Abstract
We have shown the immunogenicity and safety of synthetic carbohydrate vaccines when conjugated to the carrier keyhole limpet hemocyanin (KLH) and given with the adjuvant, QS-21, in patients with biochemically relapsed prostate cancer. To determine whether immune response could be further enhanced with stimulation by multiple antigens, a hexavalent vaccine was prepared using previously determined doses and administered in a Phase II setting to 30 high-risk patients. The hexavalent vaccine included GM2, Globo H, Lewisy, glycosylated MUC-1-32mer and Tn and TF in a clustered formation, conjugated to KLH and mixed with QS-21. Eight vaccinations were administered over 13 months. All 30 patients had significant elevations in antibody titers to at least two of the six antigens; 22 patients had increased reactivity with FACS. These serologic responses were lower than that seen previously in patients treated with the respective monovalent vaccines. The reciprocal median combined IgM and IgG antibody titers with ELISA against MUC1, Tn, TF, globo H and GM2 for these 30 patients were 640, 80, 120, 40 and 0, compared to 1280, 640, 1280, 320 and 160 seen in patients receiving individual monovalent vaccines. This hexavalent vaccine of synthetic “self” antigens broke immunologic tolerance against two or more antigens in all 30 vaccinated patients, was safe, but antibody titers against several of the antigens were lower than those seen in individual monovalent trials. No impact on PSA slope was detected. We address the relevance of the multivalent approach for prostate cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We have demonstrated the immunogenicity and safety of synthetic carbohydrate cancer antigens conjugated to the carrier keyhole limpet hemocyanin (KLH) and given as monovalent vaccines with the saponin adjuvant QS-21 in patients with biochemically relapsed prostate cancer. Tumor cell heterogeneity and heterogeneity of the human immune response in different individuals suggest that polyvalent tumor vaccines will be required for optimal efficacy. Studies on experimental animals suggest that the tolerizing effect of metastatic cancer can be diminished by vaccination when the disease is limited to micrometastases, a situation similar to the high-risk adjuvant setting after surgery or chemotherapy-induced responses, and to biochemical relapses in prostate cancer patients.
Patients with biochemically relapsed prostate cancer following primary therapy are a dichotomous group of men, as many do well for years despite rising PSAs, whereas others progress within two years after primary therapy based on the criteria of Pound et al. [1]. These “high-risk” patients were classified as having a PSA relapse within 2 years of primary therapy with PSA doubling times (PSADT) of less than 10 months, or PSA slope of >0.15, and high grade tumors such as Gleason 8, 9 or 10.
It is in this high-risk population that we investigated the impact of a multivalent vaccine with the primary goals of establishing safety and immunogenicity as manifested by development of high titer antibodies directed against the immunizing antigens, and as a secondary endpoint, whether the vaccine could impact on the PSADT or slope and time to radiographic progression. A total of 30 patients who met the above criteria for high risk with the exception of having a PSADT of less than 6 months were entered into a Phase II trial and treated with a hexavalent conjugate vaccine. The vaccine contained 6 KLH-conjugates targeting GM2, Globo H, Lewisy, Tn, TF and MUC1. Toxicity and clinical course were monitored, and serologic responses were determined and compared to responses obtained previously with the relevant monovalent vaccines.
Materials and methods
Patient selection
A total of 30 patients with prostate cancer that was histologically confirmed by the Department of Pathology at Memorial Sloan-Kettering Cancer Center were enrolled based on established high-risk parameters: biochemical progression within 2 years after primary surgery or radiation therapy (with or without neo-adjuvant androgen ablation). Patients who had intermittent hormonal treatment following primary therapy and non-castrate levels of testosterone (>50 ng/dl) were eligible. Patients must have had a Karnofsky performance status >60% with adequate organ function as defined by: WBC ≥3,500/mm3, platelet count ≥100,000 mm3, total bilirubin <2.0 mg/100 ml, or SGOT <3.0 times the upper limit of normal, creatinine ≤2.0 mg/100 ml or creatinine clearance ≥40 cc/min. Prior chemotherapy or radiation therapy within 4 weeks prior to entry into the trial was not permitted. All patients signed an IRB-approved informed consent. Baseline imaging included chest X-ray, bone scan and abdominal and pelvic CAT scan or MRI as indicated. Patients were enrolled if they had a PSADT less than 6 months and there was no evidence of radiographic progression. Patients who were post prostatectomy had to have 3 PSA determinations ≥2 weeks apart with a baseline value of 0.1. Patients who were post radiation must have had a baseline PSA of 1.0.
