Abstract
Expansive soils for residential projects are often treated with liquid ionic soil stabilizer (LISS) using deep pressurized injection method. Liquid ionic soil stabilizers have a long history of application in practice. However, there is limited knowledge and experimental evidence of their stabilization mechanism and effectiveness. This paper summarizes the research that investigated the effects of liquid ionic stabilizers on key engineering properties of a highly expansive clay from Carrollton, Texas, through comprehensive physical and mechanical testing and microscopic observation of untreated and treated soils. Test results before and after treatment were analyzed for two different treatment ratios: 1:300 and 1:150, showing a 53% swell reduction for treated soil compacted at the optimum moisture content (OMC) for treated soil, and 25% swell reduction for treated soil compacted at the OMC for the untreated soil. There was no significant improvement in unconfined compressive strength; noticeable improvement in stiffness was observed. The microscopic analysis showed a marked change in morphology and quantitative element composition, thus suggesting the occurrence of a reasonable degree of stabilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pavement distress and foundation failure are common in expansive soils and result in significant expenditures for repair and maintenance. The United States suffers billions of dollars of economic loss each year due to expansive soil problems. Chemical stabilization of these soils is usually achieved by using traditional stabilizers such as lime, cement, and fly ash. The underlying stabilization mechanisms are well known, and the standards for practice are properly documented for these stabilizers. However, they are expensive: the production of 1-ton ordinary Portland cement requires up to 150 kWh of energy, which is almost 40% of the total production cost (Khadka 2017). In addition, they are calcium-based; hence their use in sulfate-rich soils leads to excessive swelling and heaving (Hunter 1998). As the calcium present in the stabilizer reacts with sulfate and alumina in the soil, it forms a series of calcium–aluminum–sulfate hydrates; and the formed minerals, namely ettringite and thaumasite, cause a substantial increase in volume.
Non-traditional stabilizers are a potentially feasible alternative to traditional calcium-based stabilizers. They are classified into several categories: ionic, enzyme, lignosulfonates, salts, petroleum, resins, polymers, and tree resins (Tingle 1989). Liquid ionic stabilizers are non-calcium-based stabilizers, which are hypothesized to work through cation exchange and flocculation of the clay minerals that occur as a result of a change in electrolyte concentration of the pore fluid (Scholen 1992, 1995). They are intended to mimic the transformation of clay minerals into a stable phase that is expected to occur in nature over a geological timescale. The cations attached to the surface of the clay particles are replaced by liquid ionic stabilizer with small hydration cations (Xiang 2010). The process reduces the surface charge density on the clay surface and compresses the thickness of double-layer water (Liu et al. 2009). The composition of a particular liquid ionic stabilizer varies with the manufacturer; because of proprietary issues, its exact composition is seldom disclosed. They are diluted and used in conjunction with secondary products like surfactants, catalysts and UV inhibitors (Tingle 1989). The use of secondary additives can facilitate the primary stabilization mechanism by introducing a secondary stabilization mechanism. For instance, the surfactant can improve the wetting of soil particles by altering the surface charge on these particles and hence lowering the surface tension of the pore fluid.
Liquid ionic stabilizers are produced from acid concentrates diluted in water and readily applicable in the field. About 10,000 square feet of the site area can be treated to depth 10–15 feet in one single day. The transportation cost for the stabilizer is also relatively low (Katz 2001). Previous studies have demonstrated the ability of liquid ionic stabilizers to reduce the plasticity index (PI) of expansive soils and increase their strength (He 2018; Kushwaha et al. 2019; Lu and Xia 2015). Lu and Xia (2015) found that the strength of an expansive soil from Anyang, China, after treatment increased with curing days, with the maximum increase occurring in 28 days. He (2018) tested an expansive soil from Dallas, Texas, demonstrated the positive effects of the stabilizers on reducing PI, swell and increasing the unconfined compressive strength which was all improved with an increased rate of application and number of curing days. However, despite some promising results observed in the past, engineers continue to be reluctant to generalize the use of liquid ionic stabilizers, primarily because of the relative dearth of literature explaining their working mechanism. There are also differences in the test methods followed in the laboratory and the field. Consequently, the existing experimental evidence is not sufficiently reassuring as an indicator of actual field performance (Petry 1997). Rauch et al. (2003) stated that the differences in moisture content and dry unit weight among the treated and untreated soil specimens affect the interpretation of the test results. “This effect appeared to be more significant than the effects of chemical treatments (Rauch et al. 2003).” This inconclusive findings from the previous studies have been the chief motivation for the present work.
