Abstract
Soil stabilization using additives is considered as one of the sustainable alternative techniques to deal with acute material shortages. Critically reviewing the contemporary works on soil stabilization would help practitioners and researchers to comprehend the merits and demerits of each stabilization method, influential parameters, and associated constraints. Furthermore, the critical analysis might aid the authorities to develop standard protocols about the use of various additives for soil stabilization, which would persuade the industry personnel to adopt sustainable practices. This paper presents a methodical review of the present soil stabilization methods under five key areas namely, underlying chemistry, the influential factors, performance indicators, economic and environmental aspects, and industrial perspectives. Findings of the review indicate that cement-based stabilizers perform well irrespective of soil type and curing conditions, on the contrary, lime-based stabilizers require appropriate temperature and pH for strength development. The degree of stabilization depends mainly on soil type, compaction level, and curing type and condition. Most of the soils treated with different additives exhibited a reduction in plasticity index, and maximum dry density against stabilizer dosage irrespective of soil type. The typical values of unconfined compressive strength and California bearing ratio of inorganic and organic soils except for peat, treated with a 5% dosage of all common types of stabilizers, fall in between 700 and 1,500 kPa and 30–60%, respectively. Cement and cementitious blends exhibited better cost-to-strength, energy-to-strength, and CO2 emission-to-strength ratios for soils with low plasticity whereas lime-blended stabilizers seemed effective for high-plastic soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Present road construction projects often suffer from material shortage due to an inadequate supply of quality materials (Dawson et al. 1995; Huang et al. 2007). Historically, road construction materials are being extracted from natural mineral deposits (Terashi and Juran 2000; Huang et al. 2007). Heightened environmental laws have prevented exploitation of natural mineral deposits, which in turn has led to inadequate material production (Mohajerani et al. 2020; Nunes et al. 1996). Alternatively, quality materials are being transported from other regions. Transport charges, however, contribute to the escalation in material cost (Asgari et al. 2015; Pongsivasathit et al. 2019; Prusinski and Bhattacharja 1999; Terashi and Juran 2000). Besides, exploiting natural minerals and transporting materials are considered extremely impactful to the environment and unsustainable (Balaguera et al. 2018; Dawson et al. 1995; Rocha et al. 2021). Construction projects have been thereby shifting from conventional practices to cost-effective and innovative strategies. Soil stabilization is one such technique, widely deployed in road construction projects, which treats in-situ soil to enhance its inherent properties to make it suitable for construction.
Stabilization improves the engineering properties of soil including dry density, swelling potential, plasticity, Unconfined Compressive Strength (UCS), California Bearing Ratio (CBR), Modulus of Resilience (MR), and permeability (Halsted et al. 2008; Teerawattanasuk and Voottipruex 2019) of weak in-situ soils either by mechanical means or by chemical means by adding and blending foreign agents or by both (Afrin 2017; Firoozi et al. 2017; Zumrawi 2015). In mechanical stabilization, granular materials are blended and consolidated to remove excessive amounts of air and/or water, and thus form a well-graded and compacted soil stratum (Hicks 2002). When inherited properties of soil are extensively incompatible with construction, mechanical stabilization alone may not be sufficient to achieve the required standard properties for construction (Afrin 2017). In such circumstances, foreign additives are traditionally added to in-situ soil to improve soil properties (Degirmenci et al. 2007; Firoozi et al. 2017; Halsted et al. 2008; Petry and Little 2002). These additives chemically react with soil constituents to enhance the engineering properties of in-situ soil.
Table 1 summarizes the stabilization methods that deployed alternative additives to enhance engineering properties of various geotechnical elements. Commonly, mechanical stabilization accompanied by chemical stabilization was found to be effective in improving weak soils (Gomes Correia et al. 2016; Ikeagwuani and Nwonu 2019). Particularly, the problematic soils require a chemical stabilizer to enhance strength and reduce swell-shrink behavior. On the other hand, chemical stabilization could potentially harm the workmen during construction and may cause a detrimental impact on the environment (Phummiphan et al. 2016). From economic and ecological perspectives, recent studies have shown that deploying industrial by-products and agro-wastes as additives is beneficial. Detailed advantages and disadvantages of the use of mechanical and chemical stabilization methods are presented in Table 2. To select the best stabilization method, engineering knowledge, and experience are vital since there are no stringent standards that govern the application of various stabilization techniques. Nelson et al. (2015) list the following as decisive factors based on which engineers may choose the appropriate stabilization method; the expansive nature of soil, the chemical composition of the soil, the reactivity of soil, design of active zone, the presence of undesirable chemical substances, heterogeneity of soil profile and hydraulic conductivity and soil fracturing.
1.1 Mechanical Stabilization
Soils with poor grading or containing fine particles with high plasticity may be improved by mechanical stabilization (Hicks 2002). During mechanical stabilization, other granular materials with better gradation are blended with in-situ soil to alter their particle size distribution. The mix is compacted and densified by applying compaction energy using rollers, rammers and vibrators (Afrin 2017). With the addition of granular particles, internal friction is improved in soil, which contributes to the increase in shear strength (Hicks 2002). Mechanical stabilization may result in a change in the plasticity properties of the mix (Hicks 2002). Ultimately, the stability of mechanically stabilized soil greatly depends on the inherent properties of the blended soils (Afrin 2017).
1.2 Chemical Stabilization
Traditionally, weak in-situ soils are blended with chemically active compounds including cement, lime, Fly Ash (FA), and rice husk ash (RHA) (Arulrajah et al. 2018; Basha et al. 2005; Degirmenci et al. 2007; Firoozi et al. 2017; Halsted et al. 2008; Petry and Little 2002; Yaghoubi et al. 2019) to improve mechanical and durability characteristics. Besides, non-traditional stabilizers including phosphogypsum, cement kiln, eggshell powder (ESP), Silica Fume (SF), Blast Furnace Slag Cement (BFSC) and Metakaolin (MK) have been deployed in the recent past to treat weak soils (Bellum et al. 2020; Dave et al. 2020; Degirmenci et al. 2007; Eisa et al. 2021; Miller and Azad 2000; Oluwatuyi et al. 2018). FA, SF and BFSC are industrial by-products used alone or together with cement/lime to stabilize soils (Arulrajah et al. 2018; Ebrahim Asghari-Kaljahi and Mansouri 2020). MK is the calcined form of clay. Agro-wastes include Bagasse Ash (BA), Olive Fly Ash (OFA), Parawood Ash (PAW), ESP and RHA. Table 3 summarizes the oxide compositions of stabilizers determined using X-ray fluorescence (XRF). SiO2 and CaO constitute significant components of most stabilizers Cement and lime blended stabilizers have been commonly used in soil stabilization (Pongsivasathit et al. 2019; Prusinski and Bhattacharja 1999), which are often deployed to treat soils with a high fraction of clay. The primary factors that determine the degree of chemical reactions are chemical composition, morphology, and fineness of the additives (Singh et al. 2015). The rich SiO2 and Al2O3 content and the desired Si/Al ratio in FA and MK make them popular in geopolymer applications (Duxson et al. 2007; Van Jaarsveld et al. 1997). RHA and SF contain a significant amount of SiO2 that enables them to be used as silica suppliers in geopolymerization. FA and bottom ash are found to be effective pozzolans in instituting cementitious reactions. The rich Ca content in BFSC, ESP, phosphogypsum and cement kiln is found to contribute to the formation of the C–A–S–H phase and hence strength enhancement (Temuujin et al. 2009; Yip et al. 2005). The FA, BFSC and RHA were discovered to exhibit a great degree of reactivity due to their smaller sizes (Ke et al. 2015; Provis and Van Deventer 2009). The amorphous or semi-crystalline that exist in MK, SF, BFSC and FA could be another reason for higher reactivity as they can dissolve easily in alkaline conditions (Bassani et al. 2019). Another critical factor in chemical stabilization is particle shape. MK particles are of platy shape that leads to rheological issues and hence increase water demand (Li et al. 2010). The spherical shape of FA particles improves the workability of the mix and provides strong and durable binding (Duxson et al. 2007; Jitsangiam et al. 2021).
According to Unified Soil Classification System (USCS), soils containing more than 50% particles passing through the No. 200 sieve (finer than 75 μm) are classified as silt–clay materials ("ASTM D2487, Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System)," 2017). The presence of extensive amounts of clay with high plasticity brings about an increase in activity (ratio between plasticity index and clay fraction in soil). During moisture changes, highly active clays exhibit extensive swell and shrink behavior. This is due to the electrostatic charges carried by clay particles that attract water molecules to form adsorbed and double-layer of water around them. The surrounding water causes substantial expansion and contraction in the soil during moisture level changes. The Ca2+ present in cement or lime-based stabilizers retard the formation of double-layer and hence contribute to controlling excessive volume changes in soil (Halsted et al. 2008; Prusinski and Bhattacharja 1999). A thorough understanding of the underlying chemistry behind all these processes is beneficial for designing soil stabilization.
Reviewing the existing literature, the authors found that chemical and mechanical combined stabilization has been widely explored in the published literature. However, no methodical state-of-the-art review is available on soil stabilization for road construction that covers underlying chemistry, parameters influencing the stabilization process, improvements in engineering properties and economic and environmental benefits.
1.3 Review Method
The literature reviewed in this paper was selected by employing keyword combinations including, soil stabilization, pavement, soil stabilizers and engineering properties in Web of Science and Scopus platform journals from 1986 to 2023. A total of 155 publications that covered the intended scope of the review were screened out, which comprised of 154 soil-stabilizer combinations. Figure 1 depicts the statistics of the reviewed literature. Figure 1a–c show the details of the treated soils, additives, and the classification of additives. More than 75% of the soil stabilization studies were conducted on fine-grained soils with low to high plasticity. Fat clay (CH) had been explored extensively followed by Lean clay (CL). From the perspectives of stabilizers, studies that deployed cement blended stabilization dominated with 35% followed by that of hydrated lime blended with 22%.
1.4 Review Objective and Scope
From the identified literature, this comprehensive review intends to cover details of underlying chemical reactions that occur during soil stabilization with different types of stabilizers, the factors influencing stabilization, variation in performance indicators with stabilizer dosage, and economic and environmental comparison between different types of stabilizers. It is envisioned that this review would provide practitioners and researchers with a comprehensive understanding of the stabilization process of different types of soils with various types of stabilizers. Further, the presented information would assist authorities to make decisions regarding soil stabilization.
The review gives more focus on the soil stabilization process practiced in tropical weathered countries. The associated parameters and conditions that are imperative for soil stabilization in tropical conditions are therefore appraised.
1.5 Organization of the Paper
The presentation of a methodical review on soil stabilization in this paper is organized as follows. Section 2 elaborates on the details of chemical reactions that occur during the application of traditional soil stabilizers. In addition, a brief review of non-traditional soil stabilizers and their stabilization potential are also discussed. Section 3 discusses the influential parameters that affect the degree of soil stabilization and their impact on the soil stabilization process. Section 4 presents performance parameters used to measure the degree of stabilization and their variations with the quantity of stabilizers. Section 5 details the economic and environmental comparisons between various stabilizers used in soil stabilization. In Sect. 6, industrial perspectives on the use of additives have been explored. Sections 7 and 8 summarize the key findings from published literature and the way forward, respectively.
2 Soil stabilizers and Stabilization Mechanism
This section describes the underlying mechanisms and chemical reactions behind soil stabilization with cement, lime, FA, and RHA. Furthermore, a brief review of the evolution of non-traditional stabilizers in soil stabilization is presented.
2.1 Cement in Soil Stabilization
Conventionally, ordinary Portland cement (OPC), which adheres to British Standard–12, is used in soil stabilization ("BS 12: Specification for Portland cement," 1996). OPC reportedly contains the following chemical compounds required for stabilization; tricalcium silicate (C3S), dicalcium silicate (C2S), tricalcium aluminate (C3A), and tetracalcium aluminoferrite (C4AF), where C, A, F, and S simply denote CaO, Al2O3, Fe2O3, and SiO2, respectively (Barnes and Bensted 2001; Dunuweera and Rajapakse 2018; Thiery et al. 2007). These components react with water and soil at various phases during stabilization to alter the properties of in-situ soil.
2.1.1 Chemistry and Mechanism of Cement Stabilization
Four distinct chemical processes occur in the following order when OPC is added to in-situ soil containing clay, namely; cation exchange, particle restructuring, cementitious hydration, and pozzolanic reaction (Halsted et al. 2008; Prusinski and Bhattacharja 1999). All these chemical processes assist in gradual changes in properties of in-situ soil.
2.1.1.1 Cation Exchange and Reduction in Plasticity
In clay, two types of crystalline patterns are formed; one is a silica sheet formed by silicon-oxygen tetrahedron units, and the second is a gibbsite (alumina)/brucite (magnesia) sheet produced by octahedral units (Das 2008). By repeatedly stacking silica/alumina/magnesia sheets, various clay minerals are formed (Das 2008; Halsted et al. 2008). Due to the inherent atomic configuration, clay minerals carry negative charges on their surfaces and positive charges at edges. To neutralize charges, they attract water via two mechanisms; one is directly attracting water molecules by forming hydrogen bonds and the second is attracting cations which in turn attract water molecules (Das 2008; Halsted et al. 2008). The electrically attracted water surrounds clay particles and forms a double layer, which holds responsible for the plasticity property of clay. Cations vary in terms of their affinity for attraction, which contributes to the change in the thickness of the double layer (Das 2008). OPC is an excellent calcium-based additive; when blended with clay, Ca2+ is of lower affinity for attraction and tends to replace Na+ or K+, which are of a higher affinity for attraction (Halsted et al. 2008; Prusinski and Bhattacharja 1999). This exchange of cations leads to a reduction in the thickness of the double e layer, and hence loss of plasticity (Halsted et al. 2008; Prusinski and Bhattacharja 1999). Figure 2 illustrates the change in double-layer thickness when Ca2+ replace monovalent Na+. However, adsorbed water remains unaffected due to cation exchange (Das 2008).
2.1.1.2 Particle Restructuring and Shear Strength Improvement
Clay particles generally form a parallel structural orientation; each particle carries negative charges at surfaces and positive charges at edges (Das 2008). When cement is added, cations in cement induce a random edge-to-surface attraction of clay particles that activates the flocculation of clay particles (Halsted et al. 2008; Prusinski and Bhattacharja 1999). With the deposition of cement particles, flocculated clay particles then form weak bonds at the edge surface that result in agglomeration (Halsted et al. 2008; Prusinski and Bhattacharja 1999). Flocculation followed by agglomeration institutes the formation of granular particles from clay particles (Firoozi et al. 2017). Produced granular particles with improved texture assist to increase internal friction, and hence shear strength.
2.1.1.3 Cementitious Hydration and Soil Particle Binding
The typical mineralogical composition of OPC is as follows; 55–65% of C3S, 15–25% of C2S, 8–14% of, C3A, and 8–12% of C4AF (Aïtcin 2016; Barnes and Bensted 2001; Dunuweera and Rajapakse 2018; Zumrawi 2015). Less than 5% gypsum (calsulfatephate dehydrate, \({\text{C}}{\overline{\text{S}}}{\text{H}}_{{2}}\), where \({\overline{\text{S}}}\) denotes SO3) is also added to OPC to retard the early hardening of cement due to the active reaction of C3A (Aïtcin 2016). Each constituent in OPC reacts with water at different degrees and at different times. The chemical reaction between the constituents of OPC and water is called hydration, which is accountable for the setting and hardening of cement paste. The C3S phase in OPC first reacts with water and contributes to initial setting and early strength development followed by C2S (Barnes and Bensted 2001). These hydration reactions of C3S and C2S under ambient conditions form C–S–H (C3S2H3) and are given in Eqs. (1) and (2) as follows (Hoover and Ulm 2015; Prusinski and Bhattacharja 1999).
Tricalcium silicate (C3S) hydration
Dicalcium silicate (C2S) hydration
where H refers to H2O. The release of hydrated lime (HL) from both reactions maintains a pH level of about 12.5 in mortar and concrete systems (Bell 1996; Prusinski and Bhattacharja 1999). The solubility of silica and alumina increases significantly with an increase in pH greater than 12 ("ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993). At higher pH levels greater than 12, due to the higher solubility of silica and alumina, pozzolanic reactions of cement will be accelerated. If in-situ soil is of high plasticity, a significant fraction of Ca2+ is exhausted during cation exchange to reduce plasticity, thereby formation of hydrated lime will decrease that in turn will reduce pH levels and hence retard pozzolanic reactions. For such highly plastic soils, cement alone may not be sufficient for stabilization. Additional Ca2+ required for cation exchange is supplied by adding a fraction of lime to the soil at the start of stabilization, which helps to compensate for the loss in pH value of the soil ("ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993).