Vaccine preparation
Globo H, Lewisy, Tn(c) and TF(c) [clusters] were synthesized in the laboratory of Bio-Organic Chemistry headed by Dr. Sam Danishefsky [2–8]. MUC-1-32mer (-CHGVTSAPDTRPAPGSTAPPAHGVTSAPDTRPA) was synthesized in the MSKCC Core Peptide Synthesis Facility under the aegis of Dr. Paul Tempst. It was fully glycosylated (at 8 sites) with Tn using the –T2 and –T4 α-N-acetylgalactosaminyltransferases by Dr. Henrik Clausen at the University of Copenhagen, Copenhagen, Denmark. GM2 was extracted from rabbit brains as GM1, treated with beta galactosidase to yield GM2, and provided by Progenics, Inc., Tarrytown, New York.
Globo H, MUC-1-32mer, GM2, Lewis y , Tn(c), and TF(c)-KLH conjugation: these antigens were covalently attached to KLH as previously described [2–4, 7, 8]. Antigen doses were determined based on previous Phase I monovalent vaccine trials in prostate cancer patients as follows: glycosylated MUC-1-32mer, 3 μg; globo H, 10 μg; GM2, 10 μg; Ley, 10 μg; Tn(c), 3 μg; and TF(c), 3 μg. QS-21 [6, 7, 9] was used at 100 μg. Vaccines were given subcutaneously on weeks 1, 2, 3, 7, 19, 31, 43 and 55 in rotation at sites on the arms and legs.
Serologic assays
Serum samples were obtained at weeks 1, 2, 3, 7, 12, 19, 21 and 31. Serology for both immunologic studies and biochemical markers such as PSA and acid phosphatase were performed on the day of and 2 weeks after each vaccination against the following target antigens: glycosylated MUC-1-32 mer, GM-2, Globo H ceramide, desialated ovine submaxillary mucin (DOSM) expressing Tn, desialated porcine submaxillary mucin (DPSM) expressing TF and Leyceramide. IgM and IgG antibody titers were measured by ELISA, as described previously [2, 3, 10, 11]. The titer was defined as the highest dilution yielding an optical density of ≥0.1.
Flow cytometric analysis
Fluorescent-activated cell sorting(FACS) was performed as previously described [2, 3, 10, 11] to demonstrate antibody binding to the cell surface of the cell line, MCF-7, a breast carcinoma cell line known to express each of the six antigens, as well as prostate cancer cell lines DU145 and LnCAP. Pretreatment and experimental sera were read together and the pretreatment percent positive cells set to 10%. Sera with a doubling in percent positive cells and a 50% increase in mean fluorescence intensity (MFI) are considered positive.
Complement-dependent cytotoxicity
Complement-dependent cytotoxicity (CDC) was assayed on MCF-7 cell lines using a 2-h 51-chromium release assay, as previously described [10], with human complement and pre- and post-treatment sera at a dilution of 1:4 and 1:100, or with MoAb BR55 (GlycoTech, Rockville, MD) against MCF-7 at 5 μg/ml. Positive results were those wells in which post-vaccination cytotoxicity was ≥15% above the pretreatment level.
Western blot analysis
Pre- and post-treatment sera from patients with strong reactivity against MUC-1 and Globo H were used in a Western blot analysis. Purified DOSM [1 mg/ml, loading dose 5.0 μg], DPSM [1 mg/ml, loading dose 5.0 μg], Lewisy-HSA [1 mg/ml, loading dose 1.5 μg], globo H-HSA [0.5 mg/ml, loading dose 0.75 μg] and KLH [0.5 mg/ml, loading dose 0.375 μg] were loaded on a Ready Gel Tris-HLC gel (Bio-Rad, Hercules, CA) and run at 200 mV. The proteins on the gel were transferred to a polyvinylidene fluoride (PVDF) membrane (PALL, Ann Arbor, MI) for 1 h at room temperature using the Mini Trans-blot Cell (Bio-Rad). Non-specific sites on the membrane were blocked overnight at 4°C with 5% milk protein and 0.1% Tween 20 in PBS. The membrane was rinsed using 0.1% Tween 20 in PBS and incubated in pre- and post-treatment sera at 1:100 for 1 h at room temperature. The membrane was rinsed several times at room temperature using fresh changes of 0.1% Tween wash buffer, and incubated in peroxidase conjugated goat anti-human IgG, A, M (Rockland, Gilbertsville, PA) at 1:40,000 for 1 h at room temperature. Bands were detected with ECL Western blotting detection reagents (Amersham Biosciences, Corp, Piscataway, NJ).