In this work, an expansive soil from Carrollton, Texas, was selected for the experimental program. The laboratory experiments included Atterberg limits, sieve analysis, standard compaction, swell test, and unconfined compressive strength test. For the swell test, the soil specimens were compacted at different moisture content and density determined from soil compaction tests on both treated and untreated soils. Microanalysis of the soil was conducted to assess soil fabric and chemical element changes in the treated soil via a scanning electron microscope (SEM) and energy dispersive spectrometer (EDS) based analyses.
2 Materials
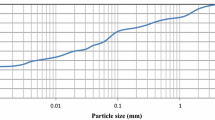
Expansive soil was collected from a depth of about 4 feet below the ground surface in Carrollton, Texas. The soil was classified as fat clay (CH) as per USCS. The grain size distribution of the soil is shown in Fig. 1, and the basic properties are listed in Table 1. More than 80% of the soil is finer than No. 200 sieve, with clay fraction accounting for 10%. The soil passing through No. 40 sieve was used for the experiments unless otherwise mentioned.
A concentrated liquid ionic stabilizer (LISS), locally available as a commercial product and comprised of sulfuric, phosphoric, and citric acids were selected. The following describes the application procedure of the LISS in the field. The acid concentrate is diluted with water and surfactant (an additive) at a selected job site before it is applied to the soil. The supplier recommends a dilution ratio of 1:300 (hereafter termed as the treatment ratio), which means that 3.79 dm3 (1 gallon) of the concentrated chemical should be mixed with 1135.62 dm3 (300 gallons) of water and 42.5 g of surfactant. For field application, the solutions of liquid ionic stabilizer, water, and surfactant are mixed in a large container and are pumped through a pipe to the hollow perforated drilling rods previously driven into the soil, as shown in Fig. 2. The foundation soil is treated with water only before the chemical injection, termed as pre-conditioning. During the chemical injection, five rods supply the diluted chemical solution through high-pressure injection at varying depths, usually extending up to 3.048 m (10 feet) depth. The injection process will be repeated until the required swell reduction is achieved.
For this lab study, two treatment ratios were initially selected. One followed the manufacture dilution ratio of 1:300 described above, and another doubled the concentration of the solution (1:150). Table 2 provides the doses of each component of the liquid ionic stabilizer used in the lab treatments. The sample preparation protocol was not intended to fully replicate the field procedure, as shown in Fig. 2; instead, a simplified approach of treatment, i.e., mixing the soil with the diluted stabilizer, was adopted. Before preparing samples for laboratory tests, oven-dried soil was mixed with the stabilizer solution at a ratio determined by the target water content to prepare wet soil samples needed for different lab tests. The wet mixed samples were allowed to equilibrate thoroughly under controlled humidity.
3 Stabilizer Effects on Atterberg’s Limit
3.1 Test Procedures (LL, PL, PI)
The ASTM D4318 standard was followed in performing Atterberg limit tests on control and treated soils. As per the guidelines, about 150 g of soil passing through number 40 sieve was used for this purpose. The dry soil was mixed with a certain amount of diluted liquid stabilizer to obtain a moisture content slightly below the liquid limit. The mixed soil was kept in a humidity-controlled room overnight in order for the stabilizer to react with the clay particles. After moisture was equilibrated, the liquid limit test was done by further mixing soil with the same diluted stabilizer. For the plastic limit, a small amount of soil prepared at the consistency of the liquid limit was allowed to dry, and the test was performed according to the standards.
3.2 Results
The liquid limit and the plastic limit of the soil relate to its consistency. Table 3 shows the result of LL and PL tests on control and treated soils. As shown in the table, the plastic limit of the soil is relatively constant before and after the treatment, whereas there is a slight change in the liquid limit with the treatment. The change, however, is not consistent as there is a slight increase in liquid limit with 1:300 treatment ratio and a decrease with 1:150 treatment ratio. The change is not significant and may not be attributed solely to the effect of stabilizer, considering the test uncertainty.