C3A, among all the components present in OPC, is highly reactive (Barnes and Bensted 2001). The hydration reaction of C3A in the absence of sulphate ions institutes rapid hardening of motor/concrete, a phenomenon referred to as ‘flash-set’, so concrete loses its slump swiftly (Aïtcin 2016; Barnes and Bensted 2001). In the presence of sulphate ions, the hydration of C3A is more retarded and it slowly forms ettringite (\({\text{C}}_{{6}} {\text{A}}{\overline{\text{S}}}_{{3}} {\text{H}}_{{{32}}}\)) that temporarily blocks its early hydration and hardening (Aïtcin 2016; Firoozi et al. 2017). Sulphate ions in OPC are supplied by gypsum. Equations (3–5) describe the two types of hydration reactions of C3A with and without sulphur (Aïtcin 2016; Firoozi et al. 2017).
Tricalcium aluminate (C3A) hydration in the absence of sulphate
Tricalcium aluminate (C3A) hydration in the presence of sulphate
Once gypsum in OPC is fully consumed, sulphate content in the solution drops below a critical value. Further hydration transforms ettringite into monosulphoaluminate (\({\text{C}}_{{4}} {\text{A}}{\overline{\text{S}}}{\text{H}}_{{{12}}}\)) as follows
The hydration of the ferrite phase in OPC is like that of C3A. When sulphate the level drops, meta-stable iron substituted AFm phases form. When the temperature rises, meta-stable AFm phases decompose into C3AH6–C3FH6. The corresponding chemical reactions are given in Eqs. (8–10) (Aïtcin 2016; Firoozi et al. 2017).
Provided that sufficient water is available, complete hydration will occur to form C–S–H and C–A–H, which will bind soil particles together. This process will turn flocculated particles into large soil grains, and hence the gradation of soil will improve (Halsted et al. 2008; Prusinski and Bhattacharja 1999). The availability of water depends on the water-to-cement ratio adopted for stabilization (Barnes and Bensted 2001; Ribeiro et al. 2016).
2.1.1.4 Pozzolanic Reaction
As described earlier, cementitious hydration forms HL, which elevates pH levels. S and A present in clay are thoroughly soluble at high pH levels. When pH increases S and A dissolve easily from clay lattice. Released S and A enter reaction with HL to initiate secondary soil stabilization. These reactions are referred to as secondary cementitious reactions or pozzolanic reactions; typically take place at a slower rate for an extended period. The pozzolanic reactions are governed by Eqs. (11) and (12).
The timeline of the soil stabilization process with cement (OPC) additive is shown in Fig. 3. It is a series of overlapping chemical reactions between clay minerals and OPC constituents. An uninterrupted supply of required constituents and conditions are to be guaranteed to achieve the targeted soil properties. As OPC contains S, soil stabilization with cement is independent of the constituents of the soil (Asgari et al. 2015).
2.1.2 Selection of Cement Stabilizer Dosage
The quantity of cement (OPC) to be blended with soil should be sufficient to the supply required chemical components to support the reactions described in Eqs. (1–12). As pointed out earlier, the clay mineral composition, especially the ones with monovalent cations (Ex. Montmorillonite) consumes a large amount of cement for initial cation exchange. The remaining portion of cement will involve in the cementitious hydration reaction and will contribute to strength development. It is therefore recommended that the minimum cement dosage used for stabilization should be more than the quantity absorbed during initial ion exchange reactions ("ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993). Table 4 details soil types along with plasticity, and respective cement dosages, and resulted in UCS of stabilized soils reported in numerous studies. The general trend shows that when plasticity increases, the optimum amount of cement required for soil stabilization increases. Cement stabilization improves UCS of non-plastic, coarse-grained soils and soils with low plasticity to be in the range of 3,000–4,800 kPa for cement dosage less than 10%. Highly plastic soils and peat showed moderate to low improvement in the UCS compared to non-plastic or low plastic soils when cement dosage was increased.
2.2 Lime in Soil Stabilization
Lime supplied in the following forms is effective for soil stabilization; hydrated lime (HL), slurry lime (SL), and quicklime (QL) (Hicks 2002). Hydrated lime is in fine powder form whereas slurry lime is a semi-liquid mixture (Hicks 2002). Quicklime is produced by the calcination of limestone at elevated temperatures (Kumar et al. 2007). Quicklime reacts with water to create hydrated lime and release a substantial amount of heat during the reaction (Firoozi et al. 2017). Hydrated lime absorbs CO2 and becomes CaCO3. The reactions of the limestone cycle are given in Eqs. (13–15).
Soil stabilization using lime undergoes almost similar reaction phases to what cement stabilization does except for hydration reactions. Ca2+ present in lime gradually replaces monovalent cations in clay, which modifies diffused hydrous double-layer around clay particles (Bell 1996). The reduction in double-layer escalates attraction between clay particles and thus attracts clay particles form flocs. On the other hand, available HL provides highly alkaline conditions in which S and A are highly soluble. Released S and A from clay minerals enter a pozzolanic reaction with HL to form C–S–H and C–A–H, as described in Eqs. (11 and 12).
It should be noted that, during lime stabilization, due to the absence of cement clinker components including C3S, C2S, C3A, and C4A, primary cementitious hydration reactions that were prominent in cement stabilization would not occur. Instead, a secondary cementitious reaction such as the pozzolanic reaction would take place. Pozzolanic reactions take place at slower rates over a long period; therefore, the rate of strength gain in lime-stabilized soils is lesser than that in cement stabilization (Bell 1996; Hicks 2002; Sariosseiri and Muhunthan 2009). For the occurrence of these reactions, there should be ample supply of required lime, clay minerals, and other pozzolanic material, and higher alkaline levels (Kassim and Chern 2018). Low temperature, low soil pH, and organic matter present in soil are believed to impede the progress of pozzolanic reactions (Hicks 2002).
As described earlier, a fraction of added lime is first adsorbed by clay minerals until the affinity of the soil for lime is satisfied (Bell 1996). This amount of lime exhausted in the initial cation exchange might be determined by the Initial Consumption of Lime (ICL) test stipulated in BS 1924–1 ("BS 1924–1: Hydraulically bound and stabilized materials for civil engineering purposes. Sampling, sample preparation and tests of materials before treatment," 2018). Rogers et al. (1997) have also prosed a method to determine ICL which was later deployed by researchers (Consoli et al. 2014). The remaining lime component enters a pozzolanic reaction to develop strength characteristics in stabilized soil. The amount of lime to be added to the soil for stabilization therefore should exceed the minimum levels that are expected to be exhausted in cation exchange (Al-Mukhtar et al. 2010; "ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993).
Respective lime dosages deployed for different soil types and the resulting UCS reported in numerous studies are detailed in Table 5. Hydrated lime and quicklime were used alternatively depending on the application. The soils with high plasticity showed significant improvement in UCS when stabilized with lime. Also, a study reported that an extremely alkaline environment might retard the lime stabilization process, which could be controlled by adding some acids (Mishra et al. 2019).
2.3 Non-traditional Soil Stabilization
2.3.1 Fly Ash in Soil Stabilization
FA is an industrial by-product created by the combustion of coal, chiefly used as a reinforcing material in bricks, concrete, and pavements (Bhattacharjee and Kandpal 2002; Fly Ash Facts for Highway Engineers 2003; Ramaji 2012; Sutcu et al. 2019). Out of four classes of FA products, classes C and F gained attention to be used in the soil stabilization process. SiO2 + Al2O3 + Fe2O3 content by weight in classes C and F is more than 70% and between 50 and 70%, respectively ("ASTM C618, Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete," 2019). While class C can be used as a stand-alone cementitious material due to its rich CaO content, class F needs the addition of other cementing agents such as cement and lime (Fly Ash Facts for Highway Engineers 2003).
The chemical reactions that occur during soil stabilization using FA resemble the reactions that occur during soil stabilization using cement/lime. Initially, the monovalent cations are replaced with Ca2+ to reduce plasticity (Christopher et al. 2000). Cementitious and pozzolanic reactions during soil stabilization with FA can be represented by Eqs. (14), (11), and (12) occur in the given order (Arman and Munfakh 1972; Tastan et al. 2011). Pozzolanic reactions in FA are slow, and happen over a long period (Ahmaruzzaman 2010). Class C FA produces more C–S–H and C–A–H than class F due to its higher CaO content (> 20% by weight) (Sridharan et al. 1997). A combination of lime and FA is commonly used in soil stabilization, which exhibits similar performance to cement-stabilized soil ("ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993). Deploying FA in road construction is reported to reduce construction costs by 10–20% (Ahmaruzzaman 2010).
2.3.2 RHA in Stabilization
RHA is produced by burning rice husk at elevated temperatures of more than 600 °C (Singh and Singh 2021). RHA has high pozzolanic content due to its rich silica concentration (Moayedi et al. 2019). Compared to traditional additives, RHA has a significantly low reaction time, which attracted researchers to study about deploying RHA for soil stabilization (Moayedi et al. 2019). RHA is often applied along with cement or lime as it does not have cementitious components. A study by Rahgozar et al. examined the usage of RHA and OPC to treat clayey sand (Rahgozar et al. 2018). The stabilized soil sample with 6% RHA and 8% OPC, cured for 28-days yielded UCS and CBR almost 25 and 18 times more than those of the values for untreated soil, respectively. A similar study by Basha et al. (2005) found that 15–20% RHA and 6–8% OPC were the optimum amounts to reduce the plasticity of residual soil to improve strength characteristics. A study reported that treating lateritic soil with high doses of RHA yielded considerable improvements compared to lime and cement (Rahman 1986).
2.3.3 SF in Stabilization
Silica fume is an industrial by-product in the manufacturing of silicon and silicon alloy (Lewis 2018; Türköz et al. 2021). Annually, 2.5 million tons of SF is produced worldwide (Lewis 2018). A study by Ahmad et al. claimed that peat treated with SF produced slightly better UCS than the ones treated with OPC (Ahmad et al. 2021a, b). Researchers found that adding SF to expansive clays improved strength characteristics while reducing the plasticity index (Phanikumar and Ramanjaneya Raju 2020; Singh et al. 2020). In geopolymer application, SF is deployed to supply silica as it contains a substantial amount of silica. Due to the amorphous structure, SF easily dissolves in an alkaline medium that promotes chemical reactions (Bassani et al. 2019). SF particles are spherical and with comparatively higher surface area, which improves the workability of the mix and provides more contact surface for chemical reactions (Türköz et al. 2021).
2.3.4 Coal Bottom Ash in Stabilization
As a result of the combustion of pulverized coal in thermal power plants, coal bottom ash (CBA) is generated as a residue (Hashemi et al. 2019). Approximately 8.5 million tons of CBA are annually generated across the globe (Hashemi et al. 2018; Ranjbar and Kuenzel 2017). The presence of silica and alumina in CBA improved strength characteristics such as UCS, and CBR, and reduced the shrink and swell potential of expansive black cotton soil (Navagire et al. 2022). Deploying coal bottom ash partially to replace OPC is a cost-effective and environmentally friendly option. However, the application of CBA possesses certain limitations as the resultant properties of stabilized soil vary, and the processing is complicated. Also, the existence of toxic heavy metals such as Ni, Cd, Zn, and Pb might cause harm to human beings and the environment, unless it is rightly handled (Hashemi et al. 2019; Zhou et al. 2022).
2.3.5 Geopolymer Technology
Production of cement, lime, FA, and RHA is considered energy-intensive, which emits harmful substances like CO2 into the environment (Durastanti and Moretti 2020; Imbabi et al. 2012; Ivanov et al. 2015; Mayooran et al. 2017; Moretti et al. 2019; J. Zhang et al. 2014a, b). To mitigate detrimental impacts on environment, sustainable practices such as replacing conventional additives with non-traditional ones have gained considerable attention in pavement construction (Consoli et al. 2020). Tingle and Santoni (2003) evaluated the usage of twelve non-traditional additives including acid, enzymes, lignosulfonate, petroleum emulsion, polymers, and a tree resin in clay soil stabilization. Soils stabilized with Lignosulfonate and polymers produced better UCS, but others exhibited trivial strength improvements. Importantly, most of the stabilized samples with said additives showed inferior performance against moisture increment.
A study reported that additive is the prime contributor to stabilization cost (Rocha et al. 2021). Accordingly, in the recent past, employing waste material for soil stabilization has been recognized as an environmentally friendly and economically advantageous strategy (Anupam et al. 2013; Imtiaz and Lovell 1992; Zhang 2013). ESP and fibers are some of the potential cost-effective replacements (Consoli et al. 2020; Rahgozar et al. 2018; Saldanha et al. 2021; Sharma et al. 2015).
ESP contains a substantial amount of calcium carbonate (CaCO3), which could be exploited to establish cementitious bonds during soil stabilization (Amaral et al. 2013; Lechtanski 2000). Consoli et al. (2020) explored the usage of quicklime and hydrated lime derived from eggshell residues for soil stabilization. The authors claimed that the mechanical properties of stabilized soil improved with the accumulation of eggshells. A similar study by Saldanha et al. (2021) revealed that eggshell limes have adequate physical–chemical–mineralogical characteristics required for soil stabilization. The study also found that deploying eggshell limes significantly reduced environmental impacts. Oluwatuyi et al. (2018) investigated using ESP and OPC to stabilize lateritic soil for highway construction. The results of the study revealed that CBR and UCS values of ESP and OPC stabilized soil were better than the soils stabilized with OPC or ESP alone. A similar study by Maduabuchi and Obikara (2018) investigated the potential of OPC and ESP mix for lateritic soil stabilization. Accumulation of ESP in this mix was found to reduce plasticity and Maximum Dry Density (MDD), whereas Optimum Moisture Content (OMC) and CBR increased.
Geopolymer technology and bio-cementation are two noteworthy strategies to be mentioned as long as state-of-the-art soil stabilization techniques are concerned (Chung et al. 2021; Consoli et al. 2020; Gowthaman et al. 2021; Maduabuchi and Obikara 2018; Naveed et al. 2020; Oluwatuyi et al. 2018). In the geopolymer technique, aluminosilicate precursors are activated using alkali activators to form an inorganic binder that agglomerates soil grains (Davidovits 1991b). The chemical reactions that occur during geopolymer formation are given in Eqs. (16) and (17) (Davidovits 1991a; Khale and Chaudhary 2007; Reddy et al. 2016; Singh 2018). Firstly, aluminosilicate precursors release Al3+ at highly alkaline conditions that subsequently form \({\text{AlO}}_{{4}}^{ - }\) tetrahedra, which attracts group I cations to balance charges (Duxson et al. 2007; Khale and Chaudhary 2007). These react with SiO2 tetrahedra and give an amorphous three-dimensional polymeric chain (Davidovits 1991b; Duxson et al. 2007; Khale and Chaudhary 2007). Equation (16) explains the formation of the intermediate form of geopolymer precursor from the synthesization of silica and alumina in the presence of NaOH solution. Equation (17) describes the formation of a geopolymer chain consisting of Polysialate (-Si–O-Al-O-) bonds.


Geopolymer technology uses RHA/FA, MK, and ESP as precursor materials and NaOH (caustic soda)/Na2SiO3 as an alkali activator (Davidovits and Sawyer 1985; Poorveekan et al. 2021). In addition, the available CaO in ESP and SiO2 in RHA contribute to the formation of C–S–H under the presence of NaOH, and hence institute strength development in stabilized soil (Amaral et al. 2013; Moayedi et al. 2019). Some studies have deployed Na2SiO3 and NaOH together as activators (Mashri et al. 2020; Shekhawat et al. 2019). Phummiphan et al. (2016) examined the stabilization potential of marginal lateritic soil using high calcium FA-based geopolymer with alternative ratios of Na2SiO3 and NaOH as alkali activators. The study revealed that at a Na2SiO3: NaOH ratio of 50:50, the maximum 90-day UCS was obtained. A similar study reported that 3–5% OPC and 4–8% FA-based geopolymer improved the mechanical characteristics of marginal lateritic soil (Teerawattanasuk and Voottipruex 2019). Tan et al. (2021a, b) treated CH-type soil with fly ash as a precursor and KOH as an alkaline activator, and further reinforced it with coir fiber. The authors concluded that the stabilized specimens exhibited improved strength parameters and failure strain. A similar study was conducted on the same soil with coir fiber coated with linseed oil and turpentine oil (Tan et al. 2021a, b). Treated fibers were found to improve compressive strength and post-peak stress behavior. Further, the microstructural analysis revealed the formation of A–S–H and C–S–H gel, which improved the interaction between the coir fiber surface and the geopolymer matrix. The application potential of soil stabilization with geopolymer technology, however, remains unevaluated for road construction.