Statistical consideration
Using the ASTRO definition, three consecutive PSA rises were considered to be a biochemical failure after radical prostatectomy or radiation therapy. The date of failure was the midpoint between the postsurgical (or post-irradiation) nadir PSA and the first of the three consecutive rises. Patients must have had a PSADT less than 6 months. PSADT was determined prior to treatment and was equal to ln(2) divided by the least squares derived slope of log PSA over time (log PSA slope > 0.15). The time interval in which PSADT was based consisted of a minimum of three PSA measurements in a 12-month interval prior to enrollment. Those who met this requirement were considered as high risk and were eligible for the trial.
Survival probabilities
The progression-free survival probabilities were estimated using the product limit method and the differences between levels within a covariate were tested using the log rank statistic. Changes in PSADT for 12 months post-treatment (compared to pre-treatment) were calculated and cross-tabulated with the treatment type. The cross-tabulations were tested for significance with Fisher’s Exact test. A total of 30 patients were evaluated with 26 meeting criteria of slope >0.15. These 26 were then used in a univariate analysis from which was calculated the 6 and 12 month post-treatment slope, requiring at least three measurements instead of the time period to estimate slope. Of the patients, 12 had enough measurements for only 6 months and 25 had enough for 12 months, the latter being used for cross-tabulation analysis.
Results
Patient profile
Patient characteristics are described in Table 1. A total of 30 patients (age range 42–82, median age 64) were accrued of whom the majority was treated with either radiation or surgery as primary therapy. Of these, 27 received all vaccinations. For greater than 50 weeks, 24 patients remained on study; 23 patients underwent radical prostatectomy alone with one patient having received neoadjuvant hormonal therapy and another patient neoadjuvant chemotherapy. Two patients received either external beam radiation or brachytherapy and three patients elected to have intermittent treatment with hormonal therapy.
Vaccine safety monitoring
No grade 4 events were reported. One patient experienced grade 1 myalgia, which was thought to be due to a concurrent viral syndrome; 23 patients developed a grade 2 reaction at the injection site consisting of induration and erythema lasting no longer than 72 h; 26 patients complained of transient grade I pain at the injection site with 7 complaining of flu-like symptoms and 9 with grade I low grade fever between 99 and 100.5°F lasting less than 24 h. Five patients developed pruritic symptoms at the injection site.
Immunological assessment
ELISA IgM and IgG antibody titers over the immunization period for all patients are depicted in Fig 1. As seen in previous trials, IgM antibodies started rising by week 3, were optimal by week 7 and started to decline by week 19 and for some antigens as late as week 31. IgM and IgG Abs were generated against MUC-1 [IgM range: 1/40 to ≥1/1280; IgG range: 1/40 to ≥1/1280] and TF(c) [IgM range: 1/40 to ≥1280; IgG range: 1/40 to ≥1/1280], with IgM being the primary Ab against Globo H [range 1/80 to ≥1/1280] and Tn(c) [range: 1/80–1/320]. At least 10 of the 27 patients who were evaluated at week 7 had antibody titers of 160 or greater against MUC1, Globo H, Tn and TF. Only five or six patients, respectively, produced antibody titers of 1/40 or greater against GM2 and Lewis Y. All patients demonstrated at least an eightfold increase in antibody titers against two of the six antigens after immunization compared to the pretreatment level; 26 patients developed a titer of at least 1/160 against two or more of the six antigens after vaccination. The median pre- and post-treatment IgM and IgG titers for each antigen in patients treated with the multivalent vaccine were compared with patients treated with the respective monovalent vaccine as seen in Table 2. IgM titers were comparable to results seen previously with monovalent vaccines at these doses against MUC1, Tn, Globo H and Lewis Y. IgM titers against TF and GM2 were at least eightfold lower with the hexavalent vaccine than seen previously with monovalent vaccines. Compared with the high titer IgM antibodies against MUC-1, Tn, Globo H, GM2 and Lewisy antigens seen in patients who received the respective monovalent vaccines, there was little or no IgG reactivity against these same antigens in sera from patients who were treated with the hexavalent vaccine.
Complement lysis
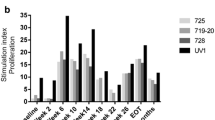
Results of initial CDC assays for all patients are shown in Fig. 2b. Sera from the seven patients with the greatest change in CDC were tested again as shown in Fig. 2a. Increase in the percent lysis with sera from these seven patients ranged between 18 and 63%.
