Abstract
As a high-efficiency and environment-friendly stabilizer material, ionic soil stabilizer (ISS) has been widely used in construction industries. However, the microstructure characteristics and mechanical behavior of the treated soil still need a case study based on vary characteristics of soils and different treatments. In this paper, microscopic and macroscopic testing methods were adopted to explore the microstructure characteristics and mechanical behavior of the acidic ISS-treated clay versus the traditional alkaline lime and cement-treated clay. The results indicate that the soil particles become much more flocculated because of the ion exchange reaction between the soil and the ISS relying on the unique molecular structure of the ISS. The unconfined compressive strength (UCS) significantly increases, but still could not satisfy the requirement of road bearing capacity. However, the UCS improves rapidly with the increase of alkaline lime and cement. In addition, there is a benefit-period when the UCS of acidic and alkaline stabilizer-treated soil is greater than that of lime and cement-treated soil, whereas the strength of lime and cement-treated soil is lower than that of the combined treated one after this period. This study investigates the microstructure characteristics and mechanical behavior of the ISS-treated clay, which provides a theoretical and scientific support for appropriate road construction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stone and gravel are widely implemented as primary road materials; however, a series of environmental pollution issues are brought out during the production processes. Hence, the local soil is considered as an alternative to satisfy the need of road materials and the environmental protection request, whereas the strength of natural soil could not reach the engineering requirement. Generally, traditional alkaline stabilizers, including lime, Portland cement, and fly ash, are utilized to enhance the soil strength after impaction (Prusinski and Bhattacharja 1999; Harris and Scullion 2009; Campbell and Jones 2011; Mutaz et al. 2011). However, these stabilizers mainly come from the mining and calcination of limestone, which requires limestone to be fired in kilns at over 1200 °C. The production process of these stabilizers highly impacts the environment. In this circumstance, the ionic soil stabilizers (ISSs) are invented to enhance the strength of soils in terms of their convenient construction process and higher stabilization function (Katz et al. 2001; Jiang et al. 2009; Onyejekwe and Ghataora 2015; Yang et al. 2016; Luo et al. 2016b). A series of microscopic and macroscopic tests are conducted to analyze the microstructure characteristics and mechanical behaviors of the ISS-treated soil, especially the treated sand soil and the treated expansive soil. It has been proven that the ISS could reduce the consumption of water and natural resources, conserve base materials, minimize maintenance cost, and also decrease the influence of the road construction.
Currently, the microscopic tests, including the scanning electron microscopy (SEM), the X-ray diffractometry (XRD), the energy-dispersive X-ray spectrometry (EDAX), and the Fourier transform infrared spectroscopy (FTIR), have been utilized to explore microstructure characteristics and the stabilized mechanical behaviors of the ISS-treated soil (Katz et al. 2001; Tingle and Santoni 2003; Eisazadeh et al. 2011; Higo et al. 2011; Latifi et al. 2014; Latifi et al. 2015). The results indicate that the ISS has significant properties of high conductivity and strong acidity. The ISS generates acidic condition where the ISS could accelerate the cementation action of the iron ion in red clay to improve the soil strength. The particles of the treated soil are contacted in a face-to-face form, which makes the soil particles’ structure closer and leads to a formation of larger particles gradually. The X-ray diffraction atlas of the treated soil illustrates that no new diffraction peak or diffraction eigenvalue was observed, which indicates there is no new mineral production after treating with the ISS (Blanck et al. 2014; Zhao et al. 2014; Luo et al. 2016a). In addition, the laboratory mechanical tests are also effective to evaluate the stabilized performance and mechanical behavior of the ISS-treated soil including the unconfined compressive strength (UCS) test, the direct shear test, the compaction test, the indirect tensile test, the freezing and thawing test, the loading fatigue test, and the moisture and temperature shrinkage test (Tingle and Santoni 2003; Argu 2008; Arabani et al. 2012; Eisazadeh et al. 2012; Rauch et al. 2002). Previous studies indicate that the ISS has significant effectiveness in enhancing the pavement performance of the soil by improving of the compressive strength during different curing time and under different compaction degrees (Argu 2008; Mgangira and Ndibewu 2010; Moloisane and Visser 2014; Tao et al. 2016).