4 Stabilizer Effects on Compaction Properties
4.1 Test Procedures (OMC, MDD)
The standard Proctor compaction test was performed to assess the relationship between the moisture content and dry density of the soil before and after the treatment according to ASTM D698. In previous studies on ionic liquid stabilizers, the standard proctor test was performed only on the untreated soil, and both the treated and untreated soil specimens were compacted at the same target optimum moisture content and maximum dry density determined from the untreated soil (Rauch et al. 2003). As the LISS was reported to affect soil compaction characteristics, a separate optimum water content for compaction of the untreated and treated soil samples was determined (He 2018). In this study, Proctor test was performed on both untreated and treated soils. The treatment of the soil was achieved by mixing the soil with diluted stabilizer solution at different moisture content and then keeping the treated soil in the moisture room to allow time for reaction. The standard Proctor test was then carried out on the following day by compacting the samples in three layers in a standard mold, as per the standard.
4.2 Results
Figure 3 shows the moisture–density curve for control and treated soil. For the treated soil, the mixture at different water content was kept overnight before compaction to ensure the reaction between soil and stabilizer has taken place. It is evident from Fig. 3 that optimum moisture content (OMC) increased and maximum dry density (MDD) decreased after treatment. A liquid ionic stabilizer needs water for an initial reaction to occur, so the increase in the OMC may have occurred because of the additional requirement of the water needed to complete the reaction. The graphs show that MDD was slightly higher for the 1:150 treatment ratio than for the 1:300 treatment ratio.
5 Stabilizer Effects on Swell Properties
5.1 Test Procedures (1-D Swell)
The one-dimensional swell test was used to determine the swell potential of control and treated soil. It is easier to perform than the three-dimensional volume change, and it quickly identifies the expansive nature of the soil. Samples with a diameter of 6.35 cm (2.5 in.) and a height of 2.54 cm (1 in.) were prepared in a single layer by static compaction via a load frame. All control and treated samples were prepared at its respective optimum moisture content (OMC) and 95% of maximum dry density (MDD), as per Fig. 3. Treated samples were also prepared at OMC and 95% of MDD of the control sample. 95% of MDD was chosen to represent a typical compaction requirement in the field. The samples were placed into an oedometer ring under a small token load of 1 psi. The sample was inundated with the distilled water, and the vertical swelling of the sample was recorded via a dial gauge. Dial readings were taken until they became virtually constant, after 3 days.
5.2 Results
Figure 4 shows the results of the one-dimensional swell test for the samples prepared at their respective OMC and 95% of MDD. It is evident that the swell potential of the control soil is rather high, as expected, reaching a value of 9.25%. However, after treatment, the swell potential (swell strain) was reduced to almost 4%. It can also be observed that neither the number of curing days nor the percentage of treatment ratios had a significant effect on the swell potential of the samples. The reduction in swell strain was about 53% of the original swell; however, the residual swell of 4% after treatment was still relatively high.
Another observation is that there were variations in the initial condition of the sample. The control sample had lower OMC and higher MDD, whereas the treated sample had higher OMC and lower MDD as shown in Fig. 3. The initial soil moisture content and degree of compaction affect the swell of expansive soil. Two more compacted soil specimens, one treated at 1:150 ratio and one untreated, were prepared by swapping the moisture content and dry unit of treated and untreated sample. The treatment ratio used for this purpose was 1:150, which has a higher concentration of chemicals compared to 1:300 treatment ratio. The purpose of the two new specimens was to eliminate the effect of moisture content and dry unit weight or degree of compaction on the effect of swell. The results are shown in Fig. 5.