Flow cytometry analysis
Patients’ sera were evaluated for reactivity to the MCF-7 cell line known to express all six antigens used in the vaccine. Low titer post-treatment IgG antibodies were induced in some patients (see Fig. 3b), but, as in the past, the serologic response consisted primarily of IgM antibodies. Sera from 22 patients demonstrated at least a doubling of the percent-positive cells and an increase in the MFI with 15 of these patients demonstrating at least a tripling of the percent-positive cells and at least a 50% increase in the MFI (Fig. 3a). FACS results with pre- and post-vaccination sera for six of these patients with a range of positive, vaccine induced, reactivity are shown in Fig. 4.
Western blot analysis
Figure 5 shows a Western blot analysis using sera from two representative patients who developed antibodies against MUC-1 and Globo H. There was no reactivity with the pre-treatment sera by ELISA or Western blot against any of the antigens. Patient 1 developed ELISA IgM anti-Globo H titers of 1/160 by week 7 and KLH antibodies, but no antibodies against DPSM, DOSM, Lewisyor Globo H. By Western blot, antibodies against both Globo H and KLH, but not any other antigens were detected, confirming the ELISA results. For patient 2, IgM anti-Globo H ELISA titers of 1/1280 and against KLH were seen by week 7, and no antibodies against other antigens. Post-treatment Western blot analysis demonstrated activity against Globo H and KLH, but also against Lewisy. Comparable Lewisy reactivity by Western, but not ELISA, was seen in three of the six patients tested.
Pre- and post-treatment Western blot analyses of sera from two patients who were serologically negative (Patient 1) and positive (Patient 2), respectively. Note that there was no pre-treatment reactivity against KLH or any other antigens. Bands are seen post-treatment, showing the presence of antibodies to Globo H, Lewisyand KLH
Progression-free survival analysis
In the univariate analysis (Table 3), N is the number in each group, and events are “progression of disease” with the median time to progression derived from the Kaplan-Meier estimate. An upper bound for the 95% confidence interval was not estimable due to the small sample size and lack of events. Gleason score, T stage and nodal involvement at the time of surgery did not appear to be associated with either survival or progression-free survival. PSA slope and pre-treatment PSA levels were treated as both continuous and categorical (threshold) variables in the univariate analyses. There was no significant effect on progression-free survival from either analysis nor was there evidence to suggest differences in post-vaccination changes in the slope, based on pre-vaccination primary therapy. Table 4 summarizes the changes in the PSA slope, based on the treatment type. There was no evidence of an effect on changes in slope from different treatments.
Discussion
Heterogeneity is characteristic of malignant cells with different antigens expressed to varying degrees on different metastases within the same patient. By combining multiple antigens in a vaccine, there is the possibility that the immune system would generate a multifaceted response in which antibodies to each antigen are produced. We found that all 30 patients had at least an eightfold titer increase and 26 developed a titer of at least 1/160 against two or more of these antigens after vaccination. Augmentation of antibody responses against multiple cell surface antigens was achieved in all of the vaccinated patients; the antibody responses against some of these antigens were lower than expected. The IgM antibody response against TF and GM2 and the IgG antibody response against MUC1, Tn and GM2 were at least eightfold lower after vaccination with the hexavalent vaccine than had been seen previously after vaccination with the respective monovalent vaccines.