As mentioned above, significant progresses have been made in the research of microstructure characteristics and mechanical behavior of ISS-treated expansive soils and sandy soils. However, different kinds of soils vary in physical and mechanical characteristics which needs a case study before the implementation of the ISS. In this paper, the studied red clay has many special properties including significant reticulate characteristics, high clay contents, low density, high liquid and plastic limit, and poor compactability, which result in many engineering hazards such as shrinkage crack, differential settlement, soil erosion, and softening. Therefore, the main objectives of this study are to analyze the microstructure characteristics of the ISS-treated clay and compare the mechanical behavior of the ISS-treated clay with the traditional lime and Portland cement–treated clay using microscopic and macroscopic testing methods. Specifically, this paper aims to
-
Investigate the microstructure characteristics of the ISS-treated clay;
-
Analyze the increasing trend of the strength of the acidic and alkali-treated clay;
-
Identify the benefit-period for the strength improvement of the ISS-treated clay.
Experimental section
Materials
Soil
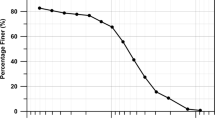
The red clay, originated from weathered shales, was selected for the test program. The average pass percentage of particle size “>0.075 mm,” “0.075–0.002 mm,” and “<0.002 mm” are 3.1%, 96.4%, and 0.5%, respectively. The particle size distribution curve is shown in Fig. 1. The organic content is less than 5%. The liquid limit and plastic limit are both 43.2% and 23.7%, respectively. The optimum water content (Wopt) and the maximum dry density (ρmax) are 16.75% and 1.792 g/cm3. The basic physical parameters were obtained by the way of particle size distribution, specific gravity test, crucial water content coefficient, loss on ignition, organic matter content, pH, optimum water rate, maximum dry density, and CBR. The results are shown in Table 1. Then the soil was classified by the way of fine soil classification method; the result presented that the typical soil was low liquid limit clay, denoted by CL (Luo et al. 2016a).
Stabilizer
The ISS adopted in this study is a kind of surface-active agents defined as EN-1 by Roadbond company in the USA. The Properties of the ionic soil stabilizer are shown in Table 2. It has a complex chemical formulation with the active ingredient of a sulfonated oil. The molecule of sulfonated oil contains a hydrophilic head and a hydrophobic tail (Fig. 2). The hydrophilic head is a sulfonic acid moiety which is completely soluble in, or miscible with, water and insoluble in most non-polar organic solvents. The hydrophilic head, which is linked via the sulfur atom to the hydrophobic tail, dissociates and produces an anionic SO3− group when the sulfonated oil disperse in water. The hydrophobic tail, which contains an assembly of carbon and hydrogen atoms, is completely insoluble in (immiscible with) water. The treatment ISS provides a direct chemical bond between the SO3− anionic and a metal cation both on the external and planar surfaces of soil particles. There is a strong connection between these bonds. Once the sulfonated oil molecules formed their associations with the clay particles, the hydrophobic tail turns toward outside of the planar surfaces. Then a protective layer is formed around the clay particle and also on its underlayer surfaces. Thus, the hydrophobic tails could squeeze water out of the clay particles without mechanical pressure. This dual behavior of the sulfonated oil could enhance the effectiveness of water reducing in clay and produce a permanent association between the ISS and the clay particles when it is adopted as a compaction aid in road construction.
The ion concentration results of the ISS coming from the plasma emission spectrometer (ICP-AES) and ion chromatograph (DX-120) are listed in Table 3. When the ISS was diluted 100 times by distilled water, there were four kinds of cations and three kinds of anions, including K+, Na+, Ca2+, Mg2+, Cl−, SO42−, and NO3−. The pH value of the diluent was less than 7. The acid solution can strengthen the cementation of the red clay and the abundant iron ion; eventually, it can enhance the strength and form a positive mechanical effect.
Experimental scheme
Microstructure characteristics of the treated soil
Analyzing microstructure characteristics of the treated soil is an effective way to investigate the stabilization mechanical behaviors of the ISS on clay soils. In this paper, the main research indicators focused on metal cation exchange, mineralogical composition change, microstructure morphology, and microvoid structure characteristic. They were investigated by the plasma emission spectrometer, the X-ray diffractometer, the scanning electron microscopy, and the surface area and pore size analyzer, respectively. The detailed experimental designs were explained as follows.
