson, M.P., Wilton, A., Kelly, W.K., Morris, M., Solit, D., Clausen, H., Livingston, P., Scher, H.I.: A polyvalent vaccine for high-risk prostate patients: ‘are more antigens better?’. Cancer Immunol. Immunother. 56, 1921–1930 (2007)
Stergiou, N., Gaidzik, N., Heimes, A.S., Dietzen, S., Besenius, P., Jäkel, J., Brenner, W., Schmidt, M., Kunz, H., Schmitt, E.: Reduced breast tumor growth after immunization with a tumor-restricted MUC1 Glycopeptide conjugated to tetanus toxoid. Cancer Immunol. Res. 7, 113–122 (2019)
Miles, D., Roché, H., Martin, M., Perren, T.J., Cameron, D.A., Glaspy, J., Dodwell, D., Parker, J., Mayordomo, J., Tres, A., Murray, J.L., Ibrahim, N.K.: Theratope® study group. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 16, 1092–1100 (2011)
Corzana, F., Busto, J.H., Marcelo, F., de Luis, M.G., Asensio, J.L., Martín-Santamaría, S., Sáenz, Y., Torres, C., Jiménez-Barbero, J., Avenoza, A., Peregrina, J.M.: Rational design of a Tn antigen mimic. Chem. Commun. (Camb.). 47, 5319–5321 (2011)
Kaiser, A., Gaidzik, N., Westerlind, U., Kowalczyk, D., Hobel, A., Schmitt, E., Kunz, H.: A synthetic vaccine consisting of a tumor-associated sialyl-T(N)-MUC1 tandem-repeat glycopeptide and tetanus toxoid: induction of a strong and highly selective immune response. Angew. Chem. Int. Ed. Eng. 48, 7551–7555 (2009)
Westerlind, U., Kunz, H.: Synthetic vaccines from tumor-associated glycopeptide antigens. Chimia (Aarau). 65, 30–34 (2011)
Hoffmann-Röder, A., Kaiser, A., Wagner, S., Gaidzik, N., Kowalczyk, D., Westerlind, U., Gerlitzki, B., Schmitt, E., Kunz, H.: Synthetic antitumor vaccines from tetanus toxoid conjugates of MUC1 glycopeptides with the Thomsen-Friedenreich antigen and a fluorine-substituted analogue. Angew. Chem. Int. Ed. Eng. 49, 8498–8503 (2010)
Fiedler, W., DeDosso, S., Cresta, S., Weidmann, J., Tessari, A., Salzberg, M., Dietrich, B., Baumeister, H., Goletz, S., Gianni, L., Sessa, C.: A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur. J. Cancer. 63, 55–63 (2016)
Bose, M., Mukherjee, P.: Potential of anti-MUC1 antibodies as a targeted therapy for gastrointestinal cancers. Vaccines (Basel). 8, 659 (2020)
Sharma, P., Allison, J.P.: Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 161, 205–214 (2015)
Shayan, G., Srivastava, R., Li, J., Schmitt, N., Kane, L.P., Ferris, R.L.: Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 6, e1261779 (2016)
Kondo, H., Hazama, S., Kawaoka, T., Yoshino, S., Yoshida, S., Tokuno, K., Takashima, M., Ueno, T., Hinoda, Y., Oka, M.: Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 28(1B), 379–387 (2008)
Butts, C., Socinski, M.A., Mitchell, P.L., Thatcher, N., Havel, L., Krzakowski, M., Nawrocki, S., Ciuleanu, T.E., Bosquée, L., Trigo, J.M., Spira, A., Tremblay, L., Nyman, J., Ramlau, R., Wickart-Johansson, G., Ellis, P., Gladkov, O., Pereira, J.R., Eberhardt, W.E., Helwig, C., Schröder, A., Shepherd, F.A., START trial team: Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 59–68 (2014)
Singer, C.F., Pfeiler, G., Hubalek, M., et al.: Austrian Breast & Colorectal Cancer Study Group. Efficacy and safety of the therapeutic cancer vaccine tecemotide (L-BLP25) in early breast cancer: results from a prospective, randomised, neoadjuvant phase II study (ABCSG 34). Eur. J. Cancer. 132, 43–52 (2020)
Oudard, S., Rixe, O., Beuselinck, B., Linassier, C., Banu, E., Machiels, J.P., Baudard, M., Ringeisen, F., Velu, T., Lefrere-Belda, M.A., Limacher, J.M., Fridman, W.H., Azizi, M., Acres, B., Tartour, E.: A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol. Immunother. 60, 261–271 (2011)