Our experience with monovalent vaccines in preclinical and clinical trials demonstrated that KLH [24] was the most effective carrier molecule and that saponins such as QS-21 [12] were the most effective adjuvants for antibody induction. Immunological tolerance was broken consistently against each of the antigens with the exception of Lewisy. Consequently, the decreased antibody response seen against several antigens in the hexavalent vaccine were unexpected. There are several possible explanations. MUC1 has been shown to down-regulate immune responses in mice [13] and there are data in man to suggest that mucins in blood may affect activation of a variety of cell types including antigen presenting cells and effector cells [26, 27]. Synthetic MUC1 peptides have been shown to have immunosuppressive effects on T cell proliferative responses as well [14–17]. MUC1 mixed with the other antigens may have affected the ability of the immune system to “see” these other antigens. While the doses of individual antigens were the lowest used previously, it may be that in polyvalent vaccines higher doses are needed. Within the context of this multivalent vaccine, the individual conjugates may (1) gain immunogenicity due to an increased population of helper T cells against the carrier protein (KLH) due to the increased KLH dose [18, 19] or (2) lose immunogenicity secondary to a decreased population of helper T cells due to anti-KLH antibodies resulting from the increased KLH dose [20, 21, 22]. Antibody response against individual antigens within a polyvalent vaccine may also be decreased by the competition for a limited number of carrier-specific helper T cells by epitopic overload [23, 24]. However, a heptavalent vaccine administered to breast and ovarian cancer patients [26], containing the same six conjugates plus sTn-KLH, resulted in more potent antibody responses with the exception of GM2. A gender difference may play some unidentified role, but it is possible that following immunosuppressive chemotherapy, these patients had fewer regulatory T cells to interfere with immune responsiveness. This does not explain why the antibody titers induced here were lower than those in prior trials in this same prostate population. A final possibility is that QS21, known to be labile [25], was less active by the time it was vialed and frozen for this trial. All three trials shared the low antibody response against GM2 in contrast to the consistently high antibody response seen in all patients on the monovalent GM2-KLH plus QS-21 trial.
The patient population used for the vaccine trial was high risk, based on their PSADT and time to PSA relapse of less than 2 years. The vaccine did not seem to impact on the time to radiographic progression of the disease. Five patients remained on the study with 16 of the 25 (64%) progressing in either bone or lymph nodes. Nine patients elected to discontinue observation due to anxiety over the rises in PSA. Although there was no impact on the rate of rise of the PSAs slopes [3, 10], there may be a subgroup for whom vaccine strategies may be more suited [27] and responses more enhanced using GM-CSF [28, 29], low dose cyclophosphamide [30] or inhibition of CTLA-4 [31]. PSA doubling time is still not an established criterion for reporting response nor are there sufficient data to suggest that a change in PSA necessarily reflects a change in the biology of the tumor. There is an on-going initiative which focuses on how to improve the readout of vaccine trials for this population. It remains unclear as to whether different readouts for different types of investigational agents may be needed as we go forward with clinical trials for this patient population, as previously discussed in a paper by Scher et al. [32].
References
Pound CR, Partin AW, Eisenberger MA et al (1999) Natural history of progression after PSA elevation following radical prostatectomy. J Am Med Assoc 281:1591–1597
Ragupathi R, Slovin SF, Adluri S et al (1999) A fully synthetic globo H carbohydrate vaccine induces a focused humoral response to prostate cancer patients: a proof of principle. Angew Chem Int Ed 38:563–566
Slovin SF, Ragupathi G, Musselli C et al (2003) Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with α-N-acetylgalactosamine-O-serine/threonine (Tn) conjugate vaccine. J Clin Oncol 21:4292–4298
Ragupathi G Koganty RR, Qiu DX et al (1998) A novel and efficient method for synthetic carbohydrate conjugate vaccine preparation: synthesis of sialyl Tn KLH conjugate using a 4-(4-N-maleimidomethyl)cyclohexane-1-carboxyl hydrazide (MMCCH) linker arm. Glycoconj J 15:217–221
Seeberger P, Cirillo PF, Hu S et al (1996) Synthesis of the pentasaccharide core structure of asparagine-linked glycoprotein oligosaccharides by the glycal assembly method. Enantiomer 1:311–323
Danishefsky SJ, Bilodeau MT (1996) Glycals in organic synthesis: the evolution of comprehensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew Chem Int Ed Engl 35:1380–1419
Helling F, Zhang S, Shang A et al (1995) GM2-KLH conjugate vaccine: increased immunogenicity in melanoma patients after administration with immunological adjuvant QS-21. Cancer Res 55:2783–2788