The change of the metal cation concentration in the clay-water solution before and after treatment was measured by using the plasma emission spectrometer (ICP-AES). The ion exchange is vital because the physical properties (e.g., plasticity) of the clay are mainly dependent on the exchangeable ions carried by the clay. For instance, the plastic properties of the clay are highly dependent on whether Na+ or Ca+2 is the exchangeable cation. Therefore, it is possible to alter the plastic characteristics of soils to meet the specific needs by carrying out specific ion exchange reactions. Treated specimens were prepared by mixing seven different mass ratios of the ISS with the original clay, including 0%, 0.01%, 0.012%, 0.014%, 0.016%, 0.018%, and 0.02%. The specimens were cured in a moisture room to trigger reactions between ISS and the soil. After that, 30 g weight of the corresponding treated soils was put into seven beakers, respectively. After being mixed with distilled water, they were stirred by a magnetic stirrer for 1 h. After 2-h standing, the supernatant liquid was acquired to conduct the plasma emission spectrometer test to determine the metal cation concentrations.
The mineralogical compositions before and after treatment were estimated by the XRD to determine whether there is a new substance generated during the treatment process. The XRD was used to analyze the structure of a material from the produced scattering pattern through the interaction of beams of radiation. The treated specimens of the XRD analysis involved seven different mass ratios of the ISS including 0%, 0.01%, 0.012%, 0.014%, 0.016%, 0.018%, and 0.02%. The mineralogical compositions were analyzed according to W.L. Bragg equation presented as Function (1) (JCPDS 1995). It indicated that if the λ value was fixed during the testing process, different d values could be determined according to the incident angles of the XRD, and then the clay mineral phase could be analyzed and identified.
where d is the spacing between diffracting planes, θ is the incident angle, and λ is the wavelength of the beam. The SEM is a common technique used to determine the morphology of materials including size, shape, orientation, and aggregation. This method could be implemented in soil stabilization studies to visualize the topographical features and observe the formation of new cementitious materials. In this study, the Hitachi S-4800 scanning electron microscope was selected to analyze morphological microstructure changes of the treated soils. The treated specimens contained seven different mixing amounts of the ISS (i.e., 0%, 0.012%, 0.014%, 0.016%, 0.018%, and 0.02%). In this technique, each specimen was sputtered with platinum for 120 s at 30 mA under high vacuum until they were completely covered and ready to be used for the microscopic morphology analysis (Eisazadeh et al. 2011).
The characteristics of specific surface area and pore microstructure of the treated soil are the essential parameters to understand the effectiveness of the ISS to clay regarding so many chemical reactions and changes occurred at the surface of the soil (Mitchell and Soga 2005). The most typical approach for evaluating the surface area of thinly separated materials was gathering the isotherm report for the physical adsorption of an inert gas and modeling the adsorption figures in terms of the Brunauer-Emmett-Teller (BET) theory (Brunauer et al. 1938; Rauch et al. 2003). In this study, the surface area of the treated soil was measured by means of the surface area and pore size analyzer (JW-BK100A). It was derived through the physical adsorption of nitrogen to analyze characteristics of the specific surface area and pore microstructure of the treated soil. After preparing the testing specimens, 0.2 g treated soil was placed in a specimen container. After degassing for 3 h at 110 °C, the nitrogen gas was pumped in, and the outer surface area and pore size were estimated in accordance with the BET technique and the BSISO 13320:2009.
Mechanical behavior of the treated soil
A series of mechanical property tests under different compaction degrees and during different curing periods were employed to explore the effectiveness of different stabilizers. Furthermore, the synergistic effect of the acidic and alkaline stabilizers was examined by the UCS.