As observed in Fig. 6, the control soil, when compacted at higher OMC (27%) and lower dry unit weight (13.8 kN/m3), yielded lower swell potential. The one-dimensional swell potential was only 5.35% as opposed to the initial value of 9.25%. However, the swell potential observed for treated soil with 1:150 treatment ratio at lower OMC (23%) and higher dry unit weight (14.5 KN/m3) was much higher, rising to 9.38% which is virtually the same value observed for control soil in the first case. When the swell potential of untreated soil at higher moisture content and lower dry unit weight (i.e., OMC and MDD of treated soil) is compared with that of treated soil compacted at the same moisture and dry unit weight, there is a net reduction of the swell potential of only 1.35%, a reduction of 25% of the original swell (5.35%). Therefore, it can be concluded that if the treated and control specimens are compacted at the same moisture and density, the swell reduction will be much less evident than when they are compacted at their OMC and MDD, respectively. Therefore, it is preferred to compact the treated soil according to the compaction curve of the treated soil, not the compaction curve of the virgin soil, as previously tested by Rauch et al. (2003). The treated soils were compacted at the same moisture and density as the control soil in the study by Rauch et al. (2003), and it is concluded that the treated soils had an insignificant swell reduction, which agrees with the findings of this study.
6 Stabilizer Effects on Unconfined Compressive Strength
6.1 Test Procedures (UCS)
Unconfined compressive strength (UCS) was performed on control and treated specimens to study the effect of stabilizer on the strength of the soil, according to ASTM D2166. Specimens of 7.26 cm (2.86 in.) diameter and 14.73 cm (5.8 in.) height were prepared by static compaction. Specimens of three types of soil, i.e., control soil, soil treated at 1:300 ratio, and soil treated at 1:150 ratio, were prepared for UCS tests. All the specimens were compacted at their respective OMC and 95% of their respective MDD, as per Fig. 3. The compaction was achieved in three layers to ensure that each layer was compressed properly. Before adding the second layer, the upper surface of the already-compacted first layer was scratched to ensure bonding between the successive layers. The treated specimens were allowed to cure in the humidity-controlled room for 7 days and 28 days prior to UCS testing. In each case, the average strength was assessed by testing at least two identically prepared specimens. Figure 6 shows a typically failed specimen.
6.2 Results
Figures 7 and 8 show the results of UCS tests performed on control and treated specimens for different curing days. Table 4 summarizes the UCS values and the corresponding moisture content and compaction density. Modulus at 50% of peak strain (E50) is also prested in Table 4 to quantify the effect of treatment on modulus. The average UCS results are presented in Fig. 9. In general, the results show that there is a decrease in soil strength after treatment. The maximum strength obtained with the control soil was 331 kPa (48 psi), which after treatment with 1:300 treatment ratio was reduced to less than 250 kPa (36 psi), regardless of curing time. After 1-day curing, the strength decreased by 40%, to almost 21 kPa (31 psi). The strength increase of the treated specimens occurs for specimens cured for 7 days. After 7 days curing, the increase of curing time has negligible effect on the strength. The maximum gain of strength is 36 kPa for 28 days of curing. Likewise, UCS results with 1:150 treatment ratio shows a loss of strength after treatment. Compared to 1:300 treatment ratio, however, there was an increase in strength, but the strength gain observed was still less than that of the control soil. Again, the effect of curing was not significant. The modulus E50 at 7 and 28 days after treatment slightly increased; However, the modulus E50 at 1 day after treatment slightly decreased as compared to the untreated samples.
Since the specimens were prepared at different OMCs and MDDs, the variation in strength could have been a function of various factors. The specimens for 1:300 and 1:150 treatment ratios were compacted at higher moisture content and lower dry density as compared with that of the control soil (see Table 4). The high moisture content and low dry density can lead to a decrease in UCS. Therefore, it is not clear whether the stabilizer caused the actual loss in strength, or whether it was due to variations in moisture content and density of the specimens prepared before and after treatment. Additional treated specimens were prepared by reducing the moisture content and increasing the maximum dry density so that all of the treated and control specimens had the same moisture content and dry density as the control soil specimen, i.e., moisture content and dry density equal to 23% and 14.5 kN/m3 (95% of MDD of the control soil) respectively. The results are shown in Fig. 10, indicating an increase in UCS strength with the new combination of moisture and density for the treated specimen. The new strength is compared to that of control soil; hence no apparent loss of strength is experienced under these particular compaction specifications. Both treated specimens exhibit higher modulus E50 and smaller strain at peak strength. The treated soils at the dosage of 1:150 have shown improved modulus, and the improvement slightly increases with the increase of curing time.