Gilewski T, Adluri S, Zhang S et al (2000) Vaccination of high risk breast cancer patients with Mucin-1 keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res 6:1693–1701
Livingston P (1995) Approaches to augmenting the immunogenicity of melanoma gangliosides: from whole melanoma cells to ganglioside-KLH conjugate vaccines. Immunol Rev 145:147–166
Slovin SF, Ragupathi G, Adluri S et al (1999) Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo hexasaccharide conjugate in man. Proc Natl Acad Sci USA 96:5710–5715
Slovin S, Ragupathi G, Clarke T et al (2003) Multivalency in a phase II prostate cancer (PC) vaccine trial: are more antigens better? Proc Am Soc Clin Oncol 22:167 (Abstr#671)
Kim SK, Ragupathi G, Musselli C, Livingston PO (1999) Comparison of the effect of different immunological adjuvants on the antibody and T cell response to immunization with MUC1-KLH and GD3-KLH conjugate vaccines. Vaccine 18:597–603
Tempero RM, VanLith L, Morikane K et al (1998) CD4+ lymphocytes provide MUC1-specific tumor immunity in vivo that is undetectable in viro and is absent in MUC1 transgenic mice. J Immunol 161:5500–5506
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: Protectin and control of the cell surface. Nat Rev 4:45–60
Agrawal B, Krantz MJ, Parker J, Longenecker BM (1998) Expression of MUC 1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res 58:4079–4081
Gimmi CD, Morrison BW, Mainprice BA et al (1996) Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human t cells. Nat Med 2:1367–1370
Wykes M, MacDonald KP, Tran M et al (2002) Quin RJ, Xing PX, Gendler SJ, et al. MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. J Leukoc Biol 72:692–701
Anderson P (1983) Antibody response to Haemophilus influenzae type b and diphtheria toxin induced by conjugates of oligosaccharides of type b capsule with the nontoxic protein CRM197. Infect Immun 39:233–238
Kurika S (1996) Priming with diphtheria-tetanus-pertussis vaccine enhances the response to the Haemophilus influenzae type b tetanus conjugate vaccine in infancy. Vaccine 14:1239–1242
Barington T, Gyhrs A, Kristensen K, Heilmann C (1994) Opposite effect of actively and passively acquired immunity to the carrier on response of human infants to Haemophilus influenzae type b conjugate vaccine. Infect Immun 62:9–14
Peters CC, Tenbergen-Meeks AM, Poolman JT et al (1974) Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect Immun 59:3504–3510
Sarvas H, Makela O, Toivanen P, Toivanen A (1974) Effect of carrier preimmunization on the anti-hapten response in chicken. Scand J Immunol 3:455–460
Fattom A, Cho YH, Chu C et al (1999) Epitopic overload at the site of injection may result in suppression of the immune response to combined capsular polysaccharide conjugate vaccines. Vaccine 17:126–133
Barington T, Skettrup M, Juul L, Heilman C (1993) Non-epitope-specific suppression of antibody response to Haemophilus influenzae type b conjugate vaccine by preimmunization with vaccine components. Infect Immun 61:432–438
Cleland JL, Kensil CR, Lim A et al (1996) Isomerization and formulation stability of the vaccine adjuvant QS-21. J Pharm Sci 85:22–28
Armstrong JL, Ragupathi G, Powell S et al (2003) Preliminary data of vaccination of high risk breast cancer (BC) patients with a heptavalent antigen: keyhole limpet hemocyanin (KLH) conjugate plus the immunologic adjuvant QS-21. Proc Am Soc Clin Oncol 675:168 (Abstr#675)
Slovin SF, Wilton A, Heller G, Scher HI (2005) Time to detectable metastatic disease in patients with a rising PSA after surgery or radiation therapy. Clin Cancer Res 11:8669–8673
Simons JW, Mikhak B, Chang JF et al (1999) Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res 59:5160–5168
Simons JW, Sacks N (2006) Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX® vaccine for prostate cancer. Urol Oncol 24(5):419–424
Livingston PO, Cunningham-Rundles S, Marfleet G et al (1987) Inhibition of suppressor-cell activity by cyclophosphamide in patients with malignant melanoma. J Biol Response Mod 6:392–403
Gerritsen W, Van den Eertwegh AJ, De Gruijl T et al (2006) A dose-escalation trial of GM-CSF-gene transduced allogeneic prostate cancer cellular immunotherapy in combination with a fully human anti-CTLA antibody (MDX-010, ipilimumab) in patients with metastatic hormone-refractory prostate cancer (mHRPC). Proc Am Soc Clin Oncol 24:100 (Abstr#2500)
Scher HI, Eisenberger M, D’Amico AV et al (2004) Eligibility and outcomes, reporting guidelines for clinical trials for patients in the state of a rising PSA: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 22:537–56
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Prostate Cancer Foundation, The PepsiCo Foundation, The Sharon Hels and Brad Reed Fund, Swim Across America, The Sara Chait Foundation.
Dr. Philip Livingston is a consultant for and shareholder in Progenics Pharmaceuticals, Inc.
Rights and permissions
About this article
Cite this article
Slovin, S.F., Ragupathi, G., Fernandez, C. et al. A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunol Immunother 56, 1921–1930 (2007). https://doi.org/10.1007/s00262-007-0335-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0335-y