The UCS is the main index to control the mechanical property, and the accuracy of UCS tests is directly affected by its testing method. The original soil was sieved by a 2-mm mesh to confirm the uniformity of the soil. The first step was to determine the optimum moisture content (OMC) for the original soil and the treated soils with different ratios of stabilizer (BS1377 1990). The second step was to mix the soil with the specified ratio of stabilizers. The third step was to determine the dry density and the moisture content by compressing the specimens in a steel cylindrical mold (50 mm diameter and 50 mm height). The required compaction was verified by a hydraulic jack using a persistent compaction (BS1377 1990). The loading pressure was ranged from 200 to 1000 KPa; otherwise, the unloading pressure was reduced to 100 KPa. The loading and unloading stages needed to be insisted for 24 h (Ahmad et al. 2011; Chew et al. 2004; Bobet et al. 2011). The fourth step included extruding the cylindrical specimens by a steel plunger, trimming and cleaning the releasing oil, placing the specimens in aplastic bottle, and wrapping with plastic wrap for several runs. These specimens of different tests were cured during different curing periods in a moisture room at 20 ± 2 °C (Zhang et al. 2012). It should be noted that all specimens were made under the OMC and maximum dry density (Ahmad 2004). The six specimens of each soil mix design and each curing time were prepared in order to verify the accuracy of the results (Latifi et al. 2015).
The optimum ISS ratio was based on the UCS test. The UCS under different compaction degrees and during different curing periods was tested to explore the effects of acidic and alkaline stabilizers. The ISS was selected as the acidic stabilizer and the lime and Portland cement were chosen as the alkaline stabilizer. The UCS under compaction degrees of 90%, 92%, 94%, 96%, and 98% was employed to discuss the relationship between compaction degrees and soil strength. The curing periods of 7 days, 28 days, 60 days, 90 days, and 180 days were adopted to evaluate the change of strength in different curing time.
Results and discussion
Stabilized mechanical behaviors analysis
The solutions of treated soil specimens are shown in Fig. 3. Ion concentration characteristics of solution
The result of supernatant of treated soil specimens in Table 4 shows that the concentrations of Ca2+ and Mg2+ in the treated soil supernatant were lower than those in the original soil supernatant. When the ratio of the ISS was 0.014%, the concentrations of Ca2+ and Mg2+ decreased rapidly. But the concentrations of Na+ and K+ increased with the incorporation of the ISS. Compared with four kinds of cation concentrations, it was found that there was a cation exchange reaction between the ISS solution and clay particles. With the decrease of the Ca2+ and Mg2+ and the increase of Na+ and K+, the divalent Ca2+ and Mg2+ were intercepted and absorbed by clay particles because of the ion exchange reaction. At the same time, the Na+ and K+ were desorbed significantly (Luo et al. 2016a).
Change in the mineral composition
The XRD patterns with different stabilizer ratios are presented in Fig. 4. After checking the characteristic spectral lines of typical non-clay minerals and clay minerals, the characteristic diffraction peaks of the quartz, feldspar, hematite, kaolinite, and illite were found in the X-ray diffraction pattern of the original soil in Fig. 4. Hence, the original soil contained the non-clay minerals of the quartz, feldspar, and hematite and the clay minerals of the kaolinite and illite. Comparing the X-ray diffraction pattern and characteristic diffraction peaks of the treated soil specimens versus those of the original soil specimen, there was no new characteristic value of the mineral diffraction peaks. Therefore, it can be indicated that there was no new mineral production after the ISS treatment. In addition, the diffraction peaks of soil still existed, whereas the individual spacing decreased slightly. It reflected that the ISS did not disintegrate or damage the clay mineral lattice structure, but it changed the cation on the surface of clay particles.
Morphological characteristics of microstructure
In order to determine the surface composition of the ISS-treated soils, the electron microscope scanning was used to analyze the morphological characteristic of the original specimens and the treated ones. The SEM images with the ISS ratios of 0%, 0.01%, 0.012%, 0.014%, 0.016%, and 0.018% are presented in Fig. 5 a to f, respectively. There was a significant change in the surface composition of clay particles after the ISS treatment. The micrographs (magnification × 15,000) showed that the microstructure of the treated soils was more compact than that of the original soil. The major structures were point-to-face and edge-to-face, whereas there was almost no turbulence structure or flocculation in the treated soil. Compared with the original specimen, the clay particles of the treated soils with different ratios of the ISS were coated and bonded together and their porosity was reduced greatly. Moreover, the major structures of treated specimens were contacted face-to-face.
According to the SEM results, different skeletons of soil particles were bonded together to form a stacked structure because the original red clay contained free iron, silicon, aluminum, and other materials. It indicated that the improvement of soil particle attraction, the aggregation of soil microscopic structures, and the decrease of pore quantity resulted in big changes in the soil and the pore structure, which made the structure contact more closely. Therefore, the ISS enhances the strength of red clay significantly.