7 Stabilizer Effects on Soil Fabric and Structure
7.1 Test Procedures (SEM)
SEM imaging is a high-resolution imaging technique that can be used for morphological examination of solid objects. It has a very high resolution of about 1 nm and can be used in observing the fabric arrangement of the soil sample. In this work, SEM imaging of control and treated soil samples were obtained to investigate whether the liquid ionic treatment made any morphological changes. SEM imaging requires special sample preparation, as the technique involves direct reflection of electrons from the surface of the soil (Sides and Barden 1970). Wrongly prepared samples may lead to damages of the soil structure and thus erroneous interpretation of false voids, fractures, particle structures.
For the preparation of the specimen, a small, cube-shaped sample was cut out of the compacted specimen with a sharp blade. It was then air-dried, as the samples with moisture are rejected by the SEM devices. After drying, the surface of the sample was peeled off with tape about 50 times to remove any broken debris from the surface, which would result in a misleading assessment of the changes in the fabric of the specimen. The sample was then mounted on an aluminum stub, with the help of double-sided carbon tape, and coated with the silver in CRC-100 sputtering system at a pressure below 0.001 millitorrs. The sample was coated to avoid charging the sample, which would cause the glazing of the resulting images. Finally, the sample was observed under a field-emission and scanning electron microscope. A Hitachi S-4800 field-emission scanning electron microscope and an S-3000N scanning electron microscope were used for imaging.
7.2 Results
The effect of a liquid ionic stabilizer, if any, would be evidenced by a change in the morphology of the treated soil, and hence the soil fabric. The SEM images in Fig. 11 were taken at 30 kV voltage and 5000 magnification, whereas the FE-SEM images in Fig. 12 were taken at 15 kV voltage and 5000 magnification. As can be observed in Fig. 11, there were some noticeable changes in the soil’s morphology before and after the treatment with 1:300 ratio. The flakes that were observed before the treatment were not present in the second picture, and there was a bead-like appearance in the soil after treatment. Similarly, as observed in Fig. 12, treatment with 1:150 treatment ratio showed changes in the morphology before and after the treatment. The particles seem to be closer together with the treatment, and the somewhat flaky edges that could be seen in the images before treatment were not visible after treatment. The flaky characteristics at the edges are typical of smectite group of minerals and often suggest expansive behavior of soil. Katz (2001) performed SEM analyses of samples of sodium montmorillonite clay treated and untreated by sulfonated oil electrolyte, an ionic stabilizer. The treatment at a mass application ratio of 1:6000 caused “some changes in the clay particles, especially on the edges of the flakes.”
8 Stabilizer Effects on Soil Mineral Composition
8.1 Test Procedures (CEC, SSA, TP, EDS)
The most important clay minerals that might be present in the soil are montmorillonite (M), illite (I), and kaolinite (K). Montmorillonite is an expansive mineral under smectite group and has a very high potential for swelling under wet conditions. Quantification of these minerals helps in understanding the expansive behavior of soil. Quantification of major minerals in the control soil sample was done indirectly through the measurement of cation exchange capacity (CEC), specific surface area (SSA), and total potassium (TP) of the soil by using the following correlations (Chittoori 2008):
Chapman (1965) presented a method for measuring CEC of the soil by saturating and washing it with ammonium acetate. The procedure involves the replacement of cations in the soil with ammonium acetate and measuring the ammonium acetate concentration in potassium chloride solution by further washing ammonium acetate in the soil with iso-propanol and potassium chloride solution. CEC was measured for 25 g of dry soil finer than sieve number 40.
The method for determining SSA of soil was first introduced by Dyal and Hendricks (1950), which was then modified by Carter et al. (1965). Applying this method, approximately 1.1 g of dry soil passing sieve number 40 was saturated with about 3 ml of EGME and the excess EGME over the surface of the soil was removed in a vacuum desiccator. The desiccant used for this purpose was EGME-CaCl2. The process took more than 48 h to reach constant weight.