2.3.6 Bio-cementation
Bio-cementation process harnesses bacteria containing active urease enzymes (Gowthaman et al. 2021; Jia He et al. 2020; Naveed et al. 2020). Soil is subjected to cultivated bacteria, a solution of urea, and calcium chloride. Bacterial action decomposes urea into carbonate and ammonium ions as given in Eq. (18) (Gowthaman et al. 2021). Supplied Ca2+ and produced carbonate ions form CaCO3 mineral within the soil framework as shown in Eq. (19) (Gowthaman et al. 2021). The bacteria cells attached to the aggregate surfaces provide nucleation to the formed CaCO3 mineral and institute crystal growth between adjacent soil particles, which results in the cementation of particles (Mujah et al. 2017).
2.3.7 Nanomaterial in Soil Stabilization
By adding nanoparticles as a foreign substance, the soil structure at the atomic level can be manipulated (Ghasabkolaei et al. 2017). Due to the high specific surface area, the nanoparticles actively engage in reactions with clay minerals, ions and organic matter (Ghasabkolaei et al. 2017; Zhang 2007). These reactions have a profound influence on the physical and chemical characteristics of treated soil (Kacha and Shah 2021; Zhang 2007). Researchers have explored the use of a variety of nanomaterial additives to stabilize weak soils including nano-silica, nano-alumina, carbon nanotube and nanofiber, and colloidal silica (Alipour et al. 2022; Alsharef et al. 2016; Gallagher and Mitchell 2002; Zhang 2007). Alipour et al. (2022) conducted studies on deploying nano-alumina and nano-silica to treat problematic soil. The authors concluded that nano-alumina reduced swelling potential in stabilized samples more than nano-silica did. From the strength perspective, nano-silica stabilized samples outperformed nano-alumina treated samples. A study incorporated carbon nanotube and nanofiber into clayey sand (Alsharef et al. 2016). A clear decrease in hydraulic conductivity was observed when the composition of carbon nanotube and nanofiber increased as they filled the pores between the soil particles. A marginal change in the plasticity index was observed. A similar reduction in hydraulic conductivity was reported by Persoff et al. when colloidal silica was used to treat sand (Persoff et al. 1999). Incorporating colloidal silica in loose sand was found to mitigate the risk of liquefaction (Gallagher and Mitchell 2002). By adding nanoparticles as a foreign substance, the soil structure at the atomic level can be manipulated. The existence of nanomaterials might alter the physical and chemical characteristics of treated soil due to significantly high specific surface area (Kacha and Shah 2021). Alipour et al. (2022) conducted studies on deploying nano-alumina and nano-silica to treat problematic soil in Iran. The authors concluded that nano-alumina contributed better to reducing swell potential. Meanwhile, the presence of nano-silica improved strength.
3 Parameters Influencing Soil Stabilization
The success of soil stabilization depends on parameters including soil type, the amount of organic matter present in the soil, sulphates and sulphide composition of the soil, state of compaction, moisture content, the temperature during stabilization, and curing (Maclean and Lewis 1963; Sherwood 1993). Appropriate control of these factors would contribute to steer strength development in stabilized soils, and to achieve desired levels of physical and mechanical characteristics (Afrin 2017; Sherwood 1993).
3.1 Soil Type
Soil is characterized by its particle size distribution and plasticity properties into granular soils/coarse-grained soils and cohesive soils/fine-grained soils ("ASTM D2487, Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System)," 2017; Das 2007; "M145‐91, Classification of soils and soil‐aggregate mixtures for highway construction purposes," 2012). Generally, granular soils require a relatively large amount of additives at the initial stages of stabilization to fill considerably large voids present between soil particles (Maclean and Lewis 1963). Strength development with the accumulation of additives is guaranteed if the additive dosage exceeds the minimum dosage requirement for filling the voids. For fine-grained soils, strength development starts immediately as additives start cementing particles together from the beginning itself (Maclean and Lewis 1963). As per the ORN31 standard, stabilizing soils with a uniformity coefficient below 5 is economically disadvantageous and the maintenance of such soil stabilization is highly expensive ("ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993).
The plasticity index is the widely accepted measure of soil expansion characteristics or swelling potential (Halsted et al. 2008). Fine soils with a plasticity index of less than 10, generally respond well to cement stabilization, whereas lime stabilization is effective if the plasticity index is greater than 10 (Hicks 2002; "ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993). For clays with extremely high swelling potential, a small fraction of lime is first added to eliminate dramatic volume changes (Croft 1967; Stocker 1972, 1974). Thereafter, cement is added to improve the mechanical characteristics of soil (Prusinski and Bhattacharja 1999).
Concisely, the particle size and plasticity index of the soil dictate the choice of appropriate additive type/s and the amount of additive to be added to stabilize the soil concerned. Lime is particularly effective in reducing plasticity rapidly in high-plastic soils, whereas cement can perform well in all types of soils.
3.2 Presence of Organic Matter
The top layers of soil often constitute decomposed organic matter up to a depth of 1.5 m (Maclean and Lewis 1963; Sherwood 1993). The presence of organic matter in in-situ soil may react with a fraction of Ca2+ to form insoluble compounds, which limits the amount of available Ca2+ for pozzolanic reactions (Tastan et al. 2011). The organic matter in soil absorbs significantly large amounts of water, which can potentially reduce the available water for cementitious hydration reactions (Hampton and Edil 1998). Thus, the presence of organic matter prevents stabilizers from developing desired strength characteristics (Zumrawi 2015). Oliveira et al. (2012) found that an increase in organic matter caused an increment in compressibility characteristics. The influence of organic content in soil on its mechanical properties including UCS and MR was found to have a hyperbolic relationship (Tastan et al. 2011).
Maclean and Lewis (1963) proposed conducting diagnostic tests to detect the presence of organic matter in the soil. This test was performed by measuring the pH value of in-situ soil one hour after adding 10% OPC and water. A measured pH value less than 12.1 indicated that organic matter present in soil was capable of preventing cement hardening (Maclean and Lewis 1963), which interpreted that cement stabilization for that particular soil type was ineffective. Ahmad et al. (2021a, b) recommended using OPC and filler materials to fill the voids in peat using cementitious products. They also emphasized the need of deciding the stabilizer dosage in treating organic soil by considering the indexed properties and the presence of humus. Chen and Wang (2006) added extra admixture to overcome the issues of organic matter in cement-stabilized soft soil.
Organic soils contain less amount of clay particles and a substantial portion of humus. Soft organic soils of different types (Pt, OL, and OL-OH) were stabilized using six different types of FA-class C and F, and OPC—type I (Tastan et al. 2011). Pt-type soil attained lower UCS and MR values than the other two. The authors concluded that CaO content and CaO/SiO2 ratio in FA affected the increase in UCS and MR. Ahmad et al. (2021a, b) used a combination of SF and OPC to treat peat soil. UCS and CBR of the stabilized specimens improved with the stabilizer dosage and time. Strength characteristics improved with the increase in SF content in the mix. This was due to the development of compact and dense structure of peat. Morphological analysis conducted on the stabilized specimens revealed that the formation of C–S–H and C–A–H led to a dense peat matrix (Ahmad et al. 2023). Also, the strength development rate in SF stabilized specimens was more rapid than that of OPC. Stabilization of peat soil is challenging and consumes a lot of money, and requires additional stabilizer dosage to initiate the process (Ahmad et al. 2021a, b). From the environmental perspective, partially replacing OPC with lime, fly ash, kaolin, etc. is considered beneficial.
The amount of organic matter present in the soil is the key to fixing the stabilizer dosage. Grassland soils, high plasticity index soils, poorly drained soils, and lowland soils are susceptible to containing substantial amount of organic matter. Such soils need additional stabilizers or extra admixture to treat organic matter present in the soil.
3.3 Sulphates and Sulphides
Sulphate ions react with calcium-based additives to form ettringite (\({\text{C}}_{{6}} {\text{A}}{\overline{\text{S}}}_{{3}} {\text{H}}_{{{32}}}\)) and with additional hydration, ettringite is transformed into monosulphoaluminate (\({\text{3C}}_{{4}} {\text{A}}{\overline{\text{S}}}{\text{H}}_{{{12}}}\)) as given in Eqs. (5) and (6) (Afrin 2017; Aïtcin 2016; Firoozi et al. 2017). Produced compounds occupy larger volumes than the reactants. This expansive nature breaks the bonds in stabilized soil (Maclean and Lewis 1963). Sulphate content in the soil can be determined according to British Standard 1377–3 ("BS 1377–3—Methods of test for Soils for civil engineering purposes—Part 3: Chemical and electro-chemical tests," 1990).
Sulphides present in industrial by-products might be oxidized in the presence of water to form sulphuric acid (\({\overline{\text{S}}\text{H}}\)). Cementitious hydration reactions form HL and C–S–H, which further reacts with \({\overline{\text{S}}\text{H}}\) to form gypsum (\({\text{C}}{\overline{\text{S}}}{\text{H}}_{{2}}\)) as shown in Eqs. (20) and (21).
Formed gypsum enters a similar reaction with cementitious products to form ettringite and monosulphoaluminate as shown in Eqs. (5) and (6).
If the pH of a system is high, ettringite can precipitate when an adequate amount of sulphate, calcium, and alumina ions are available along with water (Diaz Caselles et al. 2020).The addition of lime or cement can release HL in the system, which can potentially increase the pH of the system (Celik and Nalbantoglu 2013; Diaz Caselles et al. 2020). On the other hand, soils with significant amount of alumina and calcium ions are susceptible to the formation of ettringite and monosulphoaluminate. Therefore, the choice of additive requires considering the chemical composition of the soil and its contribution to the pH of the system to eliminate sulphate attack.
3.4 State of Compaction
Stabilized soil is compacted until it reaches MDD under OMC. For a particular soil sample, MDD and OMC can be determined according to the modified Proctor compaction test stipulated in British Standard 1377–4 ("BS 1377–4, Methods of test for—Soils for civil engineering purposes—Part 4, Compaction-related tests," 1990). For each additive content, 5–6 stabilized soil samples are to be cast with alternative moisture contents, and their corresponding dry densities are measured. By plotting dry densities against moisture contents, MDD and OMC can be determined by choosing the highest density and respective moisture content, respectively, for a designated additive content ("BS 1377–4, Methods of test for—Soils for civil engineering purposes—Part 4, Compaction-related tests," 1990). During cement stabilization, hardening reactions described in Eqs. (1) and (2) start occurring immediately when water is added to the soil cum cement mix. The hardened soil–cement mix might require additional compaction energy to obtain the required MDD, also compaction may break the bonds formed during hardening that resulting in a loss of strength. To prevent compaction from being hindered by hardening, the cement-stabilized soil samples should be immediately compacted (Afrin 2017; "ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993).
For cement-stabilized soils, generally, MDD decreases with the increase in cement content, however, OMC increases (Basha et al. 2005; Pongsivasathit et al. 2019). A similar tendency in MDD and OMC was also reported in lime-stabilized soils (Asgari et al. 2015; Rahman 1986). On the contrary, in soil stabilized with cement and FA-based mix, with increasing FA content, MDD decreased initially and then increased (Degirmenci et al. 2007; Zumrawi 2015). Basha et al. (2005) reported that MDD decreased with increasing additive content including cement, RHA, a mixture of 4% cement blended with RHA, and a mixture of 8% cement blended with RHA. OMC, however, increased in all cases. These variations in MDD could be attributed to the change in grain size distribution, specific gravity of soils and type of stabilizing agent (Nalbantoğlu 2004; Rahman 1986, 1987). Stabilized soils form large grains through the agglomeration process that consequently occupy larger spaces, which in turn increase voids between soil grains. An increase in the volume of voids contributes to a decrease in MDD. When the stabilizer is of high specific gravity, some soils have shown a decrease in MDD due to the agglomeration of grains initially. Continuous accumulation of stabilizer will start filing the voids, which would reduce the volume of voids and consequently increase MDD. Decreased levels of MDD obtained in stabilized soils imply that lower levels of compaction are required to achieve designated MDD which eventually saves money spent on compaction (Muntohar and Hantoro 2000).
Figure 4 illustrates a comparison of MDD and OMC variations between various soil types stabilized with different stabilizing agents. MDD of most of the stabilized soils range between 1.2 and 1.8 Mg/m3, and OMC values fall between 15 and 45%. With the increase in stabilizer dosage, all stabilizers except cement kiln and fly ash showed decreasing and increasing trends in MDD and OMC, respectively.
MDD and OMC variation in various soils stabilized with different stabilizer/s. GW, SM, SP, SC, OH, CH, CL, ML, and MH are standard USCS notations used to represent soil types. OPC ordinary Portland cement, RHA Rice husk ash, LME Lime, FLA C Fly ash, class C, FLA F Fly ash, class F, CKN Cement kiln dist, PHP Phosphogypsum and ESP Eggshell powder (Basha et al. 2005; Degirmenci et al. 2007; Maduabuchi and Obikara 2018; Mishra et al. 2019; Muntohar and Hantoro 2000; Nath et al. 2017; Oluwatuyi et al. 2018; Pongsivasathit et al. 2019; Rahman 1986; Rimal et al. 2019; Zumrawi 2015)
The MDD reduces with the increase in additive dosage for all typical stabilizers. Reduction in MDD could impact the strength characteristics of the stabilized mix. The stabilizer dosage is therefore carefully manipulated to satisfy the standard requirements of each application. Backfills of retaining walls are benefited from the reduction in MDD as the lateral pressure exerted by the soil on the structures decreases.
3.5 Curing
Curing in stabilized soils is performed by maintaining the required moisture levels and temperature to support cementitious reactions throughout the curing age (Mitchell and Hooper 1961). For cement-based stabilizers, cementitious hydration takes place immediately to bind soil particles together, followed by pozzolanic reactions (Aïtcin 2016). For the other stabilizers including, lime, RHA, FA, and ESP, pozzolanic reactions slowly start occurring and continue for a long period (Bell 1996; Prusinski and Bhattacharja 1999). ORN 31 standard recommends UCS tests to be done on samples sealed and moist-cured at 25 °C for 7 days and soaked for 7 days, whereas CBR tests to be carried out on samples sealed and moist-cured at 25 °C for 21 days and soaked for 7 days ("ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993).
3.5.1 Moisture Content
A sufficient amount of moisture is required during the soil stabilization process to support compaction and cementitious reactions (Afrin 2017). Compaction and stabilization are governed by OMC and water-to-cement ratio, respectively (Zumrawi 2015). Both parameters are expressed in terms of the dry weight of the soil (Zumrawi 2015).
Granular soils may be stabilized with OMC obtained from the Proctor compaction test (Maclean and Lewis 1963). The mechanical characteristics of cement-stabilized soil greatly depend on the water-to-cement ratio (Miura et al. 2001). Literature report a hyperbolic relationship between UCS and water-to-cement ratio (Chian et al. 2016; Cong et al. 2014; Miura et al. 2001) for various types of soils. However, a study by Ribeiro et al. (2016) revealed that maximum UCS resulted when the optimum water-to-cement ratio was in the range of 1.0–1.5 for cement-stabilized silty-sand. Large UCS was attained for higher cement dosage irrespective of the water-to-cement ratio. Moreover, the authors claimed that UCS developed faster due to the formation of dispersive bonds between sand grains when larger water-to-cement ratios were adopted. However, the speed of curing did not guarantee maximum strength (Ribeiro et al. 2016).
For cement stabilization of cohesive soils, the recommended moisture content is 2% below the plastic limit to yield high states of compaction and to obtain low water absorption in hardened soil–cement (Maclean and Lewis 1963). Asgari et al. (2015) found that the initial water-to-stabilizer ratio significantly affected the mechanical properties of stabilized CL-type soil. For cement and lime-treated soils, three different initial water-to-stabilizer ratios were used; 2% less than OMC (dry side), OMC, and 2% more than OMC (wet side). Soils stabilized with dry side water yielded higher UCS than wet side. Lime stabilization with hydrated lime or quicklime consumes water amounts to 20–30% of their self-weight (Hebib and Farrell 2003; Sherwood 1993). Soils with great water affinity such as clay and peat absorb plentiful water leaving insufficient water available for hydration reactions (Hebib and Farrell 2003; Hicks 2002).