Pore structure characteristics of soil particles
The surface area/pore size tester and BET nitrogen adsorption method were employed to get the surface area and the pore structure of soils. The results of surface area under different ISS treatments are presented in Fig. 6.
The result in Fig. 6 shows that the soil particle size increased significantly, which resulted in a decrease of the specific surface area. The minimum surface area of soil particles was 29.21 m2/g with the treatment of ISS ratio 0.014%. Compared with the original soil, the specific surface area of the treated soils decreased significantly. It indicated that the ISS, as an electrolyte, could dissociate charged cationic and anions. Furthermore, the Ca2+ and Mg2+ in the ISS exchanged the K+ and Na+ from the surface of clay particles, which resulted in the thickness decrease of the electric double layer and the hydrated film. These reactions increased the attractiveness of soil particles. Hence, particles became closer and closer, which promoted the aggregation and condensation of soil particles. Eventually, the treated soils formed larger particles gradually and the microstructure changed significantly. These also occurred in the scanning electron microscopic structure and the investigation of changes in ion concentration.
Figure 7 indicates the correlation between pore volume and vacuum pressure; the pore volume desorption isotherms of the treated soil had slight changes with different ratios of the ISS. Under the lower pressure conditions, each pore volume of the treated soils was smaller than that of the original soil, which indicated that the main reason for the change of the soil pore structure was the treatment of the ISS. The soil became denser with the reduction of porosities. The pore volumes with different ratios of the ISS are illustrated in Fig. 8. The total pore of treated soils with different ratios of ISS became smaller, which showed the size of soil particles changed significantly. After that, the soil particles became denser and closer with the decrease of the soil particle pores. These results are consistent with the specific surface area results.
In conclusion, a series of stabilized mechanical behaviors illustrated the decrease of the hydrated film thickness absorbed on the surface of particles and the increase of the attraction between the particles. The particle distribution became closer due to the reduction of the porosity among the soil particles. Finally, the treated soils were formed into bigger aggregates and its structure became denser. In fact, there were mainly two reasons for the decrease of hydrated film thickness, one is the ion exchange reaction, and the other is the unique molecular structure of the ISS.
Macro-mechanical property analysis of the stabilized soil
The optimum ratio of ionic stabilizer
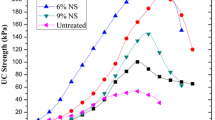
UCS tests were performed on the control soil specimens and ISS-treated ones. Five different stabilizer ratios (0.010%, 0.012%, 0.014%, 0.016%, and 0.018%) were used to treat the soil. Two compaction degrees (96% and 98%) were involved in the producing of the specimens. Seven specimens were prepared for each stabilizer ratio under each compaction degree condition. The results of the UCS are presented in Table 5 and Fig. 9. The UCS of specimens with 96% and 98% compaction degrees was considerably improved after the ISS treatment. With the improvement of the compaction degree, the effectiveness of the strength increase became more significant. The soil strength firstly enhanced quickly and then decreased slightly with the continual increase of the ISS ratio. When the ISS ratio was 0.014%, the UCS reached the maximum value. Therefore, in this study, the optimum ratio of the ISS in treating the red clay soil was 0.014%. The UCS of the soil improved with the addition of the ISS, but it still could not satisfy the strength requirements of the road base or sub-base material for heavy vehicles (base requirements ≥ 3.0 MPa; sub-base requirements ≥ 2.0 MPa). Therefore, the traditional stabilizers, such as cement and lime, were recommended as additional stabilizer to improve the strength of the treated soils when used the ISS to treat the road base material.
Unconfined compressive strength with different compaction
Based on the analysis of the soil strength with different ISS, the ISS ratio 0.014% was selected to design a series of UCS experiments with different stabilizer mixtures; the ratios of different mixtures were numbered in Table 6.