Scheid, E., Major, P., Bergeron, A., Finn, O.J., Salter, R.D., Eady, R., Yassine-Diab, B., Favre, D., Peretz, Y., Landry, C., Hotte, S., Mukherjee, S.D., Dekaban, G.A., Fink, C., Foster, P.J., Gaudet, J., Gariepy, J., Sekaly, R.P., Lacombe, L., Fradet, Y., Foley, R.: Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II trial in patients with nonmetastatic castrate-resistant prostate cancer. Cancer Immunol. Res. 4, 881–892 (2016)
Holmberg, L.A., Sandmaier, B.M.: Theratope vaccine (STn-KLH). Expert. Opin. Biol. Ther. 1, 881–891 (2001)
Carmon, L., Avivi, I., Kovjazin, R., Zuckerman, T., Dray, L., Gatt, M.E., Or, R., Shapira, M.Y.: Phase I/II study exploring ImMucin, a pan-major histocompatibility complex, anti-MUC1 signal peptide vaccine, in multiple myeloma patients. Br. J. Haematol. 169, 44–56 (2015)
Shindo, Y., Hazama, S., Maeda, Y., Matsui, H., Iida, M., Suzuki, N., Yoshimura, K., Ueno, T., Yoshino, S., Sakai, K., Suehiro, Y., Yamasaki, T., Hinoda, Y., Oka, M.: Adoptive immunotherapy with MUC1-mRNA transfected dendritic cells and cytotoxic lymphocytes plus gemcitabine for unresectable pancreatic cancer. J. Transl. Med. 12, 175 (2014)
Posey Jr., A.D., Schwab, R.D., Boesteanu, A.C., Steentoft, C., Mandel, U., Engels, B., Stone, J.D., Madsen, T.D., Schreiber, K., Haines, K.M., Cogdill, A.P., Chen, T.J., Song, D., Scholler, J., Kranz, D.M., Feldman, M.D., Young, R., Keith, B., Schreiber, H., Clausen, H., Johnson, L.A., June, C.H.: Engineered CAR T cells targeting the cancer-associated Tn-Glycoform of the membrane Mucin MUC1 control adenocarcinoma. Immunity. 44, 1444–1454 (2016)
Sasidharan Nair, V., Toor, S.M., Taha, R.Z., Ahmed, A.A., Kurer, M.A., Murshed, K., Soofi, M.E., Ouararhni, K., Alajez, N.M., Abu Nada, M., Elkord, E.: Transcriptomic profiling of tumor-infiltrating CD4+TIM-3+ T cells reveals their suppressive, exhausted, and metastatic characteristics in colorectal cancer patients. Vaccines (Basel). 8, 71 (2020)
Baghdadi, M., Nagao, H., Yoshiyama, H., Akiba, H., Yagita, H., Dosaka-Akita, H., Jinushi, M.: Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol. Immunother. 62, 629–637 (2013)
Rashidijahanabad, Z., Huang, X.: Recent advances in tumor associated carbohydrate antigen based chimeric antigen receptor T cells and bispecific antibodies for anti-cancer immunotherapy. Semin. Immunol. 47, 101390 (2020)
Katayose, Y., Kudo, T., Suzuki, M., Shinoda, M., Saijyo, S., Sakurai, N., Saeki, H., Fukuhara, K., Imai, K., Matsuno, S.: MUC1-specific targeting immunotherapy with bispecific antibodies: inhibition of xenografted human bile duct carcinoma growth. Cancer Res. 56, 4205–4212 (1996)
Johnen, H., Kulbe, H., Pecher, G.: Long-term tumor growth suppression in mice immunized with naked DNA of the human tumor antigen mucin (MUC1). Cancer Immunol. Immunother. 50, 356–360 (2001)
Wei, J., Gao, W., Wu, J., Meng, K., Zhang, J., Chen, J., Miao, Y.: Dendritic cells expressing a combined PADRE/MUC4-derived polyepitope DNA vaccine induce multiple cytotoxic T-cell responses. Cancer Biother. Radiopharm. 23, 121–128 (2008)
Brooks, N., Hsu, J., Esparon, S., Pouniotis, D., Pietersz, G.A.: Immunogenicity of a tripartite cell penetrating peptide containing a MUC1 variable number of tandem repeat (VNTR) and a T helper epitope. Molecules. 23, 2233 (2018)
Leiria Campo, V., Riul, T.B., Oliveira Bortot, L., Martins-Teixeira, M.B., Fiori Marchiori, M., Iaccarino, E., Ruvo, M., Dias-Baruffi, M., Carvalho, I.: A synthetic MUC1 Glycopeptide bearing βGalNAc-Thr as a Tn antigen isomer induces the production of antibodies against tumor cells. Chembiochem. 18, 527–538 (2017)