Appropriate moisture content is vital in attaining high strength characteristics. The amount of water to be added is dictated by parameters of compaction and stabilization. The OMC required for compaction needs to be manipulated by accommodating the moisture requirements pertaining to soil plasticity and additive consumption.
3.5.2 Temperature
In the presence of elevated curing temperatures, higher degrees of hydration reaction take place, which expedite the release of HL required for pozzolanic reactions. The temperature rise and availability of ample amount of HL accelerate the pozzolanic reaction, which promotes strength development in stabilized soils at both early and mature ages (Afrin 2017; Rao and Shivananda 2005; Zhang et al. 2014a, b). George et al. (1992) claimed that lime-stabilized soil at 50 °C resulted in more strength gain than that treated at lower temperatures. A similar influence of temperature on UCS of clay soil stabilized with lime has been reported by Bell (1996). Al-Mukhtar et al. (2010) found that increasing the curing temperature from 20 to 50 °C multiplied the rate of pozzolanic reaction by six. Zhang et al. (2014a, b) claimed that higher curing temperatures not only yielded higher short-term UCS but also ultimate UCS in cement-treated clay. In the published literature, the mechanical characteristics of stabilized soils were evaluated at temperatures ranging between 20 and 50 °C, considering typical air temperature variations in different countries.
To capitalize on the positive impact provided by elevated temperatures, studies have recommended to carryout stabilization of soil with cement and pozzolanic additives such as Lime, FA, and RHA during warmer conditions for better results (Sherwood 1993). Also, the practical implications pertaining to elevated temperatures are another concern. On the other hand, carbonation might increase with the temperature rise, which needs to be dealt with appropriately.
3.5.3 Curing Age
According to the reviewed literature, typical curing ages set to monitor the change in properties of stabilized soil are 3, 7, 14, 28, 56, and 91 days (Asgari et al. 2015; Ho et al. 2017; Latifi et al. 2017; Oluwatuyi et al. 2018; Phummiphan et al. 2016; Prusinski and Bhattacharja 1999; Rahman 1987; Rimal et al. 2019). Rarely researchers have tested the properties of stabilized soils at 1 day or 180, 224, 300, and 365 days (Kalantari and Prasad 2014; Lemaire et al. 2013; Rimal et al. 2019; Zhang et al. 2014a, b). Figure 5 illustrates the UCS development over the curing age. In all the soil-stabilizer combinations, rapid strength development is visible for up to 28 days, the rate of strength gain decreases afterward and eventually, it attains an asymptotic relation with curing age. A study compared the effect of curing age in cement and lime-stabilized soils, and found that the most significant changes occurred after 1 h curing period, and subsequent changes were marginal (Christensen 1969). In the initial stages of curing, cementitious hydration reactions are prominent in cement-based additives that contribute to rapid strength development. Pozzolanic reactions occur after cementitious hydration reaction at slower rates, which contribute to slower strength gain. In soil stabilization using stabilizers such as lime, RHA, FA, and ESP, strength development is caused by pozzolanic reactions only that provide a slower strength gain. Eventually, at the exhaust of required inputs for pozzolanic reactions, strength gain becomes zero. Horpibulsuk et al. (2010) found that, with the increase in curing age, hydration reactions increased to form cementitious products. They filled pores between granular particles and reduced total pore volume. Reduction in pores consequently contributed to an increase in strength.
Variation in unconfined compressive strengths for different soil types and stabilizers with curing age. SM, OH, CH, CL, and MH are standard USCS notations used to represent soil types. OPC ordinary Portland cement, LME Lime, FLA C Fly ash, class C, ESP Eggshell powder, SH-85 calcium-based powder additive
For all types of additives, the strength development in stabilized soil has been significant up to 28 days from the treatment. Appropriate curing conditions are to be ensured during this period to guarantee that the stabilized soil attains the maximum possible strength.
3.5.4 Curing Types
Kalantari and Prasad (2014) explored the effect of types of curing including air curing, moist curing, and moist curing with a surcharge load of 10 kPa on cement-stabilized peat samples. The authors measured the UCS of cured samples under the above conditions after curing periods of 28, 90, and 180 days, and found that moist curing with surcharge load resulted in the highest percent increase in UCS. Similarly, a study by Ho et al. (2017) compared sealed (for 91 days) and drying (sealed for 7 days and air-cured from 7th to 91st day) curing conditions for cement-stabilized sand and concluded that drying curing conditions produced the highest UCS. Different types of curing mediums including distilled water and seawater have been used by researchers (Chian et al. 2016; Xiao et al. 2014). Emarah and Seleem (2018) found that adding seawater to lime stabilization of highly expansive clay yielded a significant reduction in swelling potential and an improvement in compression potential. Table 6 provides details of curing methods used for different soil types stabilized with various stabilizing agents. Figure 6 illustrates the variation of UCS against curing age for different curing types.
Variation in unconfined compressive strengths for different curing types and soils with curing age. Pt and SP are standard USCS notations used to represent soil types; OPC ordinary Portland cement, LP Liquid polymer, A Air curing, M Moist curing, MS Moist curing with surcharge, S Sealed curing, AM Air curing and moist curing, and AH Air curing and heating at 100 °C (Ho et al. 2017; Kalantari and Prasad 2014; Rezaeimalek et al. 2017)
3.6 Carbonation
In lime stabilization, HL or C could react with atmospheric CO2 to form CaCO3, which is an insoluble content. Equations (13) and (15) govern chemical reactions of the formation of CaCO3. Similarly, in cement stabilization, cementitious hydration products including C–S–H and C–A–H formed at initial stages, are susceptible to carbonation, and their chemical reactions are shown in Eqs. (22) and (23) (Gourley and Greening 1999).
Other stabilizing agents including FA-class C, ESP, phosphogypsum, and cement kiln dust, which have rich C content also could potentially react with atmospheric CO2 to form CaCO3.
This early formation of CaCO3 retards pozzolanic reaction and strength gain, which could potentially lead to premature failure of pavements. To prevent carbonation from occurring at the pre-stabilization stage, it is important to minimize the exposure of stabilizing agents to air during manufacturing and storing processes. At post-stabilization, direct exposure of stabilized soil with air is to be curtailed by sealing it with polythene or a layer of water.
The carbonation effect can be quantified in stabilized soil by measuring the pH of stabilized soil. The presence of HL and OPC will have a pH > 12.4, C–S–H and C–A–H will have a pH ranging from 11.0 to 12.6 and CaCO3 will have pH = 8.3 (Gourley and Greening 1999). Indicators including phenolphthalein, phenol red, and dilute hydrochloric acid have been prescribed to detect the presence of C, HL, C–S–H, and C–A–H (Gourley and Greening 1999; Netterberg 1984).
Carbonation affects the durability of the stabilized soil and hence promotes progressive strength reduction, which can lead to structural failure. Calcium-rich additives are more susceptible to a higher degree of carbonation if exposed to air after stabilization. Typical additives such as OPC and lime contain significant amounts of calcium, and so do non-traditional stabilizers including FA-class C, ESP, and phosphogypsum. This emphasizes the need for proper curtailment of stabilized soil with air.
4 Engineering Properties of Stabilized Soil
The performance of soil stabilization has been historically evaluated through physical attributes including Atterberg limits and density (Amaral et al. 2013; Miller and Azad 2000; Muntohar and Hantoro 2000; Nath et al. 2017; Zumrawi 2015); mechanical parameters such as UCS, CBR, MR, indirect tensile strength, and Young’s modulus (Miller and Azad 2000; Mishra et al. 2019; Muntohar and Hantoro 2000; Oluwatuyi et al. 2018; Pongsivasathit et al. 2019; Praticò and Puppala 2012); swelling characteristics namely swelling potential and swell pressure (Nalbantoğlu 2004; Zumrawi 2015), and durability aspects including resistance to loss in strength (Oluwatuyi et al. 2018). Limiting criterion of these properties are stipulated in different standards for various pavement applications (Hicks 2002; "ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993; "SCA/5—Standard Specifications for Construction and Maintenance of roads and bridges," 2009; "Standard Specifications for Transportation materials and Methods of Sampling and Testing," 2015). Table 7 details the tests conducted to assess the performance of stabilized soil in numerous studies and the respective standards. Fundamentally, strength-related tests such as UCS, CBR, third point loading test, dynamic flexural loading, and resilient modulus are conducted on stabilized soil samples compacted at MDD or to match minimum field requirements. Atterberg limits and pH tests are conducted to monitor the change in plasticity and carbonation, respectively. Recently, advanced tests such as X-ray diffraction (XRD) analysis and Scanning Electron Microscope (SEM) are being conducted to examine mineralogical and morphological characteristics.
4.1 Physical Attributes
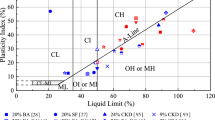
After adding stabilizing agent, soil particles undergo cation exchange and particle restructuring through which soil plasticity and density change. The changes in plasticity and density can be measured by conducting Atterberg limits and Proctor compaction tests, respectively. Density properties have already been discussed in Sect. 3.4. The Atterberg limits test is conducted according to ASTM D4318 ("ASTM D4318, Standard test methods for Liquid Limit, Plastic Limit, and Plasticity Index of soils," 2005) standard to determine the consistency limits of stabilized soil. By estimating Liquid Limit (LL) and Plastic Limit (PL), Plasticity Index (PI) can be estimated by finding the difference between LL and PL.
Figure 7 shows Atterberg limits obtained for various soil types stabilized with different stabilizing agents. Variation of LL with stabilizer dosage remains unchanged or decreases for all types of soils except OH, on the contrary PI decreased with the increase in stabilizer dosage in all soil types. As briefly noted earlier, when the stabilizing agent is added to soil, cations present that are of high affinity to water are replaced with low-affinity ones in the stabilizer. The exchange of ions retards the ability of soil particles to attract and retain water around, which turns soils less plastic. The degree of change in plasticity greatly depends on the type and amount of cations present in soil and stabilizing agents (Bell 1996; Miller and Azad 2000). An organic component present in the OH soil type consumes a large amount of water, which might have contributed to the slight rise in LL. Cement-based stabilizing agents are effective for soils with a PI less than 10, whereas lime stabilization is effective for soils with a PI above 10 (Hicks 2002; "ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993).
Variation of Liquid limit (LL) and plasticity index (PI) of different soil types with various stabilizer dosages. SC, ML, MH, CL, CH, and OH are standard USCS notations used to represent soil types; RHA Rice husk ash, LME Lime, OPC ordinary portland cement, FLA C Fly ash, class C, FLA F Fly ash, class F, CKN cement kiln dist, PHP phosphogypsum and ESP Eggshell powder (Degirmenci et al. 2007; Maduabuchi and Obikara 2018; Miller and Azad 2000; Muntohar and Hantoro 2000; Nath et al. 2017; Rahman 1986; Zumrawi 2015)
4.2 Mechanical Parameters
The structural stability of stabilized soils is assessed by determining their mechanical parameters. Historically, UCS (Amaral et al. 2013; Asgari et al. 2015; Bell 1996; Consoli et al. 2020; Degirmenci et al. 2007; Latifi et al. 2017), MR (Bhuvaneshwari et al. 2019; Ikechukwu et al. 2021; Pongsivasathit et al. 2019; Solanki et al. 2010; Tastan et al. 2011) and CBR (Basha et al. 2005; Oluwatuyi et al. 2018; Pongsivasathit et al. 2019; Zumrawi 2015) have been predominantly used in the studies to assess improvement in mechanical parameters. In addition to these three, a few studies have used indirect tensile strength (Praticò and Puppala 2012), third point loading (Pongsivasathit et al. 2019), and Young’s modulus (Cong et al. 2014; Sariosseiri and Muhunthan 2009) for evaluation.
Figure 8a shows the variation of UCS with stabilizer dosage for diverse types of soil. Cement-stabilized SC soil produced exceptionally well UCS as the readily available coarse grain in sand contributed to strength gain. For cement-based stabilizers (OPC, cement kiln), improvement in UCS with stabilizer dosage was drastic for all soil types. For 5–15% stabilizer dosage, the UCS of most of the stabilized soil lay between 700 and 1500 kPa. When cement-blended stabilizers were added to the soil, cementitious hydration occurred immediately, which could be attributed to the initial rapid strength gain. The rate of strength gain declined thereafter. On the contrary, lime stabilization showed a steep increase in UCS up to 4% dosage and then began to decline for subsequent accumulation in lime dosage. This phenomenon could be associated with the reduction in dry density of lime-stabilized soils (Bell 1996). Stabilization of organic soil with high plasticity, with FA (class C/F) up to 20% dosage achieved strengths less than 80 kPa. OPC and RHA mix stabilization showed a moderate improvement in UCS and reached a maximum UCS of 1,190 kPa at a dosage of 30%. Both RHA and FA entered a pozzolanic reaction with soil components at a slower rate, which improved the UCS of stabilized soil at a steady rate. The presence of organic matter in OH-type soil maintained a lower pH value, which heavily retarded pozzolanic reaction and strength gain in FA-stabilized soil. UCS requirements stipulated in ORN 31 for stabilized road base (CB1), stabilized road base (CB2), and stabilized sub-base (CS) are 3,000–6,000, 1,500–3,000, and 750–1,500 kPa, respectively ("ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993). SC and CL type soils stabilized with OPC at 5–10% dosage satisfied the minimum requirements for stabilized road base (CB1). For stabilized sub-base, CL, CH, ML, and MH-type soils treated with the combination of OPC, RHA, SH85, CKN, and LMN at a dosage of 5–10% might be suitable. However, another CL-type soil required more than 20% dosage of the combination of OPC and RHA to satisfy the minimum requirements for stabilized sub-base. This might be attributed to the chemical composition of the soil that exhausted a substantial portion of additives in cation exchange. Organic soil treated with fly ash was unsuitable to be used for road pavements.
Variation of mechanical properties with various stabilizer dosages. SC, SM, OH, CH, CL, ML, MH, and OH are standard USCS notations used to represent soil types; RHA Rice Husk Ash, LME Lime, FLA C Fly ash, class C, FLA F Fly ash, class F, CKN Cement Kiln dist and ESP Eggshell powder (Asgari et al. 2015; Basha et al. 2005; Bhuvaneshwari et al. 2019; Ikechukwu et al. 2021; Latifi et al. 2017; Muntohar and Hantoro 2000; Nalbantoğlu 2004; Nath et al. 2017; Oluwatuyi et al. 2018; Onyelowe et al. 2021; Praticò and Puppala 2012; Rahman1986; Rimal et al. 2019; Jaritngam et al. 2014; Soltani et al. 2017; Tastan et al. 2011; Zumrawi 2015)
Figure 8b shows the CBR variation with stabilizer dosage for diverse types of soils. CBR variation with stabilizer dosage resembles the trend observed in UCS variation. Coarse-grained soils stabilized with RHA, Lime, OPC, ESP, and OPC showed rapid improvements at the initial stage and gradually attained a saturated state. Lime stabilization, as explained earlier, increased and then declined due to the associated changes in dry density. Highly plastic soils stabilized with lime or RHA did not show notable improvement in CBR values. A minimum CBR value recommended for subgrade and sub-base applications is 15% and 30%, respectively ("ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993). MH-type soil stabilized with RHA or lime could not satisfy the minimum requirements for the stabilized pavement. Mostly, sandy soils stabilized with OPC, lime, and ESP at a dosage of less than 5% satisfied the minimum requirements for sub-base and subgrade. Surprisingly, a CH-type soil too showed drastic improvement in CBR. Sandy clay treated with an RHA dosage of more than 10% satisfied the minimum requirements for sub-base and subgrade.
The state-of-the-art world practices follow the mechanistic-empirical design of pavements, which assesses the performance of a pavement under repeated loading ("AASHTO Guide for Design of Pavement Structures," 1993). MR is one of the vital input parameters for the mechanistic-empirical design of pavements, which measures elastic characteristics of subgrade incorporating nonlinear characteristics (Fabiana et al. 2011; Qubain et al. 2000; Saberian et al. 2018). Figure 8c shows the change in MR with stabilizer dosage. Lime-stabilized soils showed a steep increase in MR even with small lime dosages. FA-based stabilizers showed a steady improvement in MR with higher stabilizer dosages. However, the final MR obtained at 30% stabilizer dosage was much less than that of lime (< 10%) stabilized soils. Due to higher CaO content, higher MR was observed in soil stabilized with FA–C compared to that of FA-F.
Young’s modulus variation with stabilizer dosage is illustrated in Fig. 8d. Young’s modulus of soil stabilized with lime-based stabilizer increased with stabilizer dosage up to 5–10% and thereafter showed a descending trend whereas cement-based stabilizer exhibited continuous improvement.