After storing the specimens in the moisture room for 7 days, the UCS tests with compaction of 90%, 92%, 94%, 96%, and 98% were carried out, and the results are presented in Fig. 10. With the increase of compaction, the UCS of specimens a and b were improved significantly. Under the low compaction (90%, 92%, and 94%), the UCS of the treated soil with the ISS was lower than that of the treated soil without the ISS for specimens c, d, e, and f (except c and d under compaction 94%). When the compaction increased to 96%, the UCS of the treated soils with the ISS was greater than that of the treated soil without the ISS. These phenomena indicated that the ISS required the compaction reaching 96% to effectively stabilize the soil. In addition, no matter under what level of compaction (including 90%, 92%, 94%, 96%, and 98%), the UCS of mixture b was greater than that of mixture a. It showed that the higher incorporation of lime (≥ 5%) lowered the compaction requirements of the ISS in clay soil.
Unconfined compressive strength with different curing time
The UCS values of treated soils with the curing periods of 7 days, 28 days, 60 days, 90 days, and 180 days are showed in Fig. 11. After curing 7 days, 28 days, and 60 days, the UCS values of b were greater than those of a. However, after curing 90 days and 180 days, the UCS values of a were greater than those of b. These also occurred in the c and d. The UCS values of e and f were greater than others during all the curing periods. Moreover, when the curing periods were 60 days, 90 days, and 180 days, the UCS values of e were greater than that of f. However, the UCS of f was greater than that of e when the curing time was less than 28 days.
The results show that the UCS of the treated soils increased steadily by adding the alkaline stabilizers (lime and cement). When the curing time increased, the UCS of the treated soil continually improved with the addition of both the acidic stabilizer (ionic stabilizer) and the alkaline stabilizers (lime and cement). In a specific period of curing time, the UCS of the acidic and alkaline stabilizer-treated soils were always greater than those of the alkaline stabilizer-treated soils. After this period, there was a strength deterioration. The UCS of the alkaline stabilizer-treated soils surpassed that of the acidic and alkaline stabilizer-treated soils. Hence, there was a benefit-period with the treatment of both acidic and alkaline stabilizers. Thus, the linear differences were obtained by the UCS results in different curing time. The results in Fig. 12 illustrate that with the coexistence of acidic and alkaline stabilizers, the benefit-periods of mixture a and b, mixture c and d, and mixture e and f were 71 days, 68 days, and 39 days, respectively.
As the pH value could reveal the H+ and OH− concentrations in solution; the solution pH values of specimens with different stabilizers were analyzed to explore the internal pH of the treated soils. Three groups of typical mixtures were selected, including a and b, c and d, and e and f, to explore the internal pH of the treated soils. After 1 day curing time, the pH values of these specimens were measured, and the results are shown in Table 7.
The results indicate that with the addition of lime and cement, the pH value of the ace increased to more than 7, whereas the pH value decreased quickly in mixtures b, d, and f. Presumably, as the coexistence of acidic and alkaline stabilizers in soil, the H+ electrolyzed from the ISS and the OH− electrolyzed from lime and cement, following, the OH− and the H+ consumed by the acid-base neutralization reaction. Eventually, the pH value of the specimens decreased significantly. The reaction equations are presented as below.
Overall, during the benefit-period, the strength of acidic and alkaline stabilizer-treated soils increased rapidly. Its rising tendency slows down after the benefit-period. On the contrary, the alkaline stabilizer-treated soils showed a slow and continuous increasing trend. Hence, the UCS of the alkaline stabilizer-treated soils was higher than that of the acidic and alkaline stabilizer-treated soils with the curing time increasing. This result could make up the shortage of the traditional alkaline stabilizers in which the early strength improvement trend was very slow. And it can also provide a technical support for the rapid opening highway construction. Considering the long-term strength, the ISS had an opposite effect on the stabilizing process of the lime and cement. Additionally, there was a benefit-period for utilizing both the acidic and the alkaline stabilizers to improve the UCS of the clay.
Conclusions
The ISS is regarded as a new high-efficiency and environment-friendly soil stabilizer material. A series of tests validated that the ISS could enhance the pavement performance of the red clay. The effective application of the ISS was illustrated by the microstructure and mechanical characteristics. The conclusions obtained from the laboratory tests before and after the treatment of different stabilizers are summarized as follows:
-
(1)
The ISS could reduce the thickness of the surface hydrated film effectively, which led to the decrease of distance and the enhancement of attraction between particles. With the particles becoming closer, the aggregate structure grew larger. Therefore, the porosities of the ISS-treated soils were reduced in the way of strengthening the structure.