The total potassium in the soil was measured through EDS. EDS directly gives the percentage of varied elements present in the soil. The characteristic x-ray released during the transition of electrons is peculiar to each element. The energy level is associated with each element, and hence the elements can be identified. The SEM setup discussed in Sect. 7 was also equipped with an energy dispersive spectrometer (EDS). An EDS analysis was performed on the samples of control and treated powder soil, passing through No. 200 sieve. The sample was mounted on aluminum stub with double-sided carbon tape before it was placed into the equipment. EDS also provides relevant information about the weathering of silica through observation of the Al:Si ratio. In the natural weathering process, silica is released from the clay and hence the Al:Si ratio increases. On the other hand, if Al is selectively extracted from the clay, the Al:Si ratio decreases (Rauch et al. 2003). The suggested mechanism of cationic exchange means that selective extraction of aluminum is followed by the decrease in the Al:Si ratio. In this work, the Al:Si ratio was determined by using the average weight percentage of the scanned matrix determined by the EDS analysis.
8.2 Results
The average cation exchange capacity of control soil was obtained to be 120.7 meq/100 g of soil. The specific surface area and total potassium were measured to be 181 m2/g and 2.06% respectively. Accordingly, montmorillonite, illite, and kaolinite comprised 43%, 34%, and 23% respectively of the soil minerals. As observed, montmorillonite was highest in composition among the major minerals which support the expansive behavior of soil. Flaky edges of soil sample observed in the SEM imaging in Fig. 11 can be attributed to the dominant presence of montmorillonite in soil.
Figure 13 shows the graphic outputs obtained from the EDS analysis of the control and the treated soil which plot the x-ray counts versus energy. Energy peaks correspond to the various elements in the observed sample and the intensity (counts/s) indicates the concentration of that element (Rauch et al. 2003). Tables 5, 6, 7 and 8 show the percentage composition of the major elements, including oxygen, silicon, aluminum, magnesium, potassium, calcium, and iron in the control and the treated soil. There was a slight decrease in the Al:Si ratio from 0.46 to 0.42 and 0.43 after the treatment with the 1:300 and 1:150 treatment ratio. However, the change is not statistically significant based on the sample variations observed in Tables 5, 6, 7 and 8. Similar findings were reported on the EDS analyses of the ionic treated soils by Rauch et al. (2003).
9 Summary and Conclusions
An expansive soil from Carrollton Texas was selected and tested with a liquid ionic soil stabilizer. The laboratory test results can be summarized as follows:
-
Montmorillonite, illite, and kaolinite comprised 43%, 34%, and 23% of the soil minerals.
-
Atterberg’s limits of the Carrollton soil were insensitive to the variation of treatment ratio. Slight changes in liquid limit were observed; however, the results were insignificant and inconsistent.
-
The ionic soil stabilizer altered the soil compaction curve by increasing the OMC from 23 to 27% and decreasing the MDD from 15.2 to 14.3 kN/m3 (1:300 treatment ratio) and 14.5 kN/m3 (1:150 treatment ratio).
-
About 53% swell reduction was observed for control and treated soil specimens when compacted at their respective OMC and MDD. However, the effect seems to be more pronounced because of the difference in OMC and MDD of the treated and control sample. When compared with control soil compacted at the OMC and maximum dry unit weight of the treated soil, the swell reduction is only as much as 25%.
-
When the soil is treated during compaction, the compaction curve of the treated soil should be used to selected moisture and compaction density to achieve maximum swell reduction.
-
The unconfined compressive strength slightly decreased after the treatment when the treated soils were compacted according to the compaction curve of treated soil. The unconfined compressive strength of treated soils is close to the untreated soil when the treated soils were compacted according to the compaction curve of the untreated soil. However, the modulus E50 of the treated soil increased after 7 days of curing for both compaction method.
-
Modifications of soil fabric and structure were observed in the SEM images. The modifications include the disappearance of the flaky structures after treatment. Though not significant, evidence of mineralogical changes of expansive clay minerals to a more stable phase was also found from the EDS results.