4.3 Swelling Potential and Swell Pressure
Figure 9 displays the variation in swelling potential with stabilizer dosage for fine-grained soils. In general, swelling potential reduced with an increase in stabilizer dosage for all types of stabilizers. Lime-based stabilizers showed a rapid reduction in swell potential during the initial stages of stabilization, compared to other stabilizers. This could be attributed to the abundantly available Ca2+ in lime-based stabilizers, which replaced cations that in turn reduce the affinity of clay particles toward water. This lowered the water-attracting capacity of clay particles. Thereafter, pozzolanic reactions occurred to form flocs in the treated soil, which eventually reduced both plasticity and swelling potential. Cement–based stabilizers immediately started hydration reactions, which gradually formed granular particles. The formation of granular particles brought down plasticity and swelling potential. Agglomeration of particles is the major factor in controlling the swell potential of clayey soils.
Variation of swelling potential with various stabilizer dosages. CH, CL, and MH are standard USCS notations used to represent soil types; OPC ordinary Portland cement, RHA Rice Husk Ash, LME Lime, FLA C Fly ash, class C, FLA F Fly ash, class F, QARHA Quicklime activated RHA, HARHA Hydrated lime activated RHA and CARHA calcite activated RHA (Muntohar and Hantoro 2000; Nalbantoğlu 2004; Onyelowe et al. 2021; Soltani et al. 2017)
High swell potential corresponds to high swell pressure, which is exhibited by soil upon inundation. Excessive swell-shrink behavior exerts fatigue load on the structural elements and eventually leads to structural failure. Calcium-rich additives are observed to be effective in reducing the swell potential of high-plasticity soils. Also incorporating lime or RHA-based additives and other additives might work well in reducing swell potential. The determination of additive dosage should take account into the amount of additive exhausted in the initial cation exchange.
4.4 Durability Aspects
Durability tests are conducted if the stabilized soil is affected by the presence of moisture (Maclean and Lewis 1963). As per the standards, loss in strength between a soaked sample and the unsoaked sample is used to quantify durability ("BS 1924–1: Hydraulically bound and stabilized materials for civil engineering purposes. Sampling, sample preparation and tests of materials before treatment," 2018; "ORN31, A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries," 1993). Studies have reported determining the ratio between UCS of samples that are cured under controlled conditions for 7 days and soaked in water for another 7 days, and samples continuously cured for 14 days under controlled conditions (Basha et al. 2005; Oluwatuyi et al. 2018) to quantify the resistance to loss in strength. As per British standard, the recommended minimum resistance to loss in strength is 80% ("BS 1924–1: Hydraulically bound and stabilized materials for civil engineering purposes. Sampling, sample preparation and tests of materials before treatment," 2018). Figure 10 depicts the variation of resistance to loss in strength with stabilizer dosage. Cement-based stabilizers exhibited better durability properties with the increase in stabilizer dosage. However, an increase in RHA beyond 10% reduced the resistance of stabilized soil to loss in strength. Dosages between 5 and 20% satisfied the minimum requirement for considered stabilized soils.
5 Economic and Environmental Perspectives
The total cost incurs during soil stabilization includes raw material cost, transport cost, and in-place stabilization cost. To assess the economic benefits, the total cost-to-strength ratio is estimated for each stabilizer as described in Eq. (24)
Energy consumption and Carbon dioxide emission are the two indicators that reveal the burden on the environment. For each stabilizer, the energy-to-strength ratio and CO2-to-strength ratio are computed as given in Eqs. (25) and (26).
The cost, energy, and CO2 emission of stabilization materials and processes have been extracted from published literature and are detailed in Table 8.
Figure 11 depicts cost-strength, energy-strength and CO2 emission-strength ratios estimated for several types of soils and stabilizers. Cement-based stabilizers, since producing higher UCS, show attractive cost-strength, energy-strength, and CO2 emission-strength ratios, and so are lime-stabilized soils. Silica fume too exhibited better ratios in treating peat soil. On the contrary, RHA and FA stabilizers exhibited poor ratios in all three categories. For high plastic soils, all ratios were better in lime-stabilized soils than in cement-stabilized soils. For coarse-grained soils with low plasticity, cement stabilization was effective.
The cost-strength, energy-strength and CO2-strength ratios of various stabilized soils. SM, SC, ML, MH, CL, CH, and OH are standard USCS notations used to represent soil types; OPC ordinary Portland cement, RHA Rice Husk Ash, LME Lime, FLA C Fly ash, class C, FLA F Fly ash, class F, CKN Cement Kiln dist and ESP Eggshell powder (Ahmad et al. 2021a, b; Asgari et al. 2015; Basha et al. 2005; Latifi et al. 2017; Nath et al. 2017; Oluwatuyi et al. 2018; Praticò and Puppala 2012; Rimal et al. 2019; Jaritngam et al. 2014)
6 Industrial Application Perspectives
Construction industries have been traditionally using cement, lime, and fly ash to stabilize weak soils (Nelson et al. 2015). Recently, a transformation from traditional techniques to more sophisticated techniques has taken place, which accounts for sustainability aspects (Gomes Correia et al. 2016). Incorporating sustainability perspectives in soil stabilization focuses on reducing carbon footprint, energy consumption, and extraction of natural resources while improving engineering characteristics of stabilized geotechnical elements cost-effectively. These multi-objective tasks often require comprehensive design, skilled personnel, and stringent monitoring mechanisms. This has become more crucial when non-traditional additives are deployed for stabilization as their properties and performance vary significantly.
Amidst the uncertainty involved with the use of non-traditional additives, industries have promoted the utilization of waste materials in soil stabilization to address economic and ecological aspects. Table 9 details the additives commonly used in soil stabilization and their annual global production. From the supply point of view, fly ash and BFSC can be considered as potential replacements for cement and lime. Since they both are waste products, they provide economic advantages too (Ikeagwuani and Nwonu 2019). On the other hand, the reuse of waste for soil stabilization helps to promote effective waste management. To sustain the merits of this practice, environmental impact assessment for the use of waste material is vital to ensure that the deployed waste material does not have any detrimental impact on the environment. However, the traditional additives edge out non-traditional additives in terms of cost/energy/emission to strength ratio.
Except for traditional additives, a comprehensive standard guideline for the use of other additives is not available. This prevents the industry from choosing cost-effective and eco-friendly alternatives for soil stabilization. It is imperative to devise a comprehensive guide for the use of several types of soil stabilizers. Enormous work on the stabilization of several types of soils has been done and published to date. These all can be gathered to develop a commonly accepted guideline for soil stabilization using several types of additives. This guide should incorporate potential threats to the environment due to the use of various types of additives and respective mitigation strategies.
7 Conclusions and Discussions
The review presented in this paper attempted to cover a wide spectrum of information on soil stabilization so that practitioners and researchers can comprehend the whole mechanism and processes behind the treatment of weak soils. This would benefit them by designing a cost-effective and eco-friendly soil stabilization process. The key areas included in the review are the chemistry behind soil stabilization, the control factors influencing stabilization, performance indicators, and economic and environmental aspects.
7.1 Chemistry Behind
The rate of change in physical and mechanical properties of stabilized soils is governed by rapid hydration and slower pozzolanic reactions. The quantity and type of ions present in soil are used to determine the type of stabilizing agent and dosage required for stabilization; especially Ca2+ has predominant control over the reactions. Cement-based stabilizers performed well irrespective of soil type and curing condition, on the contrary, lime-based stabilizers required appropriate control of temperature and pH to facilitate pozzolanic reactions. Cement and cementitious blends are more suitable for soils with low plasticity (PI < 10%), whereas lime and lime blends perform well for soils with high plasticity (PI > 10%). Soil stabilization with most of the cement/lime blended stabilizers required stabilizer dosages below 10%. FA or RHA-based stabilization required dosages in the range of 10–30% for notable improvement.
7.2 Stabilization Control Factors
The primary factors that affect the degree of stabilization are soil type (specifically the clay composition), organic matter present in the soil, compaction level, and curing. Cement or lime blended stabilizers portrayed a rapid strength gain for even small stabilizer dosages, but RHA and FA-based soil stabilization attained strength very slowly. The long-term strength development process occurs during curing. It is therefore recommended to administer the required moisture level, temperature, duration of curing, and curing type to support pozzolanic reactions that promote strength enhancement in stabilized soils.
7.3 Stabilization Performance Parameters
The changes in physical attributes including Atterberg limits and MDD reported a reduction in PI and MDD with the increase in stabilizer dosage irrespective of soil type or stabilizer. Lime and cement-based stabilizers provided a substantial reduction in PI for even small dosages of less than 5%.
The mechanical performance of stabilized soil has been monitored by determining UCS, CBR, MR, and Young’s modulus. Cement-based stabilizers continued to improve with the accumulation of stabilizer dosage, however, lime-blended stabilizers showed a steep increase initially and then began to decline. For all types of stabilizers, typical values of UCS and CBR obtained for 5% dosage, fell in between 700 and 1,500 kPa and 30–60%, respectively.
7.4 Economic and Environmental Aspects
Cement and cementitious blends exhibited better cost-strength, energy-strength, and CO2 emission-strength ratios for soils with low plasticity, whereas lime-blended stabilizers performed well for high plastic soils. This implies that stabilizing soil with low plasticity with cement blended stabilizers and high plasticity with lime is economically and environmentally advantageous. Silica fume can be considered a potential alternative to treat peat soil as it showed lower cost-strength, energy-strength, and CO2 emission-strength ratios. Also, blending more than one stabilizer is recommended to achieve a balanced performance in cost, energy, and emission aspects.
7.5 Practical Implications
Although soil stabilization is a cost-effective option to improve the engineering properties of in-situ soil, the execution of the stabilization process in the field requires attention to labour, machinery, and testing. A thorough geotechnical site investigation is inevitable to decide on the method of stabilization and to carry out the stabilization process successfully. In addition, a continuous and well-structured monitoring mechanism is vital to ensure the performance and durability of the stabilized earth during the operation. Problematic soils require the addition of chemical additives to moderate the engineering properties of the stabilized soil. The application of chemical additives needs precaution and skilled personnel to prevent the workers from health hazards and to avoid heterogeneity in the outcome. Wastes such as fly ash, RHA, and BFSC are widely available across the world where coal-fired power plants or agricultural fields are located, whereas SF, redmud, phosphogypsum, and cement kiln are only available where the specific industries are located. Also, the chemical composition of the waste drastically changes from place to place. This could affect the engineering properties of the stabilized soil. It is, therefore, a systematic laboratory and field investigation is recommended, before applying the waste for soil stabilization. These variations may impact the dosage of additives required for a stabilization task, and hence become cost prohibitive.
8 Future Directions
Although ample studies have explored cement-based and lime-based stabilizers to improve soil properties, it is evident that a few areas remain yet to be explored. Some of them are given below.
-
Further studies on the durability aspects of soil stabilization are required to examine the long-term performance perspectives.
-
The application potential of geopolymer technology for soil stabilization in road construction has not yet been thoroughly studied. It is noteworthy to mention that geopolymer technology that deploys cost-effective and eco-friendly materials are the need of the hour.
-
Performance evaluation conducted on stabilized soils considered a few representative parameters to select appropriate stabilizers and dosages. A comprehensive optimization framework that covers mechanical, economic, and environmental aspects is hard to find in the published literature. Studies are required to establish such an optimization framework.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- GW:
-
Well graded gravel
- SC:
-
Clayey sand
- SP:
-
Poorly graded sand
- SM:
-
Silty sand
- CL:
-
Inorganic clay of low plasticity
- CH:
-
Inorganic clay of high plasticity
- ML:
-
Inorganic silt of low plasticity
- MH:
-
Inorganic silt of high plasticity
- OL:
-
Organic silts and clay of low plasticity
- OH:
-
Organic silts and clay of high plasticity
- Pt:
-
Peat, muck, and other highly organic soils
- OPC:
-
Ordinary Portland cement
- RHA:
-
Rice husk ash
- CKN:
-
Cement kiln
- FLA C:
-
Fly ash—class C
- FLA F:
-
Fly ash—class F
- PHP:
-
Phosphogypsum
- ESP:
-
Eggshell powder
- LME:
-
Lime
- BFSC:
-
Blast furnace slag cement
- HL:
-
Hydrated lime
- SL:
-
Slurry lime
- QL:
-
Quick lime
- MDD:
-
Maximum dry density
- OMC:
-
Optimum moisture content
- UCS:
-
Unconfined compressive strength
- CBR:
-
California bearing ratio
- MR:
-
Modulus of resilience
- C:
-
CaO
- S:
-
SiO2
- A:
-
Al2O3
- F:
-
Fe2O3
- SEM:
-
Scanning electron microscope
- XRD:
-
X-ray diffraction
References
AASHTO (1993) Guide for design of pavement structures. American Association of State Highway and Transportation Officials, Washington, DC
Abdollahnejad Z, Kheradmand M, Pacheco-Torgal F (2017) Short-term compressive strength of fly ash and waste glass alkali-activated cement-based binder mortars with two biopolymers. J Mater Civ Eng 29(7):04017045. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001920
Afrin H (2017) A review on different types soil stabilization techniques. Int J Transp Eng Technol 3(2):19–24. https://doi.org/10.11648/j.ijtet.20170302.12