Wang, H., Yang, B., Wang, Y., Liu, F., Fernández-Tejada, A., Dong, S.: β-Glucan as an immune activator and a carrier in the construction of a synthetic MUC1 vaccine. Chem. Commun. (Camb.). 55, 253–256 (2018)
Du, J.J., Wang, C.W., Xu, W.B., et al.: Multifunctional protein conjugates with built-in adjuvant (adjuvant-protein-antigen) as cancer vaccines boost potent immune responses. iScience. 23, 100935 (2020)
Cai, H., Huang, Z.H., Shi, L., Sun, Z.Y., Zhao, Y.F., Kunz, H., Li, Y.M.: Variation of the glycosylation pattern in MUC1 glycopeptide BSA vaccines and its influence on the immune response. Angew. Chem. Int. Ed. Eng. 51, 1719–1723 (2012)
Curry, J.M., Besmer, D.M., Erick, T.K., Steuerwald, N., Das Roy, L., Grover, P., Rao, S., Nath, S., Ferrier, J.W., Reid, R.W., Mukherjee, P.: Indomethacin enhances anti-tumor efficacy of a MUC1 peptide vaccine against breast cancer in MUC1 transgenic mice. PLoS One. 14, e0224309 (2019)
Wu, X., McKay, C., Pett, C., Yu, J., Schorlemer, M., Ramadan, S., Lang, S., Behren, S., Westerlind, U., Finn, M.G., Huang, X.: Synthesis and immunological evaluation of disaccharide bearing MUC-1 Glycopeptide conjugates with virus-like particles. ACS Chem. Biol. 14, 2176–2184 (2019)
Deguchi, T., Tanemura, M., Miyoshi, E., Nagano, H., Machida, T., Ohmura, Y., Kobayashi, S., Marubashi, S., Eguchi, H., Takeda, Y., Ito, T., Mori, M., Doki, Y., Sawa, Y.: Increased immunogenicity of tumor-associated antigen, mucin 1, engineered to express alpha-gal epitopes: a novel approach to immunotherapy in pancreatic cancer. Cancer Res. 70, 5259–5269 (2010)
Guo, M., Luo, B., Pan, M., Li, M., Zhao, F., Dou, J.: MUC1 plays an essential role in tumor immunity of colorectal cancer stem cell vaccine. Int. Immunopharmacol. 85, 106631 (2020)
McDonald, D.M., Hanna, C.C., Ashhurst, A.S., Corcilius, L., Byrne, S.N., Payne, R.J.: Synthesis of a self-Adjuvanting MUC1 vaccine via Diselenide-Selenoester ligation-Deselenization. ACS Chem. Biol. 13, 3279–3285 (2018)
Glaffig, M., Stergiou, N., Hartmann, S., Schmitt, E., Kunz, H.: A synthetic MUC1 anticancer vaccine containing mannose ligands for targeting macrophages and dendritic cells. ChemMedChem. 13, 25–29 (2018)
Zhou, H., Zhang, Z., Liu, G., Jiang, M., Wang, J., Liu, Y., Tai, G.: The effect of different immunization cycles of a recombinant Mucin1-maltose-binding protein vaccine on T cell responses to B16-MUC1 melanoma in mice. Int. J. Mol. Sci. 21, 5810 (2020)
Fang, F., Ma, J., Ni, W., Wang, F., Sun, X., Li, Y., Li, Q., Xie, F., Wang, J., Zhai, R., Liu, Z., Gao, S., Tai, G.: MUC1 and maltose-binding protein recombinant fusion protein combined with bacillus Calmette-Guerin induces MUC1-specific and nonspecific anti-tumor immunity in mice. Mol. Med. Rep. 10, 1056–1064 (2014)
Mehrab Mohseni, M., Amani, J., Fasihi Ramandi, M., Mahjoubi, F., Jafaria, M., Salmanian, A.H.: Immunogenicity evaluation of recombinant edible vaccine candidate containing HER2-MUC1 against breast cancer. Iran. J. Allergy Asthma Immunol. 18, 511–522 (2019)
Du, J.J., Zou, S.Y., Chen, X.Z., et al.: Liposomal antitumor vaccines targeting Mucin 1 elicit a lipid-dependent Immunodominant response. Chem. Asian J. 14, 2116–2121 (2019)
Hossain, M.K., Vartak, A., Karmakar, P., Sucheck, S.J., Wall, K.A.: Augmenting vaccine immunogenicity through the use of natural human anti-rhamnose antibodies. ACS Chem. Biol. 13, 2130–2142 (2018)
Karmakar, P., Lee, K., Sarkar, S., Wall, K.A., Sucheck, S.J.: Synthesis of a liposomal MUC1 Glycopeptide-based immunotherapeutic and evaluation of the effect of l-Rhamnose targeting on cellular immune responses. Bioconjug. Chem. 27, 110–120 (2016)
Li, M., Wang, Z., Yan, B., Yin, X., Zhao, Y., Yu, F., Meng, M., Liu, Y., Zhao, W.: Design of a MUC1-based tricomponent vaccine adjuvanted with FSL-1 for cancer immunotherapy. Medchemcomm. 10, 2073–2077 (2019)