Approximately 25% swell reduction was observed when the treated and control soil specimens were compacted at the same OMC and MDD of the control soil. The reduction is much less as compared with the treated soils compacted at its own OMC and MDD. The possible reasons could be inertness of the stabilizer to undergo any reaction because of the insignificant dose of the stabilizer used. The mass application ratio at the applied dilution is relatively low. Field application wets the soil to near saturation and swell tests are performed after about 2 weeks of saturation. During the wetting of the soil, the soil is allowed to swell and resulted in a relatively low density. This means the application mass ratio for the soil in the field is relatively high. The moisture condition in the lab is near to optimum only, which means the mass application ratio in the laboratory could be low. This could be the reason for less impact seen in the laboratory results as opposed to the effective field performance of the stabilizer. Future laboratory studies should focus on minimizing the discrepancies in the procedures followed between laboratory and field so that more solid conclusions can be drawn.
References
Carter DL, Heilman MD, Gonzalez CL (1965) Ethylene glycol monoethyl ether for determining surface area of silicate minerals. Soil Sci 100(5):356–360
Chapman HD (1965) Cation-exchange capacity. In: Black CA (ed) Methods of soil analysis-Chemical and microbiological properties. SSSA, Madison, pp 891–901
Chittoori BC (2008) Clay mineralogy effects on long-term performance of chemically treated expansive clays. University of Texas at Arlington, Arlington, p 326
Dyal RS, Hendricks SB (1950) Total surface of clays in polar liquids as a characteristic index. Soil Sci 69(6):503–509
He S (2018) Expansive soil treatment with ionic soil stabilizer. Transp Res Rec J Transp Res Board 2672:185–194
Hunter D (1998) Lime-induced heave in sulfate-bearing clay soils. J Geotech Eng 114(2):150–167
Katz LE (2001) Mechanisms of soil stabilization with liquid ionic stabilizer. Transp Res Rec 1757:50–57
Khadka SD (2017) Strength and shrink/swell behaviour of highly plastic clay treated with geopolymer. Transp Res Rec 2672:174–184
Kushwaha P, Chauhan AS, Swami S, Swami BL (2019) Investigating the effects of nanochemical-based ionic stabilizer and co-polymer on soil properties for pavement construction. Int J Geotech Eng. https://doi.org/10.1080/19386362.2019.1635817
Lu X, Xia C (2015) Experimental study of compaction effects and proportion of expansive soil improved by ionic soil stabilizer. In: International conference on advances in energy, environment and chemical engineering (AEECE)
Liu Q, Xiang W, Zhang W, Cui D (2009) Experimental study of ionic soil stabilizer-improves expansive soil. Rock Soil Mech 8:16
Petry T (1997) Performance-based testing of chemical stabilizers. J Transp Res Board 1589:36–41
Rauch AF, Katz LE, Liljestrand HM (2003) An analysis of the mechanisms and efficacy of three liquild chemical soil stabilizers. Research report 1993-1. Center for Transportation Research, University of Texas at Austin, Austin, pp 1–204
Scholen DE (1992) Non-traditional stabilizers. Report FHWA-FLP-92-011
Scholen DE (1995) Stabilizer mechanisms in nonstandard stabilizers. In: Conference proceedings 6: sixth international conference on low-volume roads, vol 2, pp 252–260. TRB, National Research Council, Washington, DC
Sides G, Barden L (1970) The microstructure of dispersed and flocculated samples of kaolinite, illite and montmorillonite. Can Geotech J 8:391–399
Tingle JS (1989) Stabilization mechanisms of non traditional additives. Transp Res Board 1989:59–67
Xiang WC (2010) Theory and practice of ionic soil stabilizer reinforcing special clay. J Earth Sci 21:882–887
Acknowledgements
This study was supported by TX ProChemical Inc. The authors are grateful to Mr. Scott Horn for assistance in the collection of the test soil samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gautam, S., Hoyos, L.R., He, S. et al. Chemical Treatment of a Highly Expansive Clay Using a Liquid Ionic Soil Stabilizer. Geotech Geol Eng 38, 4981–4993 (2020). https://doi.org/10.1007/s10706-020-01342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-020-01342-1