Ahmad A, Sutanto MH, Ahmad NRB, Bujang M, Mohamad ME (2021a) The implementation of industrial byproduct in Malaysian peat improvement: a sustainable soil stabilization approach. Materials 14(23):7315
Ahmad A, Sutanto MH, Al-Bared MAM, Harahap ISH, Abad SVANK, Khan MA (2021b) Physio-chemical properties, consolidation, and stabilization of tropical peat soil using traditional soil additives—a state of the art literature review. KSCE J Civ Eng 25(10):3662–3678. https://doi.org/10.1007/s12205-021-1247-7
Ahmad A, Sutanto MH, Ahmad NR, Mohamad ME, Bujang M (2023) Microstructural characterization of fibric peat stabilized with portland cement and silica fume. Materials 16(1):18
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36(3):327–363. https://doi.org/10.1016/j.pecs.2009.11.003
Ahmed A, Ugai K, Kamei T (2011) Environmental evaluation for clayey soil stabilized with gypsum waste plasterboard in Japan. In: Advances in unsaturated soil, geo-hazard, and geo-environmental engineering, pp 9–17
Aïtcin PC (2016) Portland cement. In: Aïtcin P-C, Flatt RJ (eds) Science and technology of concrete admixtures. Woodhead Publishing, pp 27–51
Al-Mukhtar M, Lasledj A, Alcover J-F (2010) Behaviour and mineralogy changes in lime-treated expansive soil at 50°C. Appl Clay Sci 50(2):199–203. https://doi.org/10.1016/j.clay.2010.07.022
Alipour R, Heshmati R AA, Karimiazar J, Esazadefar N, Asghari-Kaljahi E, Bahmani SH (2022). Resistance and swelling of Tabriz marl soils stabilised using nano-silica and nano-alumina. Proc Inst Civ Eng Geotech Eng 6:1–14. https://doi.org/10.1680/jgeen.21.00016
Alsharef JMA, Taha MR, Firoozi AA, Govindasamy P (2016) Potential of using nanocarbons to stabilize weak soils. Appl Environ Soil Sci 2016:5060531. https://doi.org/10.1155/2016/5060531
Amaral MC, Siqueira FB, Destefani AZ, Holanda JNF (2013) Soil cement bricks incorporated with eggshell waste. Paper presented at the Proceedings of the Institution of Civil Engineers Waste and Resource Management
Amouzadeh Omrani M, Modarres A (2018) Emulsified cold recycled mixtures using cement kiln dust and coal waste ash-mechanical-environmental impacts. J Clean Prod 199:101–111. https://doi.org/10.1016/j.jclepro.2018.07.155
Anupam AK, Kumar P, Ransinchung RNGD (2013) Use of various agricultural and industrial waste materials in road construction. Procedia Soc Behav Sci 104:264–273. https://doi.org/10.1016/j.sbspro.2013.11.119
Arman A, Munfakh GA (1972) Lime stabilization of organic soils. Highw Res Rec 381:37–45
Arulrajah A, Mohammadinia A, Phummiphan I, Horpibulsuk S, Samingthong W (2016) Stabilization of recycled demolition aggregates by geopolymers comprising calcium carbide residue, fly ash and slag precursors. Constr Build Mater 114:864–873. https://doi.org/10.1016/j.conbuildmat.2016.03.150
Arulrajah A, Yaghoubi M, Disfani MM, Horpibulsuk S, Bo MW, Leong M (2018) Evaluation of fly ash- and slag-based geopolymers for the improvement of a soft marine clay by deep soil mixing. Soils Found 58(6):1358–1370. https://doi.org/10.1016/j.sandf.2018.07.005
Asgari MR, Baghebanzadeh Dezfuli A, Bayat M (2015) Experimental study on stabilization of a low plasticity clayey soil with cement/lime. Arab J Geosci 8(3):1439–1452. https://doi.org/10.1007/s12517-013-1173-1
Association IL (2022) Lime production. Retrieved from https://www.internationallime.org/world-lime-production
ASTM C618 (2019) Standard specification for coal fly ash and raw or calcined natural pozzolan for use in concrete. In: ASTM International, West Conshohocken
ASTM D2487 (2017) Standard practice for classification of soils for engineering purposes (unified soil classification system). In. ASTM International, West Conshohocken
ASTM D4318 (2005) Standard test methods for liquid limit, plastic limit, and plasticity index of soils. In. ASTM International, West Conshohocken
Aysha H, Hemalatha T, Arunachalam N, Murthy AR, Iyer NR (2014) Assessment of embodied energy in the production of ultra high performance concrete (UHPC). Int J Stud Res Technol Manag 2(3):113–120
Baghdadi ZA, Fatani MN, Sabban NA (1995) Soil modification by cement kiln dust. J Mater Civ Eng 7(4):218–222. https://doi.org/10.1061/(ASCE)0899-1561(1995)7:4(218)
Balaguera A, Carvajal GI, Albertí J, Fullana-i-Palmer P (2018) Life cycle assessment of road construction alternative materials: a literature review. Resour Conserv Recycl 132:37–48. https://doi.org/10.1016/j.resconrec.2018.01.003
Barnes P, Bensted J (2001) Structure and performance of cements, 2nd edn. CRC Press, London
Basha EA, Hashim R, Mahmud HB, Muntohar AS (2005) Stabilization of residual soil with rice husk ash and cement. Constr Build Mater 19(6):448–453. https://doi.org/10.1016/j.conbuildmat.2004.08.001
Bassani M, Tefa L, Russo A, Palmero P (2019) Alkali-activation of recycled construction and demolition waste aggregate with no added binder. Constr Build Mater 205:398–413. https://doi.org/10.1016/j.conbuildmat.2019.02.031
Beck K, Brunetaud X, Mertz J-D, Al-Mukhtar M (2010) On the use of eggshell lime and tuffeau powder to formulate an appropriate mortar for restoration purposes. Geol Soc Lond Spec Publ 331(1):137–145. https://doi.org/10.1144/sp331.12
Bell FG (1996) Lime stabilization of clay minerals and soils. Eng Geol 42(4):223–237. https://doi.org/10.1016/0013-7952(96)00028-2
Bellum RR, Muniraj K, Madduru SRC (2020) Characteristic evaluation of geopolymer concrete for the development of road network: sustainable infrastructure. Innov Infrastruct Solut 5(3):91. https://doi.org/10.1007/s41062-020-00344-5
Bensaifi E, Bouteldja F, Nouaouria MS, Breul P (2019) Influence of crushed granulated blast furnace slag and calcined eggshell waste on mechanical properties of a compacted marl. Transp Geotech 20:100244. https://doi.org/10.1016/j.trgeo.2019.100244
Bhatt A, Priyadarshini S, Acharath Mohanakrishnan A, Abri A, Sattler M, Techapaphawit S (2019) Physical, chemical, and geotechnical properties of coal fly ash: a global review. Case Stud Construct Mater 11:e00263. https://doi.org/10.1016/j.cscm.2019.e00263
Bhattacharjee U, Kandpal T (2002) Potential of fly ash utilisation in India. Energy 27(2):151–166. https://doi.org/10.1016/S0360-5442(01)00065-2
Bhuvaneshwari S, Robinson RG, Gandhi SR (2019) Resilient modulus of lime treated expansive soil. Geotech Geol Eng 37(1):305–315. https://doi.org/10.1007/s10706-018-0610-z
Billong N, Boubakar L, Bayiha BN, Njimbouombouo SM, Melo UC, Oti J, Kinuthia J (2020) An investigation on the suitability of hydrated building lime from travertine limestone outcrop of Bogongo, South West of Cameroon. Case Stud Construct Mater 13:e00369. https://doi.org/10.1016/j.cscm.2020.e00369
Blayi RA, Sherwani AFH, Ibrahim HH, Faraj RH, Daraei A (2020) Strength improvement of expansive soil by utilizing waste glass powder. Case Stud Construct Mater 13:e00427. https://doi.org/10.1016/j.cscm.2020.e00427
BS 12 (1996) Specification for Portland cement. In: British Standards Institution, UK
BS 1377-3 (1990) Methods of test for soils for civil engineering purposes—Part 3: chemical and electro-chemical tests In. British Standards Institution, UK
BS 1377-4 (1990) Methods of test for—soils for civil engineering purposes—Part 4, compaction-related tests. In. British Standards Institution, UK
BS 1924-1 (2018) Hydraulically bound and stabilized materials for civil engineering purposes. Sampling, sample preparation and tests of materials before treatment. British Standards Institution, UK
Cardoso FA, Fernandes HC, Pileggi RG, Cincotto MA, John VM (2009) Carbide lime and industrial hydrated lime characterization. Powder Technol 195(2):143–149. https://doi.org/10.1016/j.powtec.2009.05.017
Celik E, Nalbantoglu Z (2013) Effects of ground granulated blastfurnace slag (GGBS) on the swelling properties of lime-stabilized sulfate-bearing soils. Eng Geol 163:20–25. https://doi.org/10.1016/j.enggeo.2013.05.016
Chen H, Wang Q (2006) The behaviour of organic matter in the process of soft soil stabilization using cement. Bull Eng Geol Env 65(4):445–448. https://doi.org/10.1007/s10064-005-0030-1
Chew SH, Kamruzzaman AHM, Lee FH (2004) Physicochemical and engineering behavior of cement treated clays. J Geotechn Geoenviron Eng 130(7):696–706. https://doi.org/10.1061/(ASCE)1090-0241(2004)130:7(696)
Chiaia B, Fantilli AP, Guerini A, Volpatti G, Zampini D (2014) Eco-mechanical index for structural concrete. Constr Build Mater 67:386–392. https://doi.org/10.1016/j.conbuildmat.2013.12.090
Chian SC, Nguyen ST, Phoon KK (2016) Extended strength development model of cement-treated clay. J Geotech Geoenviron Eng 142(2):06015014. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001400
Christensen AP (1969) Cement modification of clay soils. Portland Cement Association, Skokie, Ill: Research and Development Bulletin RD002.01S
Chung H, Kim SH, Nam K (2021) Application of microbially induced calcite precipitation to prevent soil loss by rainfall: effect of particle size and organic matter content. J Soils Sediments 21(8):2744–2754. https://doi.org/10.1007/s11368-020-02757-2
Cong M, Longzhu C, Bing C (2014) Analysis of strength development in soft clay stabilized with cement-based stabilizer. Constr Build Mater 71:354–362. https://doi.org/10.1016/j.conbuildmat.2014.08.087
Consoli NC, Prietto PDM, da Silva Lopes L, Winter D (2014) Control factors for the long term compressive strength of lime treated sandy clay soil. Transp Geotech 1(3):129–136. https://doi.org/10.1016/j.trgeo.2014.07.005
Consoli NC, Caicedo AML, Saldanha RB, Filho HCS, Acosta CJM (2020) Eggshell produced limes: innovative materials for soil stabilization. J Mater Civ Eng 32(11):06020018. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003418
Croft JB (1967) The influence of soil mineralogical composition on cement stabilization. Géotechnique 17(2):119–135. https://doi.org/10.1680/geot.1967.17.2.119
Cuisinier O, Auriol JC, Le Borgne T, Deneele D (2011) Microstructure and hydraulic conductivity of a compacted lime-treated soil. Eng Geol 123(3):187–193. https://doi.org/10.1016/j.enggeo.2011.07.010
da Rocha CG, Passuello A, Consoli NC, Quiñónez Samaniego RA, Kanazawa NM (2016) Life cycle assessment for soil stabilization dosages: a study for the Paraguayan Chaco. J Clean Prod 139:309–318. https://doi.org/10.1016/j.jclepro.2016.07.219
Das BM (2007) Principles of geotechnical engineering, 7th edn. Cengage Learning, USA
Das BM (2008) Advanced soil mechanics. Taylor & Francis, NY, USA
Dave N, Sahu V, Misra AK (2020) Development of geopolymer cement concrete for highway infrastructure applications. J Eng Des Technol 18(5):1321–1333. https://doi.org/10.1108/JEDT-10-2019-0263
Davidovits J (1991a) Geopolymers. J Therm Anal 37(8):1633–1656. https://doi.org/10.1007/BF01912193
Davidovits J (1991b) Geopolymers: inorganic polymeric new materials. J Therm Anal Calorim 37(8):1633–1656. https://doi.org/10.1007/bf01912193
Davidovits J, Sawyer JL (1985) Patent No US4509985
Dawson AR, Elliott RC, Rowe GM, Williams J (1995) Assessment of suitability of some industrial by-products for use in pavement bases in the United Kingdom. Transp Res Rec 1486:114–123
Degirmenci N, Okucu A, Turabi A (2007) Application of phosphogypsum in soil stabilization. Build Environ 42(9):3393–3398. https://doi.org/10.1016/j.buildenv.2006.08.010
Diaz Caselles L, Hot J, Roosz C, Cyr M (2020) Stabilization of soils containing sulfates by using alternative hydraulic binders. Appl Geochem 113:104–494. https://doi.org/10.1016/j.apgeochem.2019.104494
Du Y-J, Jiang N-J, Liu S-Y, Horpibulsuk S, Arulrajah A (2016) Field evaluation of soft highway subgrade soil stabilized with calcium carbide residue. Soils Found 56(2):301–314. https://doi.org/10.1016/j.sandf.2016.02.012
Dunuweera SP, Rajapakse RMG (2018) Cement types, composition, uses and advantages of nanocement, environmental impact on cement production, and possible solutions. Adv Mater Sci Eng 2018:4158682. https://doi.org/10.1155/2018/4158682
Durastanti C, Moretti L (2020) Environmental impacts of cement production: a statistical analysis. Appl Sci 10(22):8212
Duxson P, Fernández-Jiménez A, Provis JL, Lukey GC, Palomo A, van Deventer JSJ (2007) Geopolymer technology: the current state of the art. J Mater Sci 42(9):2917–2933. https://doi.org/10.1007/s10853-006-0637-z
Eades JL, Grim RE (1966) A quick test to determine lime requirements for lime stabilization. Highw Res Rec 139:61–72
Ebrahim Asghari-Kaljahi ZH, Mansouri H (2020) Treatment of clayey soils with steel furnace slag and lime for road construction in the South West of Iran. In: Recent thoughts in geoenvironmental engineering. Proceedings of the 3rd GeoMEast international congress and exhibition, Egypt 2019 on sustainable civil infrastructures-the official international congress of the Soil-Structure Interaction Group in Egypt (SSIGE). Springer
Eisa MS, Basiouny ME, Fahmy EA (2021) Effect of metakaolin-based geopolymer concrete on the length of rigid pavement slabs. Innov Infrastruct Solut 6(2):91. https://doi.org/10.1007/s41062-021-00465-5
El-Attar MM, Sadek DM, Salah AM (2017) Recycling of high volumes of cement kiln dust in bricks industry. J Clean Prod 143:506–515. https://doi.org/10.1016/j.jclepro.2016.12.082
Emarah DA, Seleem SA (2018) Swelling soils treatment using lime and sea water for roads construction. Alex Eng J 57(4):2357–2365. https://doi.org/10.1016/j.aej.2017.08.009
Fabiana DCL, Rosangela DSM, Kamilla LV, Liedi B (2011) Laboratory evaluation of recycled construction and demolition waste for pavements. Construct Build Mater 66:2972–2979
Fapohunda C, Akinbile B, Shittu A (2017) Structure and properties of mortar and concrete with rice husk ash as partial replacement of ordinary Portland cement—a review. Int J Sustain Built Environ 6(2):675–692. https://doi.org/10.1016/j.ijsbe.2017.07.004
Farrant WE, Babafemi AJ, Kolawole JT, Panda B (2022) Influence of sugarcane bagasse ash and silica fume on the mechanical and durability properties of concrete. Materials 15(9):3018
Ferraz E, Gamelas JAF, Coroado J, Monteiro C, Rocha F (2018) Eggshell waste to produce building lime: calcium oxide reactivity, industrial, environmental and economic implications. Mater Struct 51(5):115. https://doi.org/10.1617/s11527-018-1243-7
Firoozi AA, Guney Olgun C, Firoozi AA, Baghini MS (2017) Fundamentals of soil stabilization. Int J Geo-Eng 8(1):8–26. https://doi.org/10.1186/s40703-017-0064-9
Fly Ash Facts for Highway Engineers (2003) Retrieved from American Coal Ash Association, U.S. Department of Transportation, Federal Highway Administration
Gallagher PM, Mitchell JK (2002) Influence of colloidal silica grout on liquefaction potential and cyclic undrained behavior of loose sand. Soil Dyn Earthq Eng 22(9):1017–1026. https://doi.org/10.1016/S0267-7261(02)00126-4
George SZ, Ponniah DA, Little JA (1992) Effect of temperature on lime-soil stabilization. Constr Build Mater 6(4):247–252. https://doi.org/10.1016/0950-0618(92)90050-9
Geraldo RH, Costa ARD, Kanai J, Silva JS, Souza JD, Andrade HMC et al (2020) Calcination parameters on phosphogypsum waste recycling. Construct Build Mater 256:119-406. https://doi.org/10.1016/j.conbuildmat.2020.119406
Ghafoori N, Spitek R, Najimi M (2016) Influence of limestone size and content on transport properties of self-consolidating concrete. Constr Build Mater 127:588–595. https://doi.org/10.1016/j.conbuildmat.2016.10.051
Ghasabkolaei N, Janalizadeh Choobbasti A, Roshan N, Ghasemi SE (2017) Geotechnical properties of the soils modified with nanomaterials: a comprehensive review. Arch Civ Mech Eng 17(3):639–650. https://doi.org/10.1016/j.acme.2017.01.010
Ghavami S, Jahanbakhsh H, Saeedi Azizkandi A, Moghadas Nejad F (2021) Influence of sodium chloride on cement kiln dust-treated clayey soil: strength properties, cost analysis, and environmental impact. Environ Dev Sustain 23(1):683–702. https://doi.org/10.1007/s10668-020-00603-6