Abdel-Aal, A.B., Lakshminarayanan, V., Thompson, P., Supekar, N., Bradley, J.M., Wolfert, M.A., Cohen, P.A., Gendler, S.J., Boons, G.J.: Immune and anticancer responses elicited by fully synthetic aberrantly glycosylated MUC1 tripartite vaccines modified by a TLR2 or TLR9 agonist. Chembiochem. 15, 1508–1513 (2014)
Lakshminarayanan, V., Thompson, P., Wolfert, M.A., Buskas, T., Bradley, J.M., Pathangey, L.B., Madsen, C.S., Cohen, P.A., Gendler, S.J., Boons, G.J.: Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. U. S. A. 109, 261–266 (2012)
Hernández-Ramírez, J., Wong-Arce, A., González-Ortega, O., Rosales-Mendoza, S.: Expression in algae of a chimeric protein carrying several epitopes from tumor associated antigens. Int. J. Biol. Macromol. 147, 46–52 (2020)
Lakshminarayanan, V., Supekar, N.T., Wei, J., McCurry, D.B., Dueck, A.C., Kosiorek, H.E., Trivedi, P.P., Bradley, J.M., Madsen, C.S., Pathangey, L.B., Hoelzinger, D.B., Wolfert, M.A., Boons, G.J., Cohen, P.A., Gendler, S.J.: MUC1 vaccines, comprised of glycosylated or non-glycosylated peptides or tumor-derived MUC1, can circumvent Immunoediting to control tumor growth in MUC1 transgenic mice. PLoS One. 11, e0145920 (2016)
Kimura, T., Finn, O.J.: MUC1 immunotherapy is here to stay. Expert. Opin. Biol. Ther. 13, 35–49 (2013)
Bafna, S., Singh, A.P., Moniaux, N., Eudy, J.D., Meza, J.L., Batra, S.K.: MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Res. 68, 9231–9238 (2008)
Gautam, S.K., Kumar, S., Cannon, A., Hall, B., Bhatia, R., Nasser, M.W., Mahapatra, S., Batra, S.K., Jain, M.: MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma. Expert Opin. Ther. Targets. 21, 657–669 (2017)
Singh, A.P., Moniaux, N., Chauhan, S.C., Meza, J.L., Batra, S.K.: Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 64(2), 622–630 (2004)
Chaturvedi, P., Singh, A.P., Chakraborty, S., Chauhan, S.C., Bafna, S., Meza, J.L., Singh, P.K., Hollingsworth, M.A., Mehta, P.P., Batra, S.K.: MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 68, 2065–2070 (2008)
Bafna, S., Kaur, S., Momi, N., Batra, S.K.: Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br. J. Cancer. 101, 1155–1161 (2009)
Liu, L., Kshirsagar, P., Christiansen, J., Gautam, S.K., Aithal, A., Gulati, M., Kumar, S., Solheim, J.C., Batra, S.K., Jain, M., Wannemuehler, M.J., Narasimhan, B.: Polyanhydride nanoparticles stabilize pancreatic cancer antigen MUC4β. J. Biomed. Mater. Res. A. (2020). https://doi.org/10.1002/jbm.a.37080
Banerjee, K., Gautam, S.K., Kshirsagar, P., Ross, K.A., Spagnol, G., Sorgen, P., Wannemuehler, M.J., Narasimhan, B., Solheim, J.C., Kumar, S., Batra, S.K., Jain, M.: Amphiphilic polyanhydride-based recombinant MUC4β-nanovaccine activates dendritic cells. Genes Cancer. 10, 52–62 (2019)
Cai, H., Palitzsch, B., Hartmann, S., Stergiou, N., Kunz, H., Schmitt, E., Westerlind, U.: Antibody induction directed against the tumor-associated MUC4 glycoprotein. Chembiochem. 16, 959–967 (2015)
Acknowledgements
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (to I.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Glycoconjugate Journal: special issue on Glycoconjugate vaccines: classic and novel approaches.
Editor: Roberto Adamo, December 31, 2020, https://www.springer.com/journal/10719
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brockhausen, I., Melamed, J. Mucins as anti-cancer targets: perspectives of the glycobiologist. Glycoconj J 38, 459–474 (2021). https://doi.org/10.1007/s10719-021-09986-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-021-09986-8