-
(2)
The XRD results indicate that there was no new mineral product in the soils after the ISS treatment. The cationic adsorbed on the surface of the soil particles exchanged ions with the ISS. Hence, the ISS did not break or disintegrate the mineral crystal lattice structure of the clay.
-
(3)
The SEM results illustrated that the microstructure of the ISS-treated soils aggregated significantly. With the increase of aggregates and the decrease of pores, the structure became denser and the soil strength became higher.
-
(4)
As the curing time and compaction increase, the UCS of both acidic and alkaline stabilizer-treated soils improved gradually. Compared with the UCS of the soils treated by different stabilizers, there was a performance benefit-period by adding the acidic and alkaline stabilizers. During this period, the strength of the treated soils with the acidic and alkaline stabilizers improved rapidly. On the contrary, the UCS of the alkaline stabilizer-treated soils would surpass that of the acidic and alkaline stabilizer-treated soil after the benefit-period.
In summary, as a kind of new high-quality road construction material, the strength of the ISS-treated soils has an advantage in a short curing time, which leads to a benefit-period during the period of stabilization. The comprehensive performance of the treated soils could arrive the best with the addition of the acidic ISS and the traditional alkaline stabilizers in the benefit-period. In this study, the researches cover the shortage of the traditional alkaline stabilizers lime and cement, which enhances the early soil strength slowly. In addition, the results provide technical support for the rapid opening highway construction. However, the single acidic ISS plays a worse stabilizing effect than the alkaline lime and cement in the long-term performance of the treated soils.
References
Ahmad KB (2004) Improvement of a tropical residual soil by electrokinetic process. Doctoral dissertation, UniversitiTekno-logi Malaysia
Ahmad KB, Taha MR, Kassim KA (2011) Electrokinetic treatment on a tropical residual soil. Proc Instit Civil Eng-Ground Improv 164(1):3–13
Arabani M, Haghi AK, Hashemi SA, Karami M, Nikookar M, Bahari M (2012) Properties of clayey soils stabilized by liquid ionic stabilizer. In Conference: 3rd International Conference on New Developments in Soil Mechanics and Geotechnical Engineering, pp. 28–30
Argu Y (2008) Stabilization of light grey and red clay subgrade soil using SA-44/LS-40 chemical and lime. Doctoral dissertation, Addis Ababa University
Blanck G, Cuisinier O, Masrouri F (2014) Soil treatment with organic non-traditional additives for the improvement of earthworks. Acta Geotech 9(6):1111–1122
Bobet A, Hwang J, Johnston CT, Santagata M (2011) One-dimensional consolidation behavior of cement-treated organic soil. Can Geotech J 48(7):1100–1115
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–319
BS1377 (1990) British standard methods of test for soils for civil engineering purposes. British Standards Institution, London
Campbell A, Jones D (2011) Soil stabilization in low-volume roads: obstacles to product implementation from additive supplier’s standpoint. Transp Res Rec 2204(1):172–178
Chew SH, Kamruzzaman AHM, Lee FH (2004) Physicochemical and engineering behavior of cement treated clays. J Geotech Geoenviron 130(7):696–706
Eisazadeh A, Kassim KA, Nur H (2011) Characterization of phosphoric acid-and lime-stabilized tropical lateritic clay. Environ Earth Sci 63(5):1057–1066
Eisazadeh A, Kassim KA, Nur H (2012) Cation exchange capacity of phosphoric acid and lime stabilized montmorillonitic and kaolinitic soils. Geotech Geol Eng 30(6):1435–1440
Harris P, Scullion T (2009) Stabilizer diffusion in swelling soils. 2009 International Foundation Congress and Equipment Expo: Contemporary Topics in Ground Modification, Problem Soils, and Geo-Support (ASCE) pp 566–573