Gomes Correia A, Winter MG, Puppala AJ (2016) A review of sustainable approaches in transport infrastructure geotechnics. Transp Geotech 7:21–28. https://doi.org/10.1016/j.trgeo.2016.03.003
Gourley CS, Greening PAK (1999) Performance of chemically stabilized roadbases: results and recommendations from studies in Southern Africa. Retrieved from Transport Research Laboratory, Crowthrone, Berkshire
Gowthaman S, Yamamoto M, Nakashima K, Ivanov V, Kawasaki S (2021) Calcium phosphate biocement using bone meal and acid urease: an eco-friendly approach for soil improvement. J Clean Prod 319:128–782. https://doi.org/10.1016/j.jclepro.2021.128782
Halsted GE, Adaska WS, Association PC, McConnell WT (2008) Guide to Cement-modified Soil (CMS). Portland Cement Association
Hampton MB, Edil T (1998) Strength gain of organic ground with cement-type binders. Soil Improvement for Big Digs, Geotechnical Special Publication No. 81. ASCE, pp 135–148
Hashemi SSG, Mahmud HB, Djobo JNY, Tan CG, Ang BC, Ranjbar N (2018) Microstructural characterization and mechanical properties of bottom ash mortar. J Clean Prod 170:797–804. https://doi.org/10.1016/j.jclepro.2017.09.191
Hashemi SSG, Mahmud HB, Ghuan TC, Chin AB, Kuenzel C, Ranjbar N (2019) Safe disposal of coal bottom ash by solidification and stabilization techniques. Constr Build Mater 197:705–715. https://doi.org/10.1016/j.conbuildmat.2018.11.123
Hawa A, Tonnayopas D, Prachasaree W, Taneerananon P (2013) Development and performance evaluation of very high early strength geopolymer for rapid road repair. Adv Mater Sci Eng 2013:764180. https://doi.org/10.1155/2013/764180
He J, Jie Y, Zhang J, Yu Y, Zhang G (2013) Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cement Concr Compos 37:108–118. https://doi.org/10.1016/j.cemconcomp.2012.11.010
He J, Gao Y, Gu Z, Chu J, Wang L (2020) Characterization of crude bacterial urease for CaCO3 precipitation and cementation of silty sand. J Mater Civ Eng 32(5):04020071. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003100
Hebib S, Farrell ER (2003) Some experiences on the stabilization of Irish peats. Can Geotech J 40(1):107–120. https://doi.org/10.1139/t02-091
Heniegal AM, Ramadan MA, Naguib A, Agwa IS (2020) Study on properties of clay brick incorporating sludge of water treatment plant and agriculture waste. Case Stud Construct Mater 13:e00397. https://doi.org/10.1016/j.cscm.2020.e00397
Hicks RG (2002) FHWA-AK-RD-01-6B, Alaska Soil Stabilization Design Guide. In E. Oregon State University. Department of Civil (Ed.). Alaska Department of Transportation and Public Facilities
Hlaváček P, Šmilauer V, Škvára F, Kopecký L, Šulc R (2015) Inorganic foams made from alkali-activated fly ash: Mechanical, chemical and physical properties. J Eur Ceram Soc 35(2):703–709. https://doi.org/10.1016/j.jeurceramsoc.2014.08.024
Ho LS, Nakarai K, Ogawa Y, Sasaki T, Morioka M (2017) Strength development of cement-treated soils: Effects of water content, carbonation, and pozzolanic reaction under drying curing condition. Constr Build Mater 134:703–712. https://doi.org/10.1016/j.conbuildmat.2016.12.065
Hoover CG, Ulm F-J (2015) Experimental chemo-mechanics of early-age fracture properties of cement paste. Cem Concr Res 75:42–52. https://doi.org/10.1016/j.cemconres.2015.04.004
Horpibulsuk S, Rachan R, Chinkulkijniwat A, Raksachon Y, Suddeepong A (2010) Analysis of strength development in cement-stabilized silty clay from microstructural considerations. Constr Build Mater 24(10):2011–2021. https://doi.org/10.1016/j.conbuildmat.2010.03.011
Hu L, He Z, Zhang S (2020) Sustainable use of rice husk ash in cement-based materials: environmental evaluation and performance improvement. J Clean Product 264:121–744. https://doi.org/10.1016/j.jclepro.2020.121744
Huang Y, Bird RN, Heidrich O (2007) A review of the use of recycled solid waste materials in asphalt pavements. Resour Conserv Recycl 52:58–73
Ikeagwuani CC, Nwonu DC (2019) Emerging trends in expansive soil stabilisation: a review. J Rock Mech Geotech Eng 11(2):423–440. https://doi.org/10.1016/j.jrmge.2018.08.013
Ikechukwu AF, Hassan MM, Moubarak A (2021) Resilient modulus and microstructure of unsaturated expansive subgrade stabilized with activated fly ash. Int J Geotech Eng 15(8):915–938. https://doi.org/10.1080/19386362.2019.1656919
Imbabi MS, Carrigan C, McKenna S (2012) Trends and developments in green cement and concrete technology. Int J Sustain Built Environ 1(2):194–216. https://doi.org/10.1016/j.ijsbe.2013.05.001
Imtiaz A, Lovell CW (1992) Use of waste materials in highway construction: state of the practice and evaluation of the selected waste products. Transp Res Rec 1345:1–9
Ivanov V, Chu J, Stabnikov V (2015) Basics of construction microbial biotechnology. In Pacheco Torgal F, Labrincha JA, Diamanti MV, Yu CP, Lee HK (eds) Biotechnologies and biomimetics for civil engineering. Springer, Cham, pp 21–56
Jaritngam S, Somchainuek O, Taneerananon P (2014) Feasibility of laterite-cement mixture as pavement base course aggregate. IJST Trans Civ Eng 38(C1):275–284
Jaturapitakkul C, Cheerarot R (2003) Development of bottom ash as pozzolanic material. J Mater Civ Eng 15(1):48–53. https://doi.org/10.1061/(ASCE)0899-1561(2003)15:1(48)
Jitsangiam P, Suwan T, Pimraksa K, Sukontasukkul P, Chindaprasirt P (2021) Challenge of adopting relatively low strength and self-cured geopolymer for road construction application: a review and primary laboratory study. Int J Pavement Eng 22(11):1454–1468. https://doi.org/10.1080/10298436.2019.1696967
Kacha SM, Shah SG (2021) Review of the nanomaterials used for the soil stabilization. Paper presented at the proceedings of the Indian geotechnical conference 2019, Singapore
Kacimi L, Simon-Masseron A, Ghomari A, Derriche Z (2006) Reduction of clinkerization temperature by using phosphogypsum. J Hazard Mater 137(1):129–137. https://doi.org/10.1016/j.jhazmat.2005.12.053
Kalantari B, Prasad A (2014) A study of the effect of various curing techniques on the strength of stabilized peat. Transp Geotech 1(3):119–128. https://doi.org/10.1016/j.trgeo.2014.06.002
Kassim KA, Chern KK (2018) Lime stabilized malaysian cohesive soils. Malays J Civ Eng 16(1):13–23
Ke X, Bernal SA, Ye N, Provis JL, Yang J (2015) One-part geopolymers based on thermally treated red Mud/NaOH blends. J Am Ceram Soc 98(1):5–11. https://doi.org/10.1111/jace.13231
Khale D, Chaudhary R (2007) Mechanism of geopolymerization and factors influencing its development: a review. J Mater Sci 42(3):729–746. https://doi.org/10.1007/s10853-006-0401-4
Kizhakkumodom Venkatanarayanan H, Rangaraju PR (2015) Effect of grinding of low-carbon rice husk ash on the microstructure and performance properties of blended cement concrete. Cement Concr Compos 55:348–363. https://doi.org/10.1016/j.cemconcomp.2014.09.021
Kumar GS, Ramakrishnan A, Hung YT (2007) Lime calcination. In: Advanced physicochemical treatment technologies. Humana Press, Totowa, pp 611–633
Kuryatnyk T, Angulski da Luz C, Ambroise J, Pera J (2008) Valorization of phosphogypsum as hydraulic binder. J Hazard Mater 160(2):681–687. https://doi.org/10.1016/j.jhazmat.2008.03.014
Latifi N, Marto A, Rashid ASA, Yii JLJ (2015) Strength and physico-chemical characteristics of fly ash-bottom ash mixture. Arab J Sci Eng 40(9):2447–2455. https://doi.org/10.1007/s13369-015-1647-4
Latifi N, Eisazadeh A, Marto A, Meehan CL (2017) Tropical residual soil stabilization: a powder form material for increasing soil strength. Constr Build Mater 147:827–836. https://doi.org/10.1016/j.conbuildmat.2017.04.115
Lechtanski VL (2000) Inquiry-based experiments in chemistry, pp 159–165
Lemaire K, Deneele D, Bonnet S, Legret M (2013) Effects of lime and cement treatment on the physicochemical, microstructural and mechanical characteristics of a plastic silt. Eng Geol 166:255–261. https://doi.org/10.1016/j.enggeo.2013.09.012
Lenka BP, Majhi RK, Singh S, Nayak AN (2021) Eco-friendly and cost-effective concrete utilizing high-volume blast furnace slag and demolition waste with lime. Eur J Environ Civ Eng 66:1–23. https://doi.org/10.1080/19648189.2021.1896581
Lewis RC (2018) Silica fume. In: De Belie N, Soutsos M, Gruyaert E (eds) Properties of fresh and hardened concrete containing supplementary cementitious materials: state-of-the-art report of the RILEM Technical Committee 238-SCM, Working Group 4. Springer, Cham, pp 99–121
Li C, Sun H, Li L (2010) A review: the comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem Concr Res 40(9):1341–1349. https://doi.org/10.1016/j.cemconres.2010.03.020
Li Y, Sun R, Liu C, Liu H, Lu C (2012) CO2 capture by carbide slag from chlor-alkali plant in calcination/carbonation cycles. Int J Greenhouse Gas Control 9:117–123. https://doi.org/10.1016/j.ijggc.2012.03.012
Lima C, Caggiano A, Faella C, Martinelli E, Pepe M, Realfonzo R (2013) Physical properties and mechanical behaviour of concrete made with recycled aggregates and fly ash. Constr Build Mater 47:547–559. https://doi.org/10.1016/j.conbuildmat.2013.04.051
Lollini F, Redaelli E, Bertolini L (2014) Effects of portland cement replacement with limestone on the properties of hardened concrete. Cement Concr Compos 46:32–40. https://doi.org/10.1016/j.cemconcomp.2013.10.016
Lovell CW, Ke T-C, Huang W-H, Lovell JE (1991) Bottom ash as a highway material. Transp Res Rec 1310:106–116
M145‐91 (2012) Classification of soils and soil‐aggregate mixtures for highway construction purposes. American Association of State Highway and Transportation Officials, Washington, DC
Maaitah ON (2012) Soil stabilization by chemical agent. Geotech Geol Eng 30(6):1345–1356. https://doi.org/10.1007/s10706-012-9549-7
Maclean DJ, Lewis WA (1963) British practice in the design and specification of cement-stabilized bases and subbases for roads. Highw Res Board 36:56–76
Maduabuchi MN, Obikara, FO (2018) Effect of egg shell powder (ESP) on the strength properties of cement-stabilization on Olokoro lateritic soil. J Waste Manag. https://doi.org/10.13140/RG.2.2.33016.29444
Mahvash S, López-Querol S, Bahadori-Jahromi A (2017) Effect of class F fly ash on fine sand compaction through soil stabilization. Heliyon 3(3):e00274. https://doi.org/10.1016/j.heliyon.2017.e00274
Mashri MOM, Johari MAM, Mijarsh MJA, Ahmad ZA (2020) Effect of various grades of Na2SiO3 on compressive strength development of eggshells powder-based alkaline activated mortar
Maslehuddin M, Al-Amoudi OSB, Shameem M, Rehman MK, Ibrahim M (2008) Usage of cement kiln dust in cement products—Research review and preliminary investigations. Constr Build Mater 22(12):2369–2375. https://doi.org/10.1016/j.conbuildmat.2007.09.005
Maslehuddin M, Al-Amoudi OSB, Rahman MK, Ali MR, Barry MS (2009) Properties of cement kiln dust concrete. Constr Build Mater 23(6):2357–2361. https://doi.org/10.1016/j.conbuildmat.2008.11.002
Mayooran S, Ragavan S, Sathiparan N (2017) Comparative study on open air burnt low- and high-carbon rice husk ash as partial cement replacement in cement block production. J Build Eng 13:137–145. https://doi.org/10.1016/j.jobe.2017.07.011
Meddah MS, Ismail MA, El-Gamal S, Fitriani H (2018) Performances evaluation of binary concrete designed with silica fume and metakaolin. Constr Build Mater 166:400–412. https://doi.org/10.1016/j.conbuildmat.2018.01.138
Mijan MA, Kim D-H, Kwak H-S (2014) Physicochemical properties of nanopowdered eggshell. Int J Food Sci Technol 49(7):1751–1757. https://doi.org/10.1111/ijfs.12451
Milani APdS, Labaki LC (2012) Physical, mechanical, and thermal performance of cement-stabilized rammed Earth–rice husk ash walls. J Mater Civ Eng 24(6):775–782. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000439
Miller GA, Azad S (2000) Influence of soil type on stabilization with cement kiln dust. Constr Build Mater 14(2):89–97. https://doi.org/10.1016/S0950-0618(00)00007-6
Mishra MC, Babu KS, Reddy NG, Dey PP, Rao BH (2019) Performance of lime stabilization on extremely alkaline red mud waste under acidic environment. J Hazard Toxic Radioact Waste 23(4):04019012. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000448
Mitchell JK, Hooper DR (1961) Influence of time between mixing and compaction on properties of lime stabilized expansive clay. Highw Res Board 304:14–31
Miura N, Horpibulsuk S, Nagaraj TS (2001) Engineering behavior of cement stabilized clay at high water content. Soils Found 41(5):33–45. https://doi.org/10.3208/sandf.41.5_33
Moayedi H, Aghel B, Abdullahi MAM, Nguyen H, Safuan A Rashid A (2019) Applications of rice husk ash as green and sustainable biomass. J Clean Prod 237:117851. https://doi.org/10.1016/j.jclepro.2019.117851
Mohajerani A, Burnett L, Smith JV, Markovski S, Rodwell G, Rahman MT et al (2020) Recycling waste rubber tyres in construction materials and associated environmental considerations: a review. Resour Conserv Recycl 155:104679. https://doi.org/10.1016/j.resconrec.2020.104679
Moretti L, Di Mascio P, Fusco C (2019) Porous concrete for pedestrian pavements. Water 11(10):2105
Mujah D, Shahin MA, Cheng L (2017) State-of-the-art review of biocementation by Microbially Induced Calcite Precipitation (MICP) for soil stabilization. Geomicrobiol J 34(6):524–537. https://doi.org/10.1080/01490451.2016.1225866
Muntohar A, Hantoro G (2000) Influence of rice husk ash and lime on engineering properties clayey subgrade. Electron J Geotech Eng 5:1–19
Murmu AL, Dhole N, Patel A (2020) Stabilisation of black cotton soil for subgrade application using fly ash geopolymer. Road Mater Pavement Des 21(3):867–885. https://doi.org/10.1080/14680629.2018.1530131
Nalbantoğlu Z (2004) Effectiveness of class C fly ash as an expansive soil stabilizer. Constr Build Mater 18(6):377–381. https://doi.org/10.1016/j.conbuildmat.2004.03.011
Nath BD, Molla MKA, Sarkar G (2017) Study on strength behavior of organic soil stabilized with fly ash. Int Sch Res Not 2017:5786541. https://doi.org/10.1155/2017/5786541
Navagire OP, Sharma SK, Rambabu D (2022) Stabilization of black cotton soil with coal bottom ash. Mater Today Proc 52:979–985. https://doi.org/10.1016/j.matpr.2021.10.447
Naveed M, Duan J, Uddin S, Suleman M, Hui Y, Li H (2020) Application of microbially induced calcium carbonate precipitation with urea hydrolysis to improve the mechanical properties of soil. Ecol Eng 153:105885. https://doi.org/10.1016/j.ecoleng.2020.105885
Nelson JD, Chao KC, Overton DD, Nelson EJ (2015) Soil treatment and moisture control. In: Foundation engineering for expansive soils (pp 258–294)
Netterberg F (1984) Rapid field test for carbonation of lime or cement-treated materials. Retrieved from National Institute for Transport and Road Research Report RS/2/84, Pretoria: Council for Scientific and Industrial Research
Nkoumbou C, Njoya A, Njoya D, Grosbois C, Njopwouo D, Yvon J, Martin F (2009) Kaolin from Mayouom (Western Cameroon): Industrial suitability evaluation. Appl Clay Sci 43(1):118–124. https://doi.org/10.1016/j.clay.2008.07.019
Nodehi M, Taghvaee VM (2022) Alkali-activated materials and geopolymer: a review of common precursors and activators addressing circular economy. Circ Econ Sustain 2(1):165–196. https://doi.org/10.1007/s43615-021-00029-w
Nunes MCM, Bridges MG, Dawson AR (1996) Assessment of secondary materials for pavement construction: technical and environmental aspects. Waste Manag 16(1):87–96. https://doi.org/10.1016/S0956-053X(96)00030-X
Okagbue CO, Onyeobi TUS (1999) Potential of marble dust to stabilise red tropical soils for road construction. Eng Geol 53(3):371–380. https://doi.org/10.1016/S0013-7952(99)00036-8
Olinic T, Olinic E (2016) The effect of quicklime stabilization on soil properties. Agric Agric Sci Procedia 10:444–451. https://doi.org/10.1016/j.aaspro.2016.09.013
Oliveira PJV, Correia AAS, Garcia MR (2012) Effect of organic matter content and curing conditions on the creep behavior of an artificially stabilized soil. J Mater Civ Eng 24(7):868–875. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000454
Oluwatuyi OE, Adeola BO, Alhassan EA, Nnochiri ES, Modupe AE, Elemile OO et al (2018) Ameliorating effect of milled eggshell on cement stabilized lateritic soil for highway construction. Case Stud Constr Mater 9:e00191. https://doi.org/10.1016/j.cscm.2018.e00191
Onyelowe K, Igboayaka C, Orji F, Ugwuanyi H, Van DB (2019) Triaxial and density behaviour of quarry dust based geopolymer cement treated expansive soil with crushed waste glasses for pavement foundation purposes. Int J Pavement Res Technol 12(1):78–87. https://doi.org/10.1007/s42947-019-0010-7
Onyelowe KC, Onyia ME, Nguyen-Thi D, Van Bui D, Onukwugha E, Baykara H et al (2021) Swelling potential of clayey soil modified with rice husk ash activated by calcination for pavement underlay by Plasticity Index Method (PIM). Adv Mater Sci Eng 2021:668–8519. https://doi.org/10.1155/2021/6688519
ORN31 (1993) A guide to the structural design of bitumen surfaced roads in tropical and sub-tropical countries. Transport Research Laboratory, UK
Panesar DK, Zhang R (2020) Performance comparison of cement replacing materials in concrete: limestone fillers and supplementary cementing materials—a review. Constr Build Mater 251:118866. https://doi.org/10.1016/j.conbuildmat.2020.118866
Panich V, Suksun H, Chairat T (2019) Bagasse ash–fly ash-geopolymer-treated soft Bangkok clay as subgrade material. Environ Geotech 66:1–8. https://doi.org/10.1680/jenge.19.00123
Pavía S, Regan D (2010) Influence of cement kiln dust on the physical properties of calcium lime mortars. Mater Struct 43(3):381–391. https://doi.org/10.1617/s11527-009-9496-9
Persoff P, Apps J, Moridis G, Whang JM (1999) Effect of dilution and contaminants on sand grouted with colloidal silica. J Geotech Geoenviron Eng 125(6):461–469. https://doi.org/10.1061/(ASCE)1090-0241(1999)125:6(461)
Petry TM, Little DN (2002) Review of stabilization of clays and expansive soils in pavements and lightly loaded structures—history, practice, and future. J Mater Civ Eng 14(6):447–460. https://doi.org/10.1061/(ASCE)0899-1561(2002)14:6(447)
Phanikumar BR, Ramanjaneya Raju e (2020) Silica fume stabilization of an expansive clay subgrade and the effect of silica fume-stabilised soil cushion on its CBR. Geomech Geoeng 15(1):64–77. https://doi.org/10.1080/17486025.2019.1620348
Phummiphan I, Horpibulsuk S, Sukmak P, Chinkulkijniwat A, Arulrajah A, Shen S-L (2016) Stabilisation of marginal lateritic soil using high calcium fly ash-based geopolymer. Road Mater Pavement Des 17(4):877–891. https://doi.org/10.1080/14680629.2015.1132632
Pongsivasathit S, Horpibulsuk S, Piyaphipat S (2019) Assessment of mechanical properties of cement stabilized soils. Case Stud Constr Mater 11:e00301. https://doi.org/10.1016/j.cscm.2019.e00301
Poorveekan K, Ath KMS, Anburuvel A, Sathiparan N (2021) Investigation of the engineering properties of cementless stabilized earth blocks with alkali-activated eggshell and rice husk ash as a binder. Constr Build Mater 277:122371. https://doi.org/10.1016/j.conbuildmat.2021.122371
Praticò FG, Puppala AJ (2012) Lime and cement treatments of subgrades in southern Italy: facing interports issues and challenges. Procedia Soc Behav Sci 53:389–398. https://doi.org/10.1016/j.sbspro.2012.09.890
Provis JL, Van Deventer JSJ (2009) 1—Introduction to geopolymers. In: Provis JL, van Deventer JSJ (eds) Geopolymers. Woodhead Publishing, pp 1–11
Prusinski JR, Bhattacharja S (1999) Effectiveness of Portland cement and lime in stabilizing clay soils. Transp Res Rec 1652(1):215–227. https://doi.org/10.3141/1652-28
Qubain BS, Seksinsky EJ, Li J (2000) Incorporating subgrade lime stabilization into pavement design. Transp Res Rec 1721(1):3–8. https://doi.org/10.3141/1721-01
Rahgozar MA, Saberian M, Li J (2018) Soil stabilization with non-conventional eco-friendly agricultural waste materials: an experimental study. Transp Geotech 14:52–60. https://doi.org/10.1016/j.trgeo.2017.09.004
Rahman MDA (1986) The potentials of some stabilizers for the use of lateritic soil in construction. Build Environ 21(1):57–61. https://doi.org/10.1016/0360-1323(86)90008-9
Rahman MA (1987) Effects of cement-rice husk ash mixtures on geotechnical properties of lateritic soils. Jpn Soc Soil Mech Found Eng 27(2):61–65. https://doi.org/10.3208/sandf1972.27.2_61
Ramaji AE (2012) A review on the soil stabilization using low-cost methods. J Appl Sci Res 8(4):2193–2196
Ranjbar N, Kuenzel C (2017) Cenospheres: a review. Fuel 207:1–12. https://doi.org/10.1016/j.fuel.2017.06.059
Rao SM, Shivananda P (2005) Role of curing temperature in progress of lime-soil reactions. Geotech Geol Eng 23(1):79. https://doi.org/10.1007/s10706-003-3157-5
Raza SA, Chandra D (1995) Strength of soil-flyash mixtures with geotextile reinforcement. Paper presented at the Indian geotechnical conference (IGC 95)
Reddy MS, Dinakar P, Rao BH (2016) A review of the influence of source material’s oxide composition on the compressive strength of geopolymer concrete. Microporous Mesoporous Mater 234:12–23. https://doi.org/10.1016/j.micromeso.2016.07.005
Rezaeimalek S, Huang J, Bin-Shafique S (2017) Evaluation of curing method and mix design of a moisture activated polymer for sand stabilization. Constr Build Mater 146:210–220. https://doi.org/10.1016/j.conbuildmat.2017.04.093
Ribeiro D, Néri R, Cardoso R (2016) Influence of water content in the UCS of soil-cement mixtures for different cement dosages. Procedia Eng 143:59–66. https://doi.org/10.1016/j.proeng.2016.06.008
Rimal S, Poudel RK, Gautam D (2019) Experimental study on properties of natural soils treated with cement kiln dust. Case Stud Constr Mater 10:e00223. https://doi.org/10.1016/j.cscm.2019.e00223
Rivera JF, Orobio A, Cristelo N, Mejía de Gutiérrez R (2020) Fly ash-based geopolymer as A4 type soil stabiliser. Transp Geotech 25:100–409. https://doi.org/10.1016/j.trgeo.2020.100409
Rocha CGD, Marin EJB, Samaniego RAQ, Consoli NC (2021) Decision-making model for soil stabilization: minimizing cost and environmental impacts. J Mater Civ Eng 33(2):06020024. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003551
Rogers CDF, Stephanie G, Roff TEJ (1997) Lime modification of clay soils for construction expediency. Proc Inst Civ Eng Geotech Eng 125(4):242–249. https://doi.org/10.1680/igeng.1997.29660
Saadaoui E, Ghazel N, Ben Romdhane C, Massoudi N (2017) Phosphogypsum: potential uses and problems—a review. Int J Environ Stud 74(4):558–567. https://doi.org/10.1080/00207233.2017.1330582
Saberian M, Li J, Nguyen B, Wang G (2018) Permanent deformation behaviour of pavement base and subbase containing recycle concrete aggregate, coarse and fine crumb rubber. Constr Build Mater 178:51–58. https://doi.org/10.1016/j.conbuildmat.2018.05.107
Saldanha RB, da Rocha CG, Caicedo AML, Consoli NC (2021) Technical and environmental performance of eggshell lime for soil stabilization. Constr Build Mater 298:123648. https://doi.org/10.1016/j.conbuildmat.2021.123648
Sariosseiri F, Muhunthan B (2009) Effect of cement treatment on geotechnical properties of some Washington State soils. Eng Geol 104(1):119–125. https://doi.org/10.1016/j.enggeo.2008.09.003
Sathiparan N (2021) Utilization prospects of eggshell powder in sustainable construction material—a review. Constr Build Mater 293:123465. https://doi.org/10.1016/j.conbuildmat.2021.123465
SCA/5 (2009) Standard Specifications for Construction and Maintenance of roads and bridges. Institute for Construction Training and Development (ICTAD), Sri Lanka
Shafiei A, Latifi Namin M (2014) Experimental investigation on the effect of hydrated lime on mechanical properties of SMA. Constr Build Mater 70:379–387. https://doi.org/10.1016/j.conbuildmat.2014.07.084
Sharma AK, Sivapullaiah PV (2016) Ground granulated blast furnace slag amended fly ash as an expansive soil stabilizer. Soils Found 56(2):205–212. https://doi.org/10.1016/j.sandf.2016.02.004
Sharma V, Vinayak HK, Marwaha BM (2015) Enhancing compressive strength of soil using natural fibers. Constr Build Mater 93:943–949. https://doi.org/10.1016/j.conbuildmat.2015.05.065
Shekhawat P, Sharma G, Singh RM (2019) Strength behavior of alkaline activated eggshell powder and flyash geopolymer cured at ambient temperature. Constr Build Mater 223:1112–1122. https://doi.org/10.1016/j.conbuildmat.2019.07.325
Sherwood P (1993) Soil stabilization with cement and lime. Transport Research Laboratory, Her Majesty Stationary Office, London
Singh NB (2018) Fly ash-based geopolymer binder: a future construction material. Minerals 8(7):299
Singh A, Singh B (2021) Characterization of rice husk ash obtained from an industrial source. J Sustain Cem Based Mater 10(4):193–212. https://doi.org/10.1080/21650373.2020.1789010
Singh B, Ishwarya G, Gupta M, Bhattacharyya SK (2015) Geopolymer concrete: a review of some recent developments. Constr Build Mater 85:78–90. https://doi.org/10.1016/j.conbuildmat.2015.03.036
Singh P, Kumar Dash H, Samantaray S (2020) Effect of silica fume on engineering properties of expansive soil. Mater Today Proc 33:5035–5040. https://doi.org/10.1016/j.matpr.2020.02.839
Solanki P, Zaman MM, Dean J (2010) Resilient modulus of clay subgrades stabilized with lime, class C fly ash, and cement kiln dust for pavement design. Transp Res Rec 2186(1):101–110. https://doi.org/10.3141/2186-11
Soltani A, Taheri A, Khatibi M, Estabragh AR (2017) Swelling potential of a stabilized expansive soil: a comparative experimental study. Geotech Geol Eng 35(4):1717–1744. https://doi.org/10.1007/s10706-017-0204-1
Sridharan A, Prashanth JP, Sivapullaiah PV (1997) Effect of fly ash on the unconfined compressive strength of black cotton soil. Proc Inst Civ Eng Ground Improv 1(3):169–175. https://doi.org/10.1680/gi.1997.010304
Standard Specifications for Transportation materials and Methods of Sampling and Testing (2015) American Association of State Highway and Transportation Officials, Washington, DC
Stocker PT (1972) Diffusion and diffuse cementation in lime and cement stabilised clayey soils—chemical aspects. Aust Road Res 5(9):6–47
Stocker PT (1974) Diffusion and diffuse cementation in lime and cement stabilised clay soils—studies of plasticity and aggregation. Aust Road Res 5(6):51–75
Sutcu M, Erdogmus E, Gencel O, Gholampour A, Atan E, Ozbakkaloglu T (2019) Recycling of bottom ash and fly ash wastes in eco-friendly clay brick production. J Clean Prod 233:753–764. https://doi.org/10.1016/j.jclepro.2019.06.017
Tan T, Huat BK, Anggraini V, Shukla SK (2021a) Improving the engineering behaviour of residual soil with fly ash and treated natural fibres in alkaline condition. Int J Geotech Eng 15(3):313–326. https://doi.org/10.1080/19386362.2018.1564854
Tan T, Huat BBK, Anggraini V, Shukla SK, Nahazanan H (2021b) Strength behavior of fly ash-stabilized soil reinforced with coir fibers in alkaline environment. J Nat Fibers 18(11):1556–1569. https://doi.org/10.1080/15440478.2019.1691701
Tastan EO, Edil TB, Benson CH, Aydilek AH (2011) Stabilization of organic soils with fly ash. J Geotech Geoenviron Eng 137(9):819–833. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000502
Teerawattanasuk C, Voottipruex P (2019) Comparison between cement and fly ash geopolymer for stabilized marginal lateritic soil as road material. Int J Pavement Eng 20(11):1264–1274. https://doi.org/10.1080/10298436.2017.1402593
Temuujin J, van Riessen A, Williams R (2009) Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J Hazard Mater 167(1):82–88. https://doi.org/10.1016/j.jhazmat.2008.12.121
Terashi M, Juran I (2000) Ground improvement—state of the art. Paper presented at the international symposium 2000, Lisbon
Thiery M, Villain G, Dangla P, Platret G (2007) Investigation of the carbonation front shape on cementitious materials: effects of the chemical kinetics. Cem Concr Res 37(7):1047–1058. https://doi.org/10.1016/j.cemconres.2007.04.002
Thomas BS, Kumar S, Arel HS (2017) Sustainable concrete containing palm oil fuel ash as a supplementary cementitious material—a review. Renew Sustain Energy Rev 80:550–561. https://doi.org/10.1016/j.rser.2017.05.128
Tingle JS, Santoni RL (2003) Stabilization of clay soils with nontraditional additives. Transp Res Rec 1819(1):72–84. https://doi.org/10.3141/1819b-10
Tsivilis S, Tsantilas J, Kakali G, Chaniotakis E, Sakellariou A (2003) The permeability of Portland limestone cement concrete. Cem Concr Res 33(9):1465–1471. https://doi.org/10.1016/S0008-8846(03)00092-9
Türköz M, Umu SU, Öztürk O (2021) Effect of silica fume as a waste material for sustainable environment on the stabilization and dynamic behavior of dispersive soil. Sustainability 13(8):4321
Van Jaarsveld JGS, Van Deventer JSJ, Lorenzen L (1997) The potential use of geopolymeric materials to immobilise toxic metals: part I. Theory Appl Minerals Eng 10(7):659–669. https://doi.org/10.1016/S0892-6875(97)00046-0
Xiao H, Lee FH, Chin KG (2014) Yielding of cement-treated marine clay. Soils Found 54(3):488–501. https://doi.org/10.1016/j.sandf.2014.04.021
Xiao R, Polaczyk P, Zhang M, Jiang X, Zhang Y, Huang B, Hu W (2020) Evaluation of glass powder-based geopolymer stabilized road bases containing recycled waste glass aggregate. Transp Res Rec 2674(1):22–32. https://doi.org/10.1177/0361198119898695
Yaghoubi M, Arulrajah A, Disfani MM, Horpibulsuk S, Darmawan S, Wang J (2019) Impact of field conditions on the strength development of a geopolymer stabilized marine clay. Appl Clay Sci 167:33–42. https://doi.org/10.1016/j.clay.2018.10.005
Yip CK, Lukey GC, van Deventer JSJ (2005) The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem Concr Res 35(9):1688–1697. https://doi.org/10.1016/j.cemconres.2004.10.042
Zhang G (2007) Soil Nanoparticles and their influence on engineering properties of soils. In: Advances in measurement and modeling of soil behavior, pp 1–13
Zhang L (2013) Production of bricks from waste materials—a review. Constr Build Mater 47:643–655. https://doi.org/10.1016/j.conbuildmat.2013.05.043
Zhang G, He J, Gambrell RP (2010) Synthesis, characterization, and mechanical properties of red mud-based geopolymers. Transp Res Rec 2167(1):1–9. https://doi.org/10.3141/2167-01
Zhang J, Liu G, Chen B, Song D, Qi J, Liu X (2014a) Analysis of CO2 emission for the cement manufacturing with alternative raw materials: a LCA-based framework. Energy Procedia 61:2541–2545. https://doi.org/10.1016/j.egypro.2014.12.041
Zhang RJ, Lu YT, Tan TS, Phoon KK, Santoso AM (2014b) Long-term effect of curing temperature on the strength behavior of cement-stabilized clay. J Geotech Geoenviron Eng 140(8):04014045. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001144
Zhou H, Bhattarai R, Li Y, Si B, Dong X, Wang T, Yao Z (2022) Towards sustainable coal industry: turning coal bottom ash into wealth. Sci Total Environ 804:149–985. https://doi.org/10.1016/j.scitotenv.2021.149985
Zumrawi MME (2015) Stabilization of pavement subgrade by using fly ash activated by cement. Am J Civ Eng Architect 3(6):218–224. https://doi.org/10.12691/ajcea-3-6-5
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Arulanantham Anburuvel: Conceptualization, Investigation, Formal analysis, Writing—original draft, Writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anburuvel, A. The Engineering Behind Soil Stabilization with Additives: A State-of-the-Art Review. Geotech Geol Eng 42, 1–42 (2024). https://doi.org/10.1007/s10706-023-02554-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-023-02554-x