Higo Y, Oka F, Kimoto S, Sanagawa T, Matsuhima Y (2011) Observation of microstructural changes and strain localization of unsaturated sands using microfocus X-ray CT. In: Advances in Bifurcation and Degradation in Geomaterials. Springer, Dordrecht, pp 37–43
JCPDS (1995) Index to the powder diffraction file. International Center for Diffraction Data, Swarthmore
Jiang T, Zhou Y, Liang S, Liu H, Han B (2009) Hydrogenolysis of glycerol catalyzed by Ru-Cu bimetallic catalysts supported on clay with the aid of ionic liquids. Green Chem 11(7):1000–1006
Katz LE, Rauch AF, Liljestrand HM, Harmon JS, Shaw KS, Albers H (2001) Mechanisms of soil stabilization with liquid ionic stabilizer. Transp Res Rec 1757(1):50–57
Latifi N, Eisazadeh A, Marto A (2014) Strength behavior and microstructural characteristics of tropical laterite soil treated with sodium silicate-based liquid stabilizer. Environ Earth Sci 72(1):91–98
Latifi N, Marto A, Eisazadeh A (2015) Analysis of strength development in non-traditional liquid additive-stabilized laterite soil from macro-and micro-structural considerations. Environ Earth Sci 73(3):1133–1141
Luo X, Qiu X, Wang Y, Wu J, Xiao S (2016a) Micro-treatment mechanism study on ionic soil stabilizer improving clay. Key Eng Mater 667:370–375
Luo X, Yang Q, Qiu X, Xiao S (2016b) Detecting and expressing of asphalt materials modulus considering temperature effect. Key Eng Mater 667:347–352
Mgangira MB, Ndibewu P (2010) Identification of microscale characteristics of treated subgrade materials and how they relate to macroscopic properties. 29th Southern African Transport Conference (SATC 2010), Pretoria, South Africa
Mitchell JK, Soga K (2005) Fundamentals of soil behavior, 3rd edn. Wiley, New York
Moloisane RJ, Visser AT (2014) Evaluation of the strength behaviour of unpaved road material treated with electrochemical-based non-traditional soil stabilisation additives. J South Afr Inst Civil Eng 56(1):28–39
Mutaz E, Shamrani MA, Puppala AJ, Dafalla MA (2011) Evaluation of chemical stabilization of a highly expansive clayey soil. Transp Res Rec 2204(1):148–157
Onyejekwe S, Ghataora GS (2015) Soil stabilization using proprietary liquid chemical stabilizers: Sulphonated oil and a polymer. Bull Eng Geol Environ 74(2):651–665
Prusinski JR, Bhattacharja S (1999) Effectiveness of Portland cement and lime in stabilizing clay soils. Transp Res Rec 1652(1):215–227
Rauch AF, Harmon JS, Katz LE, Liljestrand HM (2002) Measured effects of liquid soil stabilizers on engineering properties of clay. Transp Res Rec 1787(1):33–41
Rauch AF, Katz LE, Liljestrand HM (2003) An analysis of the mechanisms and efficacy of three liquid chemical soil stabilizers. Center for Transportation Research, The University of Texas, Austin
Tao JQ, Lin WY, Luo XH, Qiu X, Wu JH (2016) Compressive strength analysis of ionic soil stabilizer improving soil. Key Eng Mater 667:341–346
Tingle JS, Santoni RL (2003) Stabilization of clay soils with nontraditional additives. Transp Res Rec 1819(1):72–84
Yang Q, Luo X, Qiu X (2016) Analysis on mechanical behavior characteristics of stabilized clay under the coexistence condition of acidic and alkalic additives. J Highway Transpo Res Dev 6:8
Zhang T, Xu YY, Wang H (2012) Application and curing mechanism of soil stabilizer. Adv Mater Res 557:809–812
Zhao H, Ge L, Petry TM, Sun YZ (2014) Effects of chemical stabilizers on an expansive clay. KSCE J Civ Eng 18(4):1009–1017
Funding
This work was financially supported by the Natural Science Foundation of Zhejiang Province (LY18E080020 and LQ20E080009), the Key Laboratory of Infrastructure Durability and Operation Safety in Airfield of CAAC (MK201901), and the Zhejiang Provincial Key Laboratory of Urban Rail Transit Intelligent Operation and Maintenance Technology & Equipment.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Luo, X., Xu, W., Qiu, X. et al. Exploring the microstructure characteristics and mechanical behavior of the ionic soil stabilizer-treated clay. Arab J Geosci 13, 729 (2020). https://doi.org/10.1007/s12517-020-05708-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-